Abstract
Purpose
To evaluate the effectiveness of Zhang and Zheng’s InnovEyes (ZZ InnovEyes) strategy for optimizing outcomes of ray-tracing-guided laser in situ keratomileusis (LASIK) compared to the standard automated strategy.
Methods
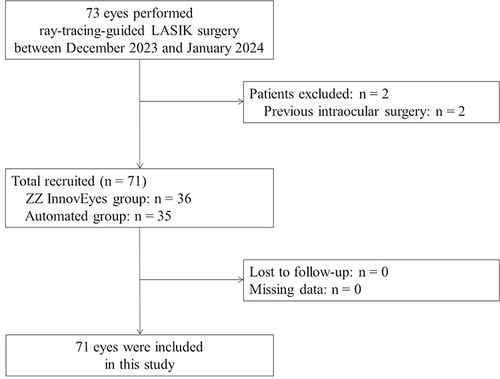
A total of 38 patients (71 eyes) undergoing therapeutic refractive surgery at Hangzhou MSK Eye Hospital were randomly assigned to the ZZ InnovEyes and automated groups using double-masked randomization. The study assessed visual acuity, refractive outcomes, and higher-order aberrations preoperatively and at 1-day, 2-week, 1-month, and 3-month follow-ups. Statistical analysis was done with Microsoft Excel and SPSS 19.0.
Results
The exposure and control groups comprised 36 and 35 eyes, respectively. The ZZ InnovEyes group demonstrated significant advantages in manifest refraction spherical equivalent (MRSE) correction compared to the automated approach group (0.13 ± 0.30 D vs 0.62 ± 0.40 D, p < 0.001), achieving 97.22% uncorrected distance visual acuity (UDVA) of 20/16 or better compared to 85.71% in the automated group at the 3-month follow-up (p = 0.08), and achieving 50.00% UDVA of 20/12.5 or better compared to 28.57% in the automated group at the 3-month follow-up (p = 0.06). Loss lines from preoperative corrected distance visual acuity to postoperative UDVA were lower in the ZZ InnovEyes group (0.00%) than the automated group (8.57%; p = 0.07). Both groups exhibited similar astigmatism corrections and higher-order aberrations.
Conclusion
The ZZ InnovEyes strategy, which incorporates manifest and wavefront refraction for ray-tracing-guided LASIK, demonstrated superior MRSE correction and potential advantages in visual acuity outcomes compared to the standard automated strategy. This study highlights the need for ongoing optimization and research in refractive surgery.
Clinical Trial Registration Number
ChiCTR2300078709.
Introduction
Corneal refractive surgery has evolved into a well-established and safe intervention method over the past three decades, with laser in situ keratomileusis (LASIK) standing out as a globally practiced technique.Citation1 Continuous refinement of theory, recognition, and technology has led to the development of accurate calculation methods, such as wavefront guided, Q-value guided, and topography guided excimer ablation modes. These methods aim to customize guided ablations and optimize postoperative visual acuity.Citation2
Among these advancements, ray-tracing guided LASIK, propelled by a new generation of algorithms, holds the promise of overcoming limitations posed by earlier algorithms, thereby enhancing clinical outcomes.Citation3 Preliminary trials of this technique in myopic individuals without prior eye surgery have reported remarkable success rates, with 98.1% achieving uncorrected visual acuity of 20/20 or better and 48% achieving 20/12.5 or better.Citation4–7 These findings, however, are based on pre-launch clinical trials. Their design limitations, such as excluding eyes with significant discrepancies between manifest and wavefront refractive measurements, which are common in the general population, warrant careful consideration.
Post-launch clinical outcomes have indicated the potential occurrence of residual refractive errors in some eyes with highly consistent wavefront refraction and subjective refraction, causing impaired uncorrected visual acuity. Despite the theoretical assertion that ray-tracing guided LASIK does not necessitate additional modifications in treatment planning beyond data validation, and despite the manufacturer also recommending automated refraction for ablation,Citation4 post-launch results suggest the need for meaningful ablation refraction adjustments.
To address these challenges and further optimize clinical outcomes, this study evaluated Zhang and Zheng’s InnovEyes (ZZ InnovEyes) strategy. This strategy takes into account factors such as age, accommodative refraction, and with-the-rule tendencies. The objective of this prospective study was to compare the postoperative outcomes of eyes treated with the ZZ InnovEyes strategy with outcomes of eyes treated using the standard automated strategy. Based on a meticulous examination of visual acuity, refractive outcomes, and higher-order aberrations, our study provides valuable insights into the potential advantages of the ZZ InnovEyes strategy for enhancing the effectiveness of ray-tracing guided LASIK.
Materials and Methods
Study Design and Participants
This prospective study analyzed a consecutive series of patients referred for therapeutic refractive surgery at Hangzhou MSK Eye Hospital between December 2023 and January 2024. The study used a double-masked randomization process to assign participants to either the ZZ InnovEyes group or the automated group. Both the examiners and patients were blinded to the treatment assignments. Sampling and group assignments were achieved using the random number generation function of SPSS software. Institutional review board approval was obtained from the Medical Research Ethics Committee of Hangzhou MSK Eye Hospital (#MSKLL20231213). The study adhered to the principles outlined in the Declaration of Helsinki. Informed written consent was obtained from all participants.
Inclusion and Exclusion Criteria
Inclusion criteria comprised age between 18 and 40 years, myopia ranging from −0.50 diopter sphere (DS) to −8.00 DS, refractive astigmatism ranging from 0.00 diopter cylinder (DC) to −3.00 DC, and corrected distance visual acuity of Snellen 20/20 or better. Exclusion criteria included the presence of keratoconus, abnormal intraocular pressure, cataract, or uveitis; dry eye that may potentially affect refraction and visual acuity outcomes; history of previous ocular or intraocular surgery; follow-up duration less than three months; presence of corneal opacities or infections; and contraindications to corneal refractive surgery.
Study Group
The ZZ InnovEyes strategy involved combining age, preoperative refraction, InnovEyes automated refraction, and target refractive power to calculate the intended refractive correction (IRC). The emmetropic was set to the target refraction of all patients. Additionally, the ZZ InnovEyes formula can be open accessed at https://www.zzcal.com/calc/en/innov_eyes_two ().
Figure 1 Application of the Zhang and Zheng InnovEyes (ZZ InnovEyes) formula illustrated through a series of screenshots. (A), Selection of the treatment zone diameter; (B), Recording the manifest refraction in the red box, the treatment 4 mm refraction in the blue box (auto-generated by the excimer device as the simulated manifest refraction), and the treatment refraction in the light blue box (generated by the detail button under the default treatment 4 mm refraction, representing the corrected lower-order refraction calculated base on the treatment pattern); (C), Input of the recorded data into the corresponding colored box to obtain the optimized treatment 4 mm refraction in the Orange box, along with the corresponding treatment refraction in the yellow box (generated by the detail button after modifying the treatment 4 mm refraction); (D), Entry of the optimized treatment 4 mm refraction into the Orange box and verification of consistency in the corresponding treatment refraction in the yellow box (a deviation of 0.01 D may occur due to rounding).
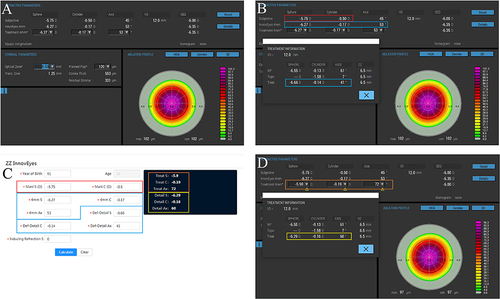
The automated group relied on machine-generated IRC without additional modifications (EX500, Alcon, Fort Worth, USA).
Preoperative and Postoperative Examinations
Comprehensive ophthalmic examinations, including uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), intraocular pressure measurement, slit-lamp microscopy, wavefront, tomography, and biometric data, were conducted using the InnovEyes sightmap system (InnovEyes sightmap, Alcon, Fort Worth, USA). Examinations were performed preoperatively and at 1-day, 2-week, 1-month, and 3-month postoperative follow-ups. The InnovEyes examinations were conducted by an independent physician with no additional role in the study.
Data Collection
Data collected included UDVA, CDVA, photopic manifest refraction, root mean square of higher-order aberrations (RMSh), vertical coma, horizontal coma, and spherical aberration in a 5.0 mm zone. Data were assessed before surgery and at the 3-month postoperative follow-up.
Statistical Analysis
Statistical analyses were conducted using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) and SPSS 19.0 (IBM, Armonk, NY, USA). The normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Continuous variables were presented as the mean ± standard deviation (SD) or median (range) based on normality. Continuous variables that followed a normal distribution, Levene’s test and paired t-tests were used for inter-group and intra-group comparisons, respectively. For continuous variables that had a skewed distribution, a Wilcoxon signed-rank test was used. Categorical variables are presented as counts and percentages [n (%)] and were analyzed using a chi-squared test. A two-sided p-value of less than 0.05 was considered statistically significant. PASS 15.0 software was used to calculate the sample size for the study.
Results
Characteristics of the Patients
A total of 38 patients (71 eyes; mean age, 27.34 ± 6.28 years; 19 males) were included in the final analysis ( and ).
Table 1 Preoperative Clinical Characteristics of Study Participants
Visual Acuity and Refraction Analysis
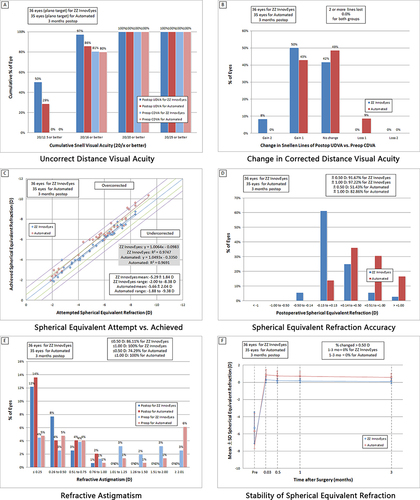
The standardized graphs in describe the visual and refractive outcomes. At the 3-month follow-up, the ZZ InnovEyes group exhibited superior outcomes, with 97.22% achieving UDVA of 20/16 or better compared to 85.71% in the automated group (p = 0.08). The ZZ InnovEyes group also showed a trend toward a better UDVA of 20/12.5 or better (50.00% vs 28.57%, p = 0.06; ). Lost lines from preoperative CDVA to month 3 UDVA were significantly lower in the ZZ InnovEyes group (0.00%) compared to the automated group (8.57%, p = 0.07; ). The ZZ InnovEyes strategy demonstrated a strong correlation between attempted and achieved manifest refraction spherical equivalent (MRSE) refraction (R2 > 0.95), with a significantly lower overcorrection ratio compared to the automated group (0.13 ± 0.30 D vs 0.62 ± 0.40 D, p < 0.001; and ). Additionally, the ZZ InnovEyes group exhibited a significantly higher proportion of MRSE within ±0.50 D compared to the automated group (p < 0.001; ). Cylindrical correction accuracy and refractive stability were similar in the two groups (, and ).
Table 2 Analysis of Residual Refraction at Three Months After Surgery
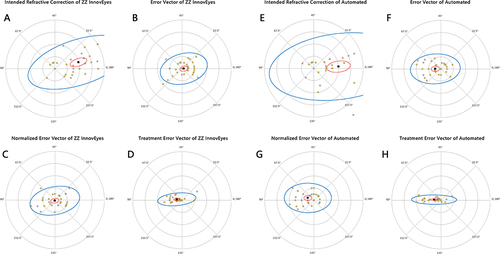
Figure 3 Standard graphs for reporting refractive surgery outcomes (2011). The six standard graphs for reporting refractive surgical outcomes show the visual (A and B), refractive (C and D), astigmatism (E), and stability (F) outcomes.
Vector Analysis
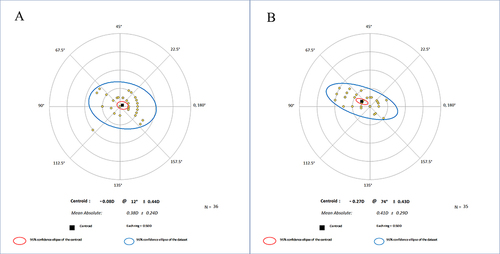
Using the Alpins vector analysis method, the mean vector residual cylinders were −0.08@12°±0.44 D for the ZZ InnovEyes strategy and −0.27@74°±0.43D for the automated strategy ().Citation8 Eydelman vector scatter plots indicated a higher level of agreement with the refractive surgery development aim in the ZZ InnovEyes group ().Citation9
Higher-Order Aberration Analysis
At the 3-month follow-up, no statistically significant differences were observed between the ZZ InnovEyes and automated groups in RMSh, vertical coma, horizontal coma, and spherical aberration in a 5 mm zone (All p > 0.05; ). Intragroup comparisons showed statistically significant differences in RMSh, spherical aberration, and vertical coma for both groups (All p < 0.05; ).
Table 3 Higher-Order Aberration in 5 Mm at Preoperative and Three Months Postoperatively
Discussion
The initial results from clinical trials on ray-tracing-guided LASIK have supported its clinical advantages, particularly in achieving postoperative UDVA. However, the current state of technology and algorithms has revealed potential areas for enhancement. This study serves as a preliminary exploration into the optimization space for this innovative technology by evaluating the feasibility of optimization strategies through a prospective study.
The findings of this study highlight the superior clinical advantages of the ZZ InnovEyes strategy for correcting MRSE. Notably, this strategy has demonstrated potential benefits in terms of UDVA and Snellen line changes compared to the automated strategy. Vector analysis of cylindrical dispersion further supports a higher level of agreement with the goals of refractive surgery, emphasizing the continuous optimization of clinical outcomes through technological upgrades and strategic refinement.
Ray-tracing-guided LASIK introduces a notable advancement by overcoming biases inherent in traditional “forward generation ablation pattern” algorithms. The introduction of the “reverse generation ablation pattern” algorithm addresses issues arising from the mathematical equation of corneal morphology used in traditional algorithms. This becomes particularly crucial given the dynamic nature of corneal factors such as aspherical changes and astigmatism, which can be inconsistent with numerical aperture or meridian alterations. Ray-tracing-guided LASIK directly calculates multi-point corneal morphology under ideal aberrations, offering a foundation for the future development direction of refractive surgery.
Ray-tracing-guided LASIK may not necessitate additional modifications or nomograms in treatment planning, provided that ocular parameters are collected accurately and the intended ablation aligns with expectations. However, the intricate nature of the human eye and the limitations of existing devices compromise its algorithmic advantages and render additional modifications meaningful. Key considerations include: 1) Ocular accommodation. The fogging function of InnovEyes devices effectively mitigates refractive overcorrection induced by ocular accommodation. However, it may not be suitable for individuals who are insensitive to fogging or those exhibiting strong accommodation induced by near-field stimuli. 2) Target refraction. Customizing target refraction based on age appears more reasonable, considering the insufficient stability of axial length development in adolescents, the gradual decline of ocular accommodation in the elderly, and the progressive tendency of corneal without-the-rule astigmatism. Presently, the ray-tracing algorithm consistently sets the target refraction to zero, irrespective of spherical equivalent or cylindrical power. 3) Ablation efficiency. The accuracy of the built-in nomogram or epithelial wound healing compensation algorithm requires confirmation through long-term clinical results, introducing a potential bias. 4) Other factors. Various other factors, such as surgeon expertise, environmental conditions, and corneal biomechanical stability, contribute to the complexity of the procedure. In summary, while ray-tracing-guided LASIK holds promise, addressing these factors is crucial to optimize outcomes and ensure the procedure’s efficacy.
In theory, the ZZ InnovEyes strategy, incorporating manifest refraction, wavefront refraction, and ray-tracing algorithms, aims to mitigate biases arising from measurement errors in spherical and cylindrical diopters. Its primary objective is to prevent the overcorrection of myopia and with-the-rule astigmatism, which thereby enhances the accuracy of refractive procedures. For a more comprehensive evaluation of the ZZ InnovEyes strategy, the target refraction was set to zero in this study. First, clinical outcomes between the two strategies revealed that the ZZ InnovEyes strategy significantly optimizes MRSE. Second, while the ratios of UDVA of 20/16 or better, 20/12.5 or better, and the loss of lines from preoperative CDVA to postoperative UDVA showed no statistical differences, the ZZ InnovEyes strategy hints at potential clinical advantages when relaxing the statistical significance level (P) to 0.1. Third, regarding astigmatism correction and postoperative higher-order aberrations, both groups demonstrated similar clinical results.
An intragroup pre- and post-operation comparison, primarily focusing on changes in higher-order aberrations, revealed increased RMSh, a negative shift in spherical aberration, and a negative shift in vertical coma. First, the postoperative increase in RMSh contradicts pre-launch multicenter research outcomes,Citation4 likely attributable to the expanded analysis area from 4 mm to 5 mm, chosen based on previous research experience.Citation10 Second, the negative shift in spherical aberration, maintained at a lower absolute value, aligns with a higher proportion of postoperative UDVA gain from preoperative CDVA. Notably, the Zernike representation’s limitation in reflecting the weights of visual impairment was addressed through indirect confirmation in this study, highlighting the highest influence of spherical aberrations among all high-order aberrations.Citation11 Third, the sustained higher absolute value of the negative shift in vertical coma suggests a slight upward shift in the ablation center compared to the corneal vertex. Similar observations have been described in EX500 excimer treatments using corneal topography for iris registration,Citation12 with potential explanations rooted in gravity-induced Kappa angle shifts from a sitting position to a supine position.
A deeper analysis of the ray-tracing-guided algorithm reveals its combination of advantages from the previous three custom-guided algorithms: wavefront-guided, topography-guided (Contoura), and Q-value-guided algorithms. First, the wavefront-guided algorithmCitation13 assesses ocular aberration instead of corneal aberration, but the ablation center is based on corneal vertices and also compensates for cyclotorsion. Second, the topography-guided algorithm or ContouraCitation14 manifests excellent clinical outcomes, but lacks effective strategies for eliminating spherical aberrations. Third, the Q-value-guided algorithmCitation15,Citation16 has an excellent strategy for modifying spherical aberrations, but lacks quantitative Q-value setting under ocular spherical aberration guidance. In addition, it eliminates the restriction that the target Q values have to be greater than −1. While the ray-tracing algorithm shows promise, limitations include: 1) the unavailability of a hyperopia correction; and 2) a fixed target RMSh at zero.Citation13 We need to balance the issue between visual acuity and depth of field, especially for those with insufficient accommodation; and 3) challenges in acquiring wavefront data from eyes with high higher-order aberrations using a Hartmann-Shack wavefront sensor. Therefore, there is still room for optimization in ray-tracing-guided LASIK.
This study has several limitations, including a small sample size that was obtained from a single-center, a short follow-up period necessitating longer-term assessments for stability, potential optometrist-induced bias due to the absence of masking during refraction, and the lack of comparison with other types of corneal refractive surgery, emphasizing the need for further research.
Conclusions
In conclusion, this study demonstrates that the ZZ InnovEyes strategy, which integrates manifest and wavefront refraction for ray-tracing-guided LASIK, effectively optimizes the MRSE, indicating enhanced precision in refractive correction. This strategy significantly reduced the overcorrection ratio and provided more accurate and consistent refractive outcomes. Additionally, the results suggest that the ZZ InnovEyes strategy offers potential advantages in improving visual acuity compared to the standard InnovEyes automated strategy.
Abbreviations
LASIKlaser in situ keratomileusis; ZZ InnovEyes, Zhang & Zheng’s InnovEyes; DS, diopter sphere; DC, diopter cylinder; IRC, intended refractive correction; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; IOP, intraocular pressure; RMSh, root mean square of higher-order aberrations; SD, standard deviation; MRSE, manifest refraction spherical equivalent; Q-value, corneal asphericity value.
Ethics Approval and Informed Consent
This study received ethical approval from the Medical Research Ethics Committee of Hangzhou MSK Eye Hospital (#MSKLL20231213) and was conducted in accordance with the principles outlined in the Declaration of Helsinki. Prior to participating in the study, all participants were provided with detailed information regarding the potential risks and benefits of the procedure. Written informed consent was obtained from each participant, indicating their voluntary agreement to participate in the study. Adherence to ethical guidelines ensured the protection of participants’ rights and welfare throughout the research process.
Consent for Publication
All participants provided written consent for the publication of the study results.
Disclosure
The authors declare that they have no competing interests in this work.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kugler LJ, Wang MX. Lasers in refractive surgery: history, present, and future. Appl Opt. 2010;49(25):F1–F9. doi:10.1364/AO.49.0000F1
- Zhang J, Zheng L, Zheng C, Sun P. A comparison of three cylindrical treatment strategies for Topography-Guided LASIK: manifest, topographic, and ZZ VR cylinders. Clin Ophthalmol. 2023;17:1335–1345. doi:10.2147/OPTH.S408101
- Mrochen M, Bueeler M, Donitzky C, Seiler T. Optical ray tracing for the calculation of optimized corneal ablation profiles in refractive treatment planning. J Refract Surg. 2008;24(4):2.
- Kanellopoulos AJ, Maus M, Bala C, et al. International multicenter, myopic and myopic astigmatism femto LASIK, customized by automated ray-tracing ablation profile calculation: a postmarket study. Clin Ophthalmol. 2024;18:525–536. doi:10.2147/OPTH.S435581
- He G, Bala C. Ray-tracing-guided myopic LASIK: real-world clinical outcomes. J Cataract Refract Surg. 2023;49(11):1140–1146. doi:10.1097/j.jcrs.0000000000001286
- Cummings AB, Kelly GE. Optical ray tracing-guided myopic laser in situ keratomileusis: 1-year clinical outcomes. Clin Ophthalmol. 2013;7:1181–1191. doi:10.2147/OPTH.S44720
- Schumacher S, Seiler T, Cummings A, Maus M, Mrochen M. Optical ray tracing-guided laser in situ keratomileusis for moderate to high myopic astigmatism. J Cataract Refract Surg. 2012;38(1):28–34. doi:10.1016/j.jcrs.2011.06.032
- Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75. doi:10.1016/S0886-3350(97)80153-8
- Eydelman MB, Drum B, Holladay J, et al. Standardized analyses of correction of astigmatism by laser systems that reshape the cornea. J Refract Surg. 2006;22(1):81–95. doi:10.3928/1081-597X-20060101-16
- Zhang J, Shao J, Cao X, Zhang Y, Zheng L. Defocus curve and satisfaction of patients with presbyopia After LASIK using the differential modulation of binocular longitudinal spherical aberration. Clin Ophthalmol. 2023;17:3531–3542. doi:10.2147/OPTH.S437324
- Skrzypecki J, Izdebska J, Ordon AJ, Przybek-Skrzypecka J, Szaflik JP. Spherical aberrations and their role in modern ophthalmology. Clin Exp Optom. 2023;106(7):703–710. doi:10.1080/08164622.2022.2160235
- Zhang J, Zheng L, Zhao X, Sun Y, Feng W, Yuan M. Corneal aberrations after small-incision lenticule extraction versus Q value-guided laser-assisted in situ keratomileusis. Medicine. 2019;98(5):2.
- Manche E, Roe J. Recent advances in wavefront-guided LASIK. Curr Opin Ophthalmol. 2018;29(4):286–291. doi:10.1097/ICU.0000000000000488
- Onishi AC, Lee-Choi C, Marvasti AH. Topography-guided excimer laser ablation. Curr Opin Ophthalmol. 2023;34(4):296–302. doi:10.1097/ICU.0000000000000957
- Zhang KP, Fang X, Zhang Y, Chao M. Comparison of Q-value-guided laser-assisted in situ keratomileusis and standard laser in situ keratomileusis for myopia: a meta-analysis. Medicine. 2020;99:45.
- Bueler M, Mrochen M, inventors; Alcon Inc, assignee. Computer program for ophthalmological surgery. United States patent US 20080033408 A1. 2008.