Abstract
Background
The purpose of this study was to determine whether a combination of vitamins B6, B9, and B12 is an effective intervention for reducing the signs and symptoms of nonproliferative diabetic retinopathy.
Methods
Ten subjects with type 2 diabetes mellitus (n = 20 eyes) with clinically diagnosed mild to moderate nonproliferative diabetic retinopathy were recruited from a private practice ophthalmology clinic for this open-label, uncontrolled, prospective six-month study. Metanx® vitamin tablets (containing 3 mg L-methylfolate calcium, 35 mg pyridoxal-5′-phosphate, and 2 mg methylcobalamin) were administered at a dosage of two tablets daily. Primary outcome indicators were the percent change in mean retinal sensitivity threshold measured by macular microperimetry and the percent change in mean central retinal thickness measured by spectral-domain optical coherence tomography.
Results
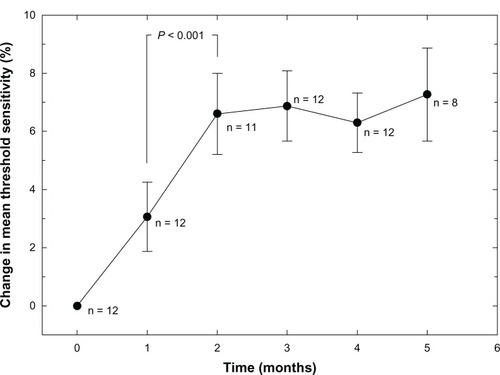
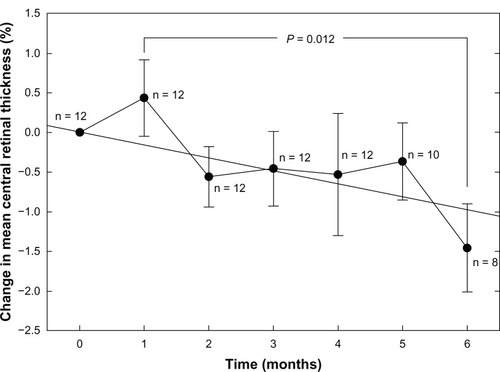
Three subjects were lost to follow-up. In the remaining seven subjects, two of 14 eyes had foveal edema that prevented microperimetry measurements due to poor fixation. The remaining 12 eyes showed a nonlinear improvement in mean threshold retinal sensitivity (P < 0.001). Overall change in mean central retinal thickness in 14 eyes was linear (R2 = 0.625; P = 0.034), with a significant reduction between one and six months (P = 0.012).
Conclusion
In this pilot study, the Metanx intervention appeared to have some beneficial effects with respect to reducing retinal edema and increasing light sensitivity in subjects with nonproliferative diabetic retinopathy.
Introduction
Patients with long-standing type 2 diabetes mellitus often develop diabetic retinopathy in later life.Citation1 Commonly seen signs and symptoms of diabetic retinopathy include visible lesions on the retina (microaneurysms, hemorrhages, and axoplasmic deposits), distorted central vision due to diabetic macular edema, and regions of dimmed or missing vision (scotomas).Citation2 Nonproliferative diabetic retinopathy is the most prevalent form of the disease, although more advanced proliferative forms of diabetic retinopathy occur with neovascularization and substantial visual deficits.Citation2 A major research goal in ophthalmology has been to discover inexpensive and effective treatments for diabetic retinopathy, particularly at an early stage of the disease when good vision is still present. However, reducing or eliminating predisposing factors such as smoking, poor diet, obesity, hypertension, and lack of exercise is essential in reducing the incidence of diabetes mellitus and diabetic retinopathy.Citation3
Many of the signs and symptoms of diabetes mellitus are caused by elevated levels of plasma homocysteine, a sulfur-containing intermediate metabolic product of the essential amino acid, methionine, that circulates throughout the body by way of the vascular system.Citation4 Normally, homocysteine is metabolically catalyzed to cysteine by various enzymes that are dependent on the B vitamins,Citation5–Citation7 and cysteine is converted to glutathione, a powerful antioxidant. However, when homocysteine is incorporated into proteins by a disulfide or amide linkage (S-homocysteinylation or N-homocysteinylation), it creates a product that generates significant oxidative stress and inflammation throughout the body.Citation4 An elevated level of homocysteine is known to be toxic to the pericytes and endothelial cells lining the wall of blood vessels, and can lead to vascular thrombosis and microaneurysms.
Individuals with diabetes mellitus are in a particularly high-risk group for elevated homocysteine, and over time they tend to exhibit the signs and symptoms associated with vascular wall cell damage from oxidative stress. For example, reduced vitamin B availability has been associated with sensory and peripheral motor nerve dysfunction in diabetes mellitus.Citation8 In particular, individuals with long-term or poorly controlled diabetes often experience diabetic peripheral neuropathy in the distal portions of the limbs where blood circulation is poorest. Likewise, diabetics have an increased mortality risk associated with cardiovascular disease, either due to or exacerbated by the presence of homocysteine.Citation7,Citation9
Vitamins B6, B9, and B12 are known to be essential for maintaining the overall integrity of the vascular system, with B9 (folate) also contributing to adequate absorption of other essential nutrients. The effective use of vitamin B supplements has been described for prevention of cardiovascular disease in patients with kidney disease.Citation10 Likewise, it has been reported that patients taking vitamin B supplements, in particular folate, have significant improvement in their signs and symptoms of diabetic peripheral neuropathy.Citation11–Citation17 A commercially available vitamin B compound reported to reduce anesthesia, paresthesia, causalgia, and other symptoms of homocysteine associated with vascular damage is Metanx® (Pamlab, Covington, LA, USA). Metanx is a biologically active formulation of vitamin B that does not have to be processed by the liver as would be the case for over-the-counter synthetic vitamins. It is labeled as a medical food by the US Food and Drug Administration and is available only by prescription. Each Metanx tablet contains 3 mg of L-methylfolate calcium (vitamin B9 as Metafolin®, Merck KGaA, Darmstadt, Germany), 35 mg pyridoxal-5′-phosphate (vitamin B6), and 2 mg of methylcobalamin (vitamin B12). A standard daily dose of Metanx is two tablets.
In a study of intervention using Metanx in 11 patients with diabetic peripheral neuropathy, 82% of patients reported improvement in sensory function and 73% had improved epidermal nerve fiber density based on tissue punch biopsies.Citation14 In a more recent study of patients with diabetic peripheral neuropathy plasma homocysteine was decreased by 2.7 ± 3.0 μmol/L from baseline in a Metanx intervention group as compared with a 0.5 ± 2.4 μmol/L increase in homocysteine in the control group (P = 0.0001) over a six-month period.Citation17 Given that multiple independent studies have shown that a majority of patients with diabetic peripheral neuropathy have been helped by vitamin B intervention with no significant side effects, we hypothesized that patients with nonproliferative diabetic retinopathy may also be helped with respect to their visual function (reduced scotomas) and/or retinal physiology (reduced diabetic macular edema). In order to establish preliminary evidence of the efficacy of vitamin B in treating nonproliferative diabetic retinopathy, and to estimate the sample size for a larger, randomized placebo-controlled study of a vitamin B intervention, several patients with nonproliferative diabetic retinopathy newly prescribed Metanx were followed for up to six months.
Materials and methods
This prospective, open-label, single-arm study was conducted in a private practice ophthalmology clinic in accordance with the tenets of the Declaration of Helsinki. Informed consent using written materials and an interview process was obtained from known patients with nonproliferative diabetic retinopathy selected to be test subjects. Test subjects were not paid to participate, but did receive the Metanx vitamin B intervention at no cost during the course of the study. Investigators performed the study independently and without financial incentives from the manufacturer.
Ten adult patients who were previously diagnosed by an experienced retinologist to have mild to moderate nonproliferative diabetic retinopathy in both eyes were recruited into the study as test subjects. The subjects agreed to take standard daily doses of Metanx and to undergo monthly assessment of their health and vision using standard nonexperimental clinical devices and vision screening methods. Selected participants did not have signs of significant ocular disease other than nonproliferative diabetic retinopathy and no significant health concerns other than type 2 diabetes mellitus. In particular, none of the participants were glaucoma suspects or diagnosed with glaucoma. No restrictions were placed on participant age, gender, or racial background; however, all were required to have well controlled diabetes, based on their most recent glycosylated hemoglobin (HbA1c) records. None of the participants reported using tobacco products or alcohol during the course of the study. The eyes included in this study were not being treated with laser or antivascular endothelial growth factor injections, but were being monitored by regularly scheduled examinations.
Ocular and general physical health were assessed at baseline and on a monthly basis thereafter using standard clinical testing for evaluating vision and ocular pathology, including high-contrast best-corrected visual acuity, intraocular pressure (IOP) by applanation tonometry (Tono-Pen® XL, Reichert Technologies, Depew, NY, USA), threshold light sensitivity by macular microperimetry (MAIA®, CenterVue Spa, Padova, Italy), and central retinal thickness by spectral-domain optical coherence tomography (Spectralis®, Heidelberg Engineering, Carlsbad, CA, USA). Because of the small n value of this pilot study, the percent change relative to baseline values of the mean threshold sensitivity and the mean central retinal thickness were used as primary outcome indicators.
The microperimetry device measured mean threshold sensitivity in dB at 37 discrete locations within the central 10 degrees of the macula using a Goldman III stimulus target. According to the manufacturer, the background luminance of the microperimeter is 4 apostilb, with a maximum luminance of 1000 apostilb for the stimulus target (1.27 candelas/m2 and 318.47 cd/m2, respectively). The device randomly presents the test target at different stimulus locations and varies the target luminance using a 4–2 staircase algorithm. The overall dynamic range of the MAIA device is 36 dB, and the threshold between normal and subnormal vision is considered to be 25 dB. Mean central retinal thickness was measured in μm in the central two degrees of the macula by spectral-domain optical coherence tomography. The Spectralis device has an axial depth resolution of ±3.9 μm.
Additional information about the general health of the subjects was gathered during the course of the study, including resting heart rate, blood pressure, body weight, and most recent HbA1c readings. Changes in medication, doses, diet, and health since the last visit were recorded. All quantitative data were recorded in spreadsheets, statistically analyzed, and plotted using SigmaPlot® version 11.0 (Systat Software, San Jose, CA, USA). Because of the small n value of this pilot study, both eyes of each patient were tested and used for analysis, except where noted. In addition, because each eye in the study had different baseline values for retinal threshold sensitivity and retinal thickness, the month-to-month changes for each metric are expressed as percentages of change relative to the baseline value. By expressing change as a percentage from baseline, it is easier to represent the change graphically in a small sample group. However, the variance at baseline becomes zero by this adjustment, and statistical comparisons based on variance must then be computed based on the first month of treatment.
Intervention with biologically active vitamin B was in the form of a daily dose of Metanx which began on the day following the baseline evaluation for subjects who were successfully recruited into the study. Subjects were instructed to take one tablet twice daily with morning and evening meals. If a dose was missed, subjects were instructed to take the regular dose at the next scheduled time, but to maintain a record of any missed doses. At the end of each monthly evaluation, patients were resupplied with an additional month’s supply of tablets.
Results
Three of the original 10 subjects recruited were lost to follow-up. One lost subject’s chief complaint was of dyspepsia after taking the vitamin B tablet in the morning and that for another was of increased tingling sensation in the legs after being on Metanx for two weeks. An increase in somatosensation after initiating Metanx has been reported to be a temporary side effect attributed to an improvement in vascular and neurologic function. On the recommendation of the primary care physician, the subject withdrew from the study. A third subject was lost to follow-up because of pneumonia requiring a period of hospitalization, at which point Metanx was stopped. Due to the washout of vitamin B during this dose interruption, the subject was not returned to the study. The remaining seven subjects continued with the study, with no side effects reported.
The mean age at baseline for the remaining subjects was 60.8 ± 8.1 years. Summary statistics of their baseline and final biometric measurements are given in . Best-corrected visual acuity readings are reported in log of the minimum angle of resolution (logMAR) units. There was a small decline in best-corrected visual acuity during the study period (equivalent to approximately three Snellen letters), but this was not statistically significant given the overall variability between subjects. There were no significant changes to any of the biometric readings followed during the study, and in particular, there were no reported changes in HbA1c readings, indicating that glucose was being controlled. None of the subjects reported a significant change to their overall health or diet during the course of the study. With the exception of an occasional missed dose, all subjects successfully followed the correct Metanx dosing schedule.
Table 1 Biometric measurements
Microperimetry findings
Fourteen eyes from seven patients were available for testing; however, two eyes from two subjects (2/14, 14%) were not included in the final microperimetry analysis because of foveal edema that caused poor fixation of the microperimetry target and an inability to complete the MAIA examinations. A failure to complete examination meant that an accurate mean threshold sensitivity could not be computed for all stimulus points. The remaining eyes (12/14, 86%) were included in the final analysis.
shows the group mean percent change in retinal threshold sensitivity relative to the baseline value. The trend for improvement is distinctly nonlinear. There is an initial two-month increase in sensitivity to threshold light stimuli of approximately 7%, followed by a plateau effect for the next three months. Data for months 2–5 were significantly different from both the baseline data and the one-month data (P < 0.001) using analysis of variance. The microperimetry device was not available for data collection at the six-month period for all subjects due to unanticipated relocation of the instrument. Because of the low n value (only two eyes) and high variance, the six-month data are not shown.
Retinal spectral-domain optical coherence tomography findings
indicates a linear trend of decreasing central retinal thickness expressed as a percentage from baseline (R2 = 0.625; P = 0.034; n = 14 for all test periods). While the overall change relative to baseline is small, the difference between the one-month and six-month data is significant (P = 0.012; Mann-Whitney rank sum test) and indicates an approximately 2% decline in mean central retinal thickness over this period. Unlike the threshold sensitivity data, the trend does not appear to plateau.
Discussion
Recent clinical studies have investigated homocysteine as a factor or cofactor involved in diabetic retinopathy,Citation18–Citation27 as well as in other ocular diseases, including age-related macular degeneration,Citation28,Citation29 central retinal vein occlusion,Citation30 and nonarteritic ischemic optic neuropathy.Citation31 Hyperglycemia during diabetes increases production of vascular superoxide, which inactivates expression of nitric oxide by endothelial cells and contributes to vascular degradation.Citation13,Citation32,Citation33 Therefore, intervention with vitamin B could make an important contribution toward enhancing the production of nitric oxide. A recent porcine animal model has demonstrated that homocysteine inhibits dilation of retinal arterioles via the nitric oxide-mediated pathway, and thereby facilitates development of retinal vascular disease, such as diabetic retinopathy.Citation34 Therefore, we believe that there is sufficient scientific evidence to suggest a role for homocysteine in vascular ocular disease, and that a study of vitamin B as an intervention in the management of diabetic retinopathy is warranted.
In our pilot study, daily use of biologically active vitamin B over a period of several months resulted in a small but significant improvement in mean retinal threshold sensitivity for eyes with mild to moderate nonproliferative diabetic retinopathy. The increase in threshold sensitivity was distinctly nonlinear, with an initial two-month increase followed by a plateau at this elevated state. While nonlinear curves in subjective testing can sometimes be attributed to learning during testing, the simple nature of microperimetry examination usually limits learning to the first few presentations of the test stimulus. The MAIA device includes a brief learning routine at the start of each examination. In addition, the combination of averaging many data points from each eye and the fact that examinations are separated by one-month intervals would tend to eliminate learning as a variable. Retinal threshold sensitivity is linked to phototransduction and synaptic transmission of neural signals. Therefore, the possibility exists that the presence of endogenous nitric oxide from vitamin B intervention could enhance photoreceptor activity and thereby enhance threshold sensitivity to light.Citation35 This also might explain the early nonlinear improvement in threshold retinal sensitivity seen in this study.
This study also reveals a small but significant decrease in mean retinal thickness of eyes with nonproliferative diabetic retinopathy. In contrast with the nonlinear response seen with mean threshold sensitivity, the reduction in central retinal thickness was essentially linear over the course of the study. Any decrease in retinal thickness must be linked to both the repair of any sources of vascular leakage, as well as moving fluid out of the edematous tissue, which should be a relatively slow, linear process. Therefore, we speculate that a significant reduction in homocysteine does not necessarily translate into an immediately significant effect on pre-existing diabetic macular edema. Further, the ability of the retina to recover from pre-existing vascular damage will depend on the overall severity of the damage. Consequently, there may be physiologic limits to the efficacy of vitamin B intervention to invoke repairs even if homocysteine can be decreased and the availability of nitric oxide increased. Although our interpretation of the results of the study are speculative, it paves the way for future studies, and provides a connection to previous laboratory studies in animal models and human testing for the treatment of diabetic peripheral neuropathy. While the observed effect was small over a six-month period (about 1.5%), when extrapolated over time, the result takes on more clinical significance. In addition, the majority of eyes examined had limited amounts of diabetic macular edema at baseline.
Study limitations
The current protocol has the limitations typical of any clinical pilot study. Among these are the lack of subject and observer masking, with a placebo control and a small n value. These limitations will be addressed in a Phase II clinical trial. The introduction of a placebo control group will also require sampling data to be restricted to a single test eye. In the current study, we combined the results of both eyes in a manner analogous to the practice of combining skin biopsy data from both legs of a patient with diabetic neuropathy. The nature of retinopathy is such that vascular damage can be variable and randomly located across the retina. Therefore, combining information from both eyes during observational studies can give a more complete assessment of functional damage to the total visual system in individuals with known disease. In such protocols, use of both eyes for data collection is acceptable.Citation36
The current study was based on a dosage deemed to be safe and effective for the treatment of diabetic peripheral neuropathy. Further study is needed to determine an appropriate dosage specifically for patients with diabetic retinopathy, and whether the dosage should be adjusted according to the severity of the diabetic retinopathy present. The current study does not address the use of other supplements that may be used synergistically with B vitamins in order to provide additional retinal protection or efficacy in the reversal of retinopathy signs and symptoms.
Finally, it would be helpful to have known whether the concentration of homocysteine was affected during the course of this study. Elevated homocysteine levels have been found in the aqueous and vitreous humors of those afflicted with diabetic retinopathy, but such samples are impractical to obtain in living subjects except under unique circumstances.Citation23,Citation25 Tracking of systemic homocysteine can be done by blood analysis, and future testing of vitamin B intervention for diabetic retinopathy should include this parameter.Citation20–Citation22
Conclusion
Preliminary data indicate that intervention with biologically active vitamin B may influence the progression of nonproliferative diabetic retinopathy, presumably through the metabolic pathway of homocysteine. A reduction of homocysteine may permit a physiologic environment more suitable for the repair of vascular structures of the retina; however, it may be impossible to repair severely damaged tissue fully. Nevertheless, interventions that help to protect the eye and retain existing vision are critical for those with diabetes.
Both mean retinal threshold sensitivity and mean central retinal thickness were significantly improved by two and six months, respectively; however, the limited nature of this pilot study prevented us from eliminating potential confounding factors that could contribute to type I error, such as a placebo effect or a greater awareness in subjects of their overall health during the study. The study also did not allow us to compare our results with the expected decline in primary outcome indicators within an untreated nonproliferative diabetic retinopathy group during the same six-month period. Nevertheless, the data are supportive of the need for a larger, randomized, placebo-controlled study that can verify any benefits of vitamin B in patients with nonproliferative diabetic retinopathy.
Author contributions
MKS performed the data analysis for the study and wrote the final manuscript. MKS prepared the informed consent documents. NFN initiated the study, selected the test subjects, followed each of the cases during the course of the study, and assisted in reviewing the final version of the manuscript for accuracy. AGJ and LRP provided diagnostic evaluation of their patients for the study, contributed to the revision of the draft, and reviewed the final manuscript for accuracy.
Disclosure
Pamlab provided the Metanx used in this study at no cost, and paid for institutional review board fees. NFN has been a speaker for Ellex (Minneapolis, MN, USA), which distributes the MAIA microperimeter in the US. EyeCare 20/20 provided clinical testing at no cost for this study. Otherwise, no author has a financial interest or a conflict of interest in this work.
References
- FongDSAielloLPFerrisFLKleinRDiabetic retinopathyDiabetes Care2004272540255315451934
- American Academy of Ophthalmology Retina/Vitreous Panel, Preferred Practice Patterns CommitteeDiabetic retinopathySan Francisco, CAAmerican Academy of Ophthalmology Available from: http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=d0c853d3-219f-487b-a524-326ab3cecd9aAccessed May 31, 2013
- DeshpandeADHarris-HayesMSchootmanMEpidemiology of diabetes and diabetes-related complicationsPhys Ther2008881254126418801858
- Perła-KajánJTwardowskiTJakubowskiHMechanisms of homocysteine toxicity in humansAmino Acids20073256157217285228
- StangerOWonischWEnzymatic and non-enzymatic antioxidative effects of folic acid and its reduced derivatesSubcell Biochem20125613116122116698
- HoffmanMHypothesis: hyperhomocysteinemia is an indicator of oxidant stressMed Hypotheses2011771088109321963358
- StangerOHerrmannWPietrzikKDACH-LIGA homocysteine (German, Austrian and Swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendationsClin Chem Lab Med2003411392140314656016
- LookerHCFagot-CampagnaAGunterEWHomocysteine and vitamin B(12) concentrations and mortality rates in type 2 diabetesDiabetes Metab Res Rev20072319320116845688
- EbesununMOObajobiEOElevated plasma homocysteine in type 2 diabetes mellitus: a risk factor for cardiovascular diseasesPan Afr Med J2012124822937188
- SudchadaPSaokaewSSridetchSIncampaSJaiyenSKhaithongWEffect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysisDiabetes Res Clin Pract20129815115822727498
- LeishearKBoudreauRMStudenskiSARelationship between vitamin B12 and sensory and motor peripheral nerve function in older adultsJ Am Geriatr Soc2012601057106322690982
- GonzálezRPedroTMartinez-HervasSPlasma homocysteine levels are independently associated with the severity of peripheral polyneuropathy in type 2 diabetic subjectsJ Peripher Nerv Syst20121719119622734904
- BagheriMJahromiBMZamaniAFolic acid may be a potential addition to diabetic foot ulcer treatment – a hypothesisInt Wound J2011865866021854546
- JacobsAMChengDManagement of diabetic small-fiber neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5′-phosphateRev Neurol Dis20118394721769070
- Miranda-MassariJRGonzalezMJJimenezFJAllende-VigoMZDucongeJMetabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom controlCurr Clin Pharmacol2011626027322082324
- LeishearKFerrucciLLauretaniFVitamin B12 and homocysteine levels and 6-year change in peripheral nerve function and neurological signsJ Gerontol A Biol Sci Med Sci20126753754322156506
- FonsecaVALaveryLAThethiTKMetanx in type 2 diabetes with peripheral neuropathy: a randomized trialAm J Med201312614114923218892
- VaccaroOIngrossoDRivelleseAGrecoGRiccardiGModerate hyperhomocysteinaemia and retinopathy in insulin-dependent diabetesLancet1997349110211039107272
- HoogeveenEKKostensePJEysinkPEHyperhomocysteinemia is associated with the presence of retinopathy in type 2 diabetes mellitus: the Hoorn studyArch Intern Med20001602984299011041907
- HuangEJKuoWWChenYJHomocysteine and other biochemical parameters in type 2 diabetes mellitus with different diabetic duration or diabetic retinopathyClin Chim Acta200636629329816343469
- ChoHCThe relationship among homocysteine, bilirubin, and diabetic retinopathyDiabetes Metab J20113559560122247902
- GoldsteinMLeibovitchIYeffimovIGavendoSSelaBALoewensteinAHyperhomocysteinemia in patients with diabetes mellitus with and without diabetic retinopathyEye (Lond)20041846046515131674
- CoralKAngayarkanniNGomathyNBharathselviMPukhrajRRupakRHomocysteine levels in the vitreous of proliferative diabetic retinopathy and rhegmatogenous retinal detachment: its modulating role on lysyl oxidaseInvest Ophthalmol Vis Sci2009503607361219369240
- SatyanarayanaABalakrishnaNPitlaSStatus of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemiaPLoS One20116e2674722069468
- LimCPLooAVKhawKWPlasma, aqueous and vitreous homocysteine levels in proliferative diabetic retinopathyBr J Ophthalmol20129670470722353698
- WrightADMartinNDodsonPMHomocysteine, folates, and the eyeEye (Lond)20082298999318064053
- ChangHHLinDPChenYSIntravitreal homocysteinethiolactone injection leads to the degeneration of multiple retinal cells, including photoreceptorsMol Vis2011171946195621850169
- ChristenWGGlynnRJChewEYAlbertCMMansonJEFolic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular StudyArch Intern Med200916933534119237716
- GopinathBFloodVMRochtchinaEWangJJMitchellPHomocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degenerationAm J Clin Nutr512013 [Epub ahead of print.]
- NarayanasamyASubramaniamBKarunakaranCHyperhomocysteinemia and low methionine stress are risk factors for central retinal venous occlusion in an Indian populationInvest Ophthalmol Vis Sci2007481441144617389469
- StangerOWegerMObeidRImpairment of homocysteine metabolism in patients with retinal vascular occlusion and non-arteritic ischemic optic neuropathyClin Chem Lab Med2005431020102516197292
- StangerOWegerMInteractions of homocysteine, nitric oxide, folate and radicals in the progressively damaged endotheliumClin Chem Lab Med2003411444145414656024
- TousoulisDKampoliAMStefanadisCDiabetes mellitus and vascular endothelial dysfunction: current perspectivesCurr Vasc Pharmacol201210193222112354
- OmaeTNagaokaTTananoIYoshidaAHomocysteine inhibition of endothelium-dependent nitric oxide-mediated dilation of porcine retinal arterioles via enhanced superoxide productionInvest Ophthalmol Vis Sci2013542288229523425693
- SavchenkoABarnesSKramerRHCyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxideNature19973906946989414163
- FanQTeoYYSawSMApplications of advanced statistics in ophthalmologyInvest Ophthalmol Vis Sci2011526059606521807933