Abstract
Purpose
To evaluate dry eye disease (DED) signs and symptoms six months after a single treatment with Localized Heat Therapy (LHT) (TearCare, Sight Sciences) for patients previously treated for six months with cyclosporine (0.05%) ophthalmic emulsion (CsA) BID (Restasis, Allergan).
Setting
Nineteen ophthalmic and optometric practices in 11 US states.
Design
Multicenter, cross-over, six month extension to the SAHARA randomized, controlled trial (RCT). Included patients were those randomized to CsA in Phase 1 of the SAHARA RCT.
Methods
This was the second phase of the SAHARA RCT in which, following the 6-month endpoint, all patients that had been randomized to CsA discontinued CsA and were treated with LHT and subsequently followed for an additional six months. Outcome measures at 12 months for CsA patients crossed over to LHT included TBUT, OSDI and MGSS.
Results
One hundred and sixty-one patients (322 eyes) were analyzed. Mean (SD) baseline TBUT prior to CsA was 4.4 (1.2) seconds, 5.6 (2.6) at 6 months which improved to 6.6 (3.2) and 6.1 (2.8) seconds (both P < 0.001) at 9 and 12 months (3, 6 months post LHT). Mean (SD) OSDI was 50.0 (14.9) at baseline and 34.2 (21.5) after CsA. With LHT at 6 months, this improved to 30.0 (20.6) and 31.0 (19.5) at 9 and 12 months (P = 0.162 vs month 6, P < 0.0001 vs baseline). MGSS was 7.1 (3.2) at baseline, 13.3 (8.2) at the end of CsA treatment which improved to 17.4 (8.8) and 16.1 (9.0) at 9 and 12 months; both P <0.001.
Conclusion
SAHARA showed 6-month superiority of LHT to CsA in clinical signs and non-inferiority in symptom scores. This extension shows that patients treated with CsA for 6 months can achieve meaningful additional improvement in signs and symptoms lasting for as long as 6 months following a single LHT treatment without the need for topical prescription therapy.
Introduction
Dry eye disease (DED) is a chronic affliction involving the ocular surface, with an estimated prevalence of between 7 and 15% in the US and up to 50% in other regions.Citation1,Citation2 Meibomian gland dysfunction (MGD) is generally considered to be the leading cause of dry eye accounting for at least half and up to 86% of DED.Citation3–6 MGD is a common, chronic, and progressive disorder in which the structure and/or function of the meibomian glands at the margin of the eyelids is abnormal and compromised resulting in decreased meibum secretion, and consequently, evaporative dry eye.Citation7
The SAHARA study was a randomized controlled clinical trial designed to compare the effectiveness of localized heat therapy, gland expression and lid debridement (TC, TearCare, Sight Sciences, Menlo Park, CA, USA) with cyclosporine 0.05% ophthalmic emulsion (CsA; Restasis; Allergan, an AbbVie company, Dublin, Ireland), the leading prescription pharmaceutical, in treating the signs and symptoms of DED. Phase 1 of the study was a head-to-head comparison of these treatments with an endpoint at six months after initiation of treatment, TC at baseline and month 5, CsA BID for six months.
In phase 2 of SAHARA, reported here, our aim was to demonstrate that cessation of CsA and treatment with a single TC procedure would result in outcomes six months later that replicated what was observed in phase 1 for the TC treatment arm. Specifically, maintenance of the improved symptoms (and possible improvement) and improvement of DED signs.
Methods
Study Design
SAHARA was a 24-month prospective multicenter, randomized, controlled trial described previously in the report of phase 1 results.Citation8 The study was IRB approved (WCG IRB Puyallup WA, USA) and was listed on the NIH clinical trials registry (clinicaltrials.gov NCT04795752) prior to the enrollment of any patients. The trial followed the principles of the Declaration of Helsinki and all patients provided written informed consent prior to participation.
Study Population
The SAHARA study enrolled adult patients that reported experiencing dry eye symptoms in the 3–6 months preceding screening and use of artificial tears or lubricants to relieve these symptoms for at least one month prior to screening. Both eyes had to meet specific criteria including anesthetized Schirmer score (>5 mm and <15 mm), moderate-to-severe OSDI score (23–79), TBUT between 1 and 7 seconds), a meibomian gland secretion score (MGSS) of <12, 15 expressible meibomian glands in each lower lid, and best-corrected visual acuity (BCVA) at least 20/200. The anesthetized Schirmer testing was selected because it measures basal rather than reflexive tearing which, we believe, is more likely to detect abnormally low amounts of tearing. Patients were excluded if they had been treated with: (within 60 days) cyclosporine or lifitegrast (within 30 days), oral tetracyclines or azithromycin, topical ophthalmic medications including antibiotics, ocular hypotensives, steroids, non-steroidal anti-inflammatory drugs (within 7 days), antihistamines. Any previous use of isotretinoin was prohibited. Systemic medications known to cause ocular dryness (eg, tricyclic antidepressants) were allowed, but the dosing regimen must have been stable for a minimum of 30 days. Other exclusions included office-based treatments for dry eye within 12 months such as intense pulsed light, TC, or thermal pulsation; planned or previous (within 12 months) eye or eyelid surgery, meibomian gland expression within 6 months, blepharoexfoliation or debridement within 3 months, punctal occlusion or plugs, tear neurostimulator within 2 weeks, or any meibomian gland probing. Patients with a history of herpetic eye disease, active or recurrent inflammation, clinically significant anterior blepharitis with or without caollarettes or flakes, or other conditions of the eye and adnexa that could confound study results were also excluded. Contact lenses could not be worn during the study period or within 2 weeks of baseline.
In phase 1 of the SAHARA trial patients had been randomized 1:1 to either TC or to CsA and followed for six months to the study primary endpoint. In phase 2 of the study, beginning at the 6 month visit, patients in the CsA arm stopped use of CsA and were treated with TC to be followed for an additional six months. At the 6 month visit, patients completed the OSDI, Symptom Assessment in Dry Eye questionnaire (SANDE), and Eye Dryness Score (EDS) visual analog scale and were queried regarding frequency of use of artificial tears or lubricants. Patients then underwent a thorough ophthalmic examination including assessment of BCVA, slit-lamp exam, TBUT, corneal and conjunctival staining, Schirmer test (STS), MGSS, IOP and recording of any adverse events (AE). Over the ensuing 6 month follow-up patients were seen 1 day, 1 week, and at 1, 3 and 6 months following their 6 month TC procedure with a complete examination and assessment (as detailed above) at each visit except the 1-day post TC where the exam was limited to BCVA, slit-lamp exam, IOP and AE assessment. Patients that had been randomized to TC in phase 1 of SAHARA continued follow-up through a total of 24 months with additional TC treatment provided when warranted according to specific protocol-defined OSDI and TBUT criteria. This portion of the study is currently ongoing and will be the subject of a subsequent report.
TearCare Treatment
The TC treatment was described in detail in our previous report.Citation8 In brief, eyelid margins underwent debridement under topical anesthesia. Makeup and other foreign materials were removed with a non-moisturizing make-up removal wipe and allowed to dry. Upper and lower SmartLids were applied to the patient’s eyelids and secured to the temples. The SmartLids were then connected to the SmartHub, and the TC procedure was initiated with temperature increasing automatically every 30s until reaching the final meibum melting temperature of 45°C which was held for 15 minutes. At this point, the SmartHub stopped the heating and the SmartLids were removed. Repeat debridement was performed if warranted and four lid meibomian gland expression using the Clearance Assistant Plus device was performed as described previously.Citation8 TC treatment was performed once only, at the beginning of the cross-over period (Month 6 study visit).
Endpoints
Endpoints for the phase 2 cross-over (CsA to TC) portion of the study were the change from baseline (TC treatment at month 6) at the 12 month visit after 6 months of follow-up for TBUT, MGSS, number of meibomian glands yielding any liquid secretions (MGYLS), number of meibomian glands yielding clear secretions (MGYCS), STS, OSDI, SANDE, EDS, and corneal and conjunctival staining. Data from both eyes were analyzed for eye-level outcomes. Analysis was with t-tests (two-sample, two-sided alpha of 0.05) except when assumptions of normality or equal variance were violated, and the Mann–Whitney rank sum test was used. All statistical analyses were conducted using SigmaStat 4.0 (Grafiti LLC, Palo Alto, CA, USA). Safety analysis included the frequency of AE and changes in BCVA and IOP.
Methodology for TBUT, MGSS, and corneal and conjunctival staining was exactly as previously described.Citation8 TBUT was measured at the slit lamp under cobalt blue illumination approximately 30 seconds after fluorescein installation. MGSS followed the method of Korb and Blackie.Citation9 Corneal and conjunctival staining were assessed as described in Gupta et al with fluorescein (cornea) and lissamine green (conjunctiva).Citation10
Results
One hundred sixty-three patients randomized and treated with CsA during phase 1 of the trial were crossed over to TC treatment at the 6-month visit. Two of these patients discontinued during follow-up after cross-over (1 at 1 month post-cross-over due to patient decision; 1 at 3 months post cross-over for reason given as “other”; neither was due to an AE) leaving 161 available for analysis. Compliance with CsA dosing during phase 1 was considered good based on the number of units issued, an average (SD) of 5.7 (1.2) units per patient over the 6-month period; 96% used ≥5. Each 5.5 mL bottle was intended to provide a 1 month supply. Demographics and baseline characteristics were reported previously;Citation8 average age 56.8 years, majority female (74%), majority White (87%), pre-study mean OSDI 50 (14.9), pre-study TBUT 4.5 sec (1.2) and 4.3 sec (1.2) for OD and OS, respectively. Values for pre-phase 1 of the study and pre-phase 2 (at month 6 prior to cross-over) for each of the outcome variables are provided in .
Table 1 Study Baseline and Cross-Over Baseline Ocular Characteristics
Ocular Symptoms
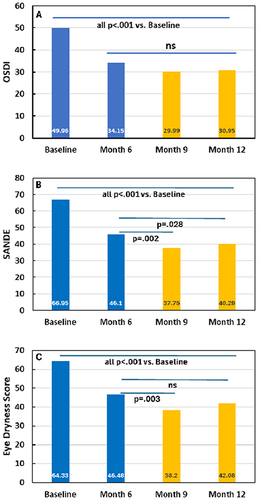
OSDI had improved for CsA patients from the pre-study baseline mean of 49.96 (SD 14.87, median 50.00) to 34.15 (21.45, 31.80) at the end of the 6 month CsA treatment period (p < 0.001) with small, additional, non-statistically significant decreases at three and six months after cessation of CsA and a single TC treatment (). In contrast to the OSDI, SANDE scores showed statistically significant improvement after the cross over to TC. At the cross-over visit mean SANDE was 46.10 (SD 24.23, median 46.13) and decreased to 37.75 (25.22, 35.28, p = 0.002) and 40.29 (25.71, 37.99, p = 0.028) at 3 and 6 months post cross over, respectively (). EDS also showed improvement after crossover; 46.48 at the cross-over visit (SD 24.80, median 48.00), 38.20 (24.86, 35.00; p = 0.003) three months later (study month 9), and 42.08 (26.23, 41.00, p = 0.129 ns) which did not reach statistical significance at six months after cross-over to TC ().
Figure 1 Ocular symptoms for patients randomized to CsA during phase 1 (blue bars) and phase 2, post-crossover (gold bars). A. OSDI (ocular surface disease index), (B) SANDE (symptom assessment questionnaire in dry eye), (C) EDS (eye dryness score). CsA = cyclosporine ophthalmic emulsion (Restasis), Ns = not significant. P values are versus either baseline or cross-over baseline as indicated using a Mann–Whitney rank sum test.
Ocular Signs
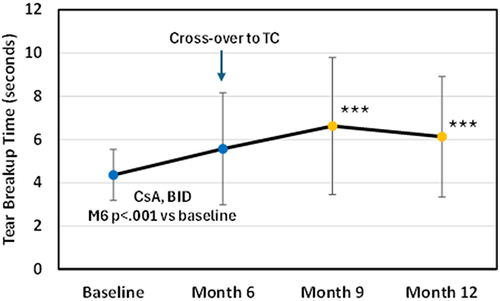
In phase 1 of SAHARA, 6 months of treatment with CsA resulted in a significant improvement in TBUT (p < 0.0001), however significantly less than what was observed for TC (observed mean difference in improvement 1.49 seconds).Citation8 As shown in , after the cross-over to TC, TBUT improved by 1.1 seconds 3 months later, and improvement persisted (0.6 seconds) at Month 12, six months later. Both of these timepoints were statistically significantly better than the cross-over baseline (p < 0.001).
Figure 2 TBUT (tear breakup time) for CsA group pre-and post crossover to TC. CsA (cyclosporine ophthalmic emulsion (Restasis)), TC (TearCare). ***P<0.001 versus month 6 using a Mann–Whitney rank sum test.
Statistically significant improvements in all other measures of signs following cross-over to TC were observed at study month 9 and month 12 with the exception of STS (not measured at month 9) which was numerically better by an average 1.0 second, but not statistically (p = 0.08) (). However when compared to the pre-study baseline, the difference (12.0 seconds vs 9.3 seconds) was significant (p < 0.001).
Table 2 Ocular Signs Outcomes
Signs and Symptoms Compared to TC
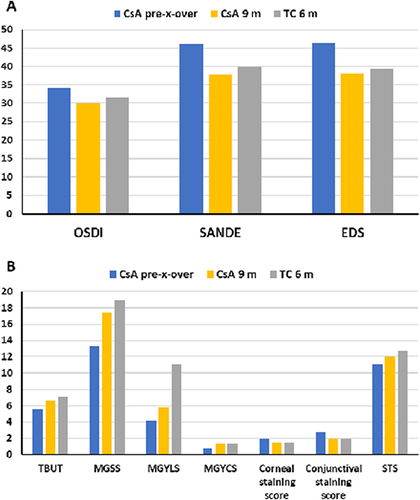
shows the observed mean values for symptoms () and signs () at the end of phase 1 of the study for both TC and CsA alongside the values reached for the cross-over cohort three months after stopping CsA and receiving a single treatment with TC. The three months post cross-over outcomes (study month 9) are presented rather than the outcomes at six months post-cross-over (study month 12) to better describe peak TC effect for these patients and because the outcomes for the phase 1 TC group (patients randomized to TC) at study month 6 was at peak effect; just one month after a TC treatment. The peak treatment effect for TC is reached by one month and sustained through at least three months but begins to diminish by six months.Citation8,Citation11 Mean OSDI, SANDE, and EDS for the cross-over patients improved and closely matched the means observed at month 6 for the TC treatment group. Similarly, TBUT, MGSS, MGYCS, corneal and conjunctival staining scores, and STS all improved and closely approximated the six-month TC values. The single exception was MGYLS which showed improvement (p < 0.001 vs month 6, ) but was intermediate between the six month values for CsA and TC.
Figure 3 Ocular symptoms (A) and signs (B) for CsA at six months (pre-crossover) and at nine months (3 months post-crossover) compared to TC randomized patients at the six month (phase 1 of study) endpoint.
Artificial Tear and Lubricant Use
We documented patient usage of OTC artificial tear or lubricant products by asking about their usage pattern since the previous visit. Most patients did not use tear or lubricant products; at months 1, 3, and 6 following the cross-over to TC there were 24%, 23%, and 30% reporting use, respectively, compared with 38% in the CsA arm in phase 1 of the study. The frequency of use for these patients was low, generally 1 administration per day on about 8 of every 10 days.
Adverse Events
There were 11 patients (6.7%) that experienced a total of 18 ocular or serious AE during the cross-over follow-up interval. These were primarily mild (15 of 18, 83%) with two graded as moderate (11%, abdominal pain due to gallstones; worsening of glaucoma) and one graded severe (6%, death due to gall bladder cancer). Two AE (11%) were judged to be possibly or definitely related to treatment; burning sensation which resolved in 1 day, and loss of BCVA (10 letters < than pre-study) which occurred 1 day after the TC procedure but was resolved when checked 1 week later. The most common AE was loss of BCVA (5 of 18) with an incidence of 3%. There were two serious AE, the death mentioned previously (occurring approximately 1 month after study completion) and a prior hospitalization for gallstones occurring in the same patient. No AE resulted in patient discontinuation. A listing of these AE is provided in .
Table 3 Ocular and Serious Adverse Events
Discussion
In phase 1 of SAHARA, the study purpose was to compare common and clinically relevant outcomes with TC versus CsA in eyes with DED associated with MGD following six months of treatment. Both treatment groups had similar clinically meaningful and statistically significant improvements in measures of ocular symptoms, but TC was found to be superior in key ocular signs including TBUT, MGSS, and other measures of meibomian gland function.Citation8 In phase 2 of SAHARA, patients that had been randomized to CsA in phase 1, ceased using CsA and received a single TC treatment with the goal of showing that symptom improvement over the 1st 6 months would be maintained without CsA over the next 6 months and that additional improvements in signs, matching what was observed for the phase 1 TC treatment group, could be reached. Both of these goals were achieved. There was no significant difference from month 6 at months 9 and 12 for OSDI, and there was significant improvement at these time points for SANDE and EDS (month 9 only). In terms of signs, TBUT and all other measures were statistically significantly improved at months 9 and 12 with the sole exception of STS which was numerically, but not significantly better. These improvements resulted in group means that, for nine of ten of these measures, closely matched what was achieved for the TC group in phase 1. Assessment post cross-over at months 9 and 12 (3 and 6 months after cessation of CsA) ensured that the treatment effect observed could be fairly ascribed to TC as the effects of topical cyclosporine do not persist beyond a few weeks.Citation12
A reasonable hypothesis regarding the cross-over group would have been that their outcomes post-TC would be even better than what was observed for TC in phase 1 because they were receiving TC treatment with a less severe baseline. That the outcomes were similar for a cohort that had a baseline built on a foundation of six months of treatment with CsA, and for a cohort that did not (CsA or other topical prescription product use within 60 days were exclusion criteria), suggests that TC effectiveness is not reliant on any prior DED treatment and that better outcomes for patients can be achieved earlier in a patient’s DED treatment journey.
While cross-over to TC resulted in improvements in symptoms, and with statistical significance for SANDE and EDS, it is fair to question whether or not the improvement has clinical significance. Miller et al found that a 7 point change in OSDI corresponded to a “minimal clinically important difference (MCID)”.Citation13 While the change in mean OSDI score between months 6 and 12 for the crossover cohort did not meet the MCID criteria (34.1 to 31.0), at the patient level 67% of score decreases (59 of 88) met MCID criteria.
We monitored adjunctive use of artificial tears and lubricants for the phase 2 patients just as we did during phase 1 of SAHARA. It is interesting to compare usage before and after the cross-over. In our previous report, we commented on the unexpected early improvement in symptoms for patients randomized to CsA, and the relatively low use of additional drops. Our speculation was that the oily nature of the CsA vehicle provided palliative benefits beyond the pharmacological action of cyclosporine. Given that dosing with CsA (and therefore its vehicle) was abruptly terminated at the month 6 visit, it would not have been surprising if adjunctive lubricant use had increased in the cross-over period. In fact, the opposite was observed; adjunctive drop use decreased in this cohort.
The nature and frequency of AE that occurred in this study confirm and extend the excellent safety for TC. In previous studies of TC fewCitation10,Citation14,Citation15 or noCitation11,Citation16 AE were observed, and these were non-serious, mild, and generally self-resolving. However, the present study provides a larger group of patients exposed to TC treatment to better characterize the safety profile; 172 with 2 treatments in phase 1, and 163 with 1 treatment in the phase 2 cross-over portion of the study. The total number of treatment emergent AE was similar for the two TC cohorts, 19 in phase 1Citation8 and 18 in phase 2. Just two were considered related or possibly related to treatment in phase 1 (conjunctival hyperemia and eyelid irritation)Citation8 as were two in phase 2 (burning sensation, BCVA decrease). All of these were mild, transient, resolved without treatment, and did not interrupt patient continuation in the study. The most frequent AE we observed was BCVA decrease. In the single incidence that was considered related to treatment, the decrease was noted 1 day following TC, and resolved 1 week later. The investigator speculated that it was likely due to “change in the tear film”.
There are strengths and weaknesses in any clinical study. SAHARA was an RCT with masked assessments, multicenter including optometric and ophthalmic practices, and a large sample size with adequate statistical power to test the superiority hypotheses at the 6 month endpoint. Phase 2 of SAHARA was a pre-planned crossover to TC for the CsA treatment group. Attrition at crossover and through subsequent follow-up was very low. There were 173 originally randomized to CsA, 163 that crossed over at six months, and 161 at the 12 month visit, attrition of just under 7%. With observations at 9 and 12 months, the treatment effect reported here was solely due to TC. In phase 2 of SAHARA, signs assessments were not assessor masked as they were in phase 1 so assessor bias cannot be ruled out. However, the signs assessments were (at least in part) objective (eg, counts of glands, stopwatch readings, etc.), and it is not obvious whether any unintentional assessor bias would favor TC or CsA. Furthermore, the very close approximation of the outcomes for signs following crossover and for the phase 1 TC group (where assessments were masked) argues against significant bias. Crossover patients received only a single TC treatment, whereas in phase 1, the TC cohort received a second treatment at 5 months. The second treatment was to ensure a fair comparison between TC and CsA with both treatments at peak effect. From previous studies, we know that the treatment effect for TC has started to diminish by 6 months.Citation11,Citation14 This means that the values reported herein for the crossover patients at 12 months likely underestimate the maximum benefit of TC in this population.
In summary, patients treated previously with CsA can realize additional clinically meaningful improvements in the signs and symptoms of DED when treated with TC. These improvements persist for at least 6 months post-treatment in the absence of continued CsA use. The clinical effectiveness of TC appears to be the same regardless of prior treatment with CsA and therefore results consistent with those reported here could be expected when TC is used as a first-line DED option. The TC safety profile was found to be excellent with only two mild AE deemed related to treatment occurring in 163 patients. Taken together, TC is a safe, effective option, not reliant on patient adherence, for the treatment of DED associated with MGD.
Disclosure
BA, MB, JL, TC, BS, JE, and SK were Investigators and received research support for the study from the study sponsor, Sight Sciences, Inc. BA is a consultant for Alcon, Allergan, Sight Sciences, Carl Zeiss Meditech, Tarsus, and Dompe. MB is a consultant for and reports honoraria from Allergan and Sight Sciences. JL is a consultant for Allergan and Sight Sciences; she is also on advisory boards for Allergan, Bausch and Lomb and Sun Pharmaceuticals. TC reports grant support, consulting, and speaking for Sight Sciences and reports non-financial support from Allergan. BS is a consultant and speaker for Sight Sciences and Allergan. SK is a consultant and speaker for Allergan and a consultant for Sight Sciences. JED jr and TR are employees of Sight Sciences. The authors report no other conflicts of interest in this work.
Data Sharing Statement
The authors do not intend to share participant-level data. Other queries or requests should be directed to the corresponding author (JED).
References
- Farrand KF, Fridman M, Stillman I, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
- Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
- Tong L, Chaurasia SS, Mehta JS, Beuerman RW. Screening for meibomian gland disease: its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore. Invest Ophthalmol Vis Sci. 2010;51(7):3449–3454. doi:10.1167/iovs.09-4445
- Viso E, Gude F, Rodríguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population-based study in Spain. Cornea. 2011;30(1):1–6. doi:10.1097/ICO.0b013e3181da5778
- Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064. doi:10.1167/iovs.10-6997g
- Ayres BD, Bloomenstein MR, Loh J, et al. A randomized, controlled trial comparing tearcare® and cyclosporine ophthalmic emulsion for the treatment of dry eye disease (SAHARA). Clin Ophthalmol. 2023;17:3925–3940. doi:10.2147/OPTH.S442971
- Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147. doi:10.1097/ICO.0b013e3181814cff
- Gupta PK, Holland EJ, Hovanesian J, et al. TearCare for the treatment of meibomian gland dysfunction in adult patients with dry eye disease: a masked randomized controlled trial. Cornea. 2022;41:417–426. doi:10.1097/ICO.0000000000002837
- Badawi D. A novel system, TearCare, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683–694. doi:10.2147/OPTH.S160403
- Williams DL. A comparative approach to topical cyclosporine therapy. Eye. 1997;11(4):453–464. doi:10.1038/eye.1997.126
- Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi:10.1001/archophthalmol.2009.356
- Badawi D. TearCare system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189–198. doi:10.2147/OPTH.S191588
- Karpecki P, Wirta D, Osmanovic S, Dhamdhere K. A prospective, post-market, multicenter trial (CHEETAH) suggested tearcare system as a safe and effective blink-assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:4551–4559. doi:10.2147/OPTH.S285953
- Chester T. A single-center retrospective trial of a blink-assisted eyelid device in treating the signs and symptoms of dry eye. Optom Vis Sci. 2021;98(6):605–612. doi:10.1097/OPX.0000000000001711
