Abstract
Background
The purpose of this study was to evaluate the effects of intravitreal bevacizumab injection in the treatment of macular thickness and reduced visual acuity in patients with branch retinal vein occlusion.
Methods
In this retrospective study, we evaluated 15 eyes of 15 consecutive patients diagnosed with branch retinal vein occlusion between May 2008 and June 2011 at our institution. Detailed ophthalmologic examination, optical coherence tomography, and/or fundus fluorescein angiography was performed at diagnosis and during follow-up. A 1.25 mg intravitreal bevacizumab injection was administered to all patients on average 2.73 (1–7) times. Visual acuity and macular thickness were evaluated before and after treatment.
Results
Eleven patients were female (73.3%) and four were male (26.6%). The mean patient age was 62.6 years. The patients were followed for a mean of 11.4 (range 4–24) months. Mean best-corrected visual acuity was 0.92 ± 0.52 logMAR (logarithm of the minimum angle of resolution) before treatment and 0.66 ± 0.42 logMAR at the last examination. The increase in visual acuity was found to be statistically significant (P = 0.031). Mean macular thickness was 395.46 ± 106.55 μm before treatment and 302.26 ± 84.6 μm after the final treatment. The decrease in macular thickness was statistically significant (P < 0.001).
Conclusion
Intravitreal bevacizumab injection was effective for treatment of retinal vein branch occlusion.
Introduction
Retinal vein disease is the second most common retinal vascular disorder after diabetic retinopathy and can cause severe vision loss. Dilated fundus examination is enough for diagnosis of branch retinal vein occlusion (BRVO). Findings on fundus examination include retinal hemorrhage, increased venous folding and calibration, soft or hard exudates, and retinal edema. BRVO is divided in two subgroups as ischemic or nonischemic, according to the width of capillary nonperfusion regions (ie, five disc spaces). Prolongation of the retinal circulation is seen on fluorescein angiography in both subgroups, with retinal areas of nonperfusion being more prevalent in ischemic BRVO.Citation1
With regard to other vasoactive factors, ischemia due to vascular obstruction causes excessive release of vascular endothelial growth factor from the retina, with consequent disruption of the blood–retina barrier.Citation2 This increased vascular permeability, which is proportional to the levels of vascular endothelial growth factor, causes macular edema. In cases that do not regress spontaneously, the main purpose of treatment is to reduce the duration of edema, thereby minimizing the damage to photoreceptors. Therefore, an agent inhibiting vascular endothelial growth factor may be effective in the treatment of macular edema due to BRVO.Citation3–Citation7 This study assessed the efficacy of intravitreal bevacizumab as a single agent in the treatment of macular edema and reduced visual acuity secondary to BRVO.
Materials and methods
In this retrospective study, we evaluated 15 eyes from 15 consecutive patients diagnosed with nonischemic BRVO and treated with intravitreal bevacizumab injection between May 2008 and June 2011 at our institution. Detailed ophthalmoscopic examination, optical coherence tomography, and/or fundus fluorescein angiography workups were done at diagnosis and during follow-up. Patients with ischemic venous branch obstruction or neovascularization of the iris, retina, or disc, and those undergoing laser photocoagulation, vitreoretinal surgery, or application of intravitreal triamcinolone acetonide/antivascular endothelial growth factor were excluded from participation in this study. Prior to administration of bevacizumab, macular thickness in four patients was 245–300 μm, which is macular edema (but not clinically significant), and in another eleven patients macular thickness was 300–550 μm, which is clinically significant macular edema. All of the patients were informed in detail about the side effects of the drug and application alone, and informed consent was taken before treatment. Bevacizumab was administered intravitreally to all patients at a dose of 1.25 mg/0.05 mL under sterile operating room conditions. Injection was done using a 30-gauge needle in the upper temporal quadrant at a distance of 3.5–4 mm from the limbus, just after inserting a drop of 0.5% proparacaine hydrochloride and 5% povidone iodine. Each patient was instructed to apply 3% ofloxacin drops four times a day for 7 days post-injection. All eyes were observed for complications following treatment and evaluated for visual acuity using a Snellen chart (logMAR; logarithm of the minimum angle of resolution), slit-lamp, intraocular pressure (Goldmann applanation tonometry), and spectral domain optical coherence tomography on day 1 and at 1, 2, and 3 months.
Statistical analysis
All statistical studies were performed using Statistical Package for Social Sciences for Windows version 15 software (SPSS Inc., Chicago, IL, USA). The paired t-test was used for comparative analyses. Results were evaluated as being statistically significant at P < 0.05 with a 95% confidence interval.
Results
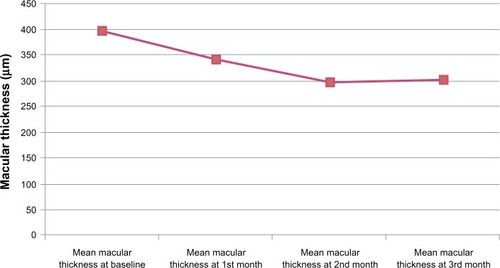
Of the patients included in the study, 11 (73.3%) were female and four (26.7%) were male. Their mean age was 62.6 (53–82) years. During a mean follow-up of 11.4 months, the mean number of bevacizumab injections administered per eye was 2.73 ± 1.66 (). The mean visual acuity score using the Snellen chart was 0.92 ± 0.52 logMAR before injection, 0.86 ± 0.50 logMAR at 1 month, 0.75 ± 0.49 logMAR at 2 months, and 0.66 ± 0.42 logMAR at 3 months (). There was a statistically significant increase (P < 0.05) in mean visual acuity at 3 months compared with baseline, but not at 1 or 2 months. At the 3-month follow-up, an increase in visual acuity was seen in nine of 15 eyes (60%), with no change in three (20%) eyes and a decrease in three (20%) eyes when compared with baseline. Mean central macular thickness was 395.466 ± 106.559 μm at baseline, 340.866 ± 131.101 μm at 1 month, 297.200 ± 81.693 μm at 2 months, and 302.266 ± 84.602 μm at 3 months (). In the average central macular thickness of all patients, a statistically significant decrease was observed (P < 0.05).
Figure 1 Mean visual acuity of patients and statistical significance of changes.
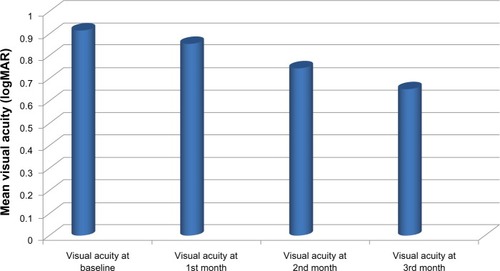
Table 1 Demographic characteristics of patients
Discussion
Macular edema is the most common complication of BRVO, and is the most serious in terms of causing vision loss. In cases of macular edema secondary to BRVO, intravitreal bevacizumab usually decreases macular edema and increases visual acuity.
The patients in this study had a mean pretreatment macular thickness of 395.46 ± 106.55 μm. After treatment, the final mean macular thickness was 302.26 ± 84.60 μm, and the reduction was found to be statistically significant (P < 0.001).
The optimal dose of intravitreal bevacizumab in the treatment of BRVO is not yet determined. Wu et al reported no significant difference with regard to number of injections and anatomic and functional results in patients with BRVO treated using 1.25 mg and 2.5 mg doses of intravitreal bevacizumab.Citation8
In this study, optimal activity was attained by a mean of 2.73 injections. In nine patients (60%), the mean number of injections was 1.66, and in six patients (40%) was 4.33 to obtain optimal activity. These numbers indicate that treatment requirements can change according to the severity of the disease and/or individual differences. Therefore, it can be said there is no standard dosing for intravitreal bevacizumab injection.
Kang et al reported a significant increase in visual acuity in patients with complete IS/OS (inner segment-outer segment) junction line and external limiting membrane after treatment with intravitreal bevacizumab in their study involving 59 eyes. Although an increase in visual acuity was observed in eyes with a disrupted IS/OS junction line and external limiting membrane, the difference was not statistically significant. Integrity of the IS/OS junction line and external limiting membrane was shown to be the best predictor of visual acuity after treatment.Citation9
Although assessment and follow-up of the IS/OS band and integrity of the external limiting membrane was not done in our study, the mean pretreatment visual acuity was 0.92 ± 0.52 logMAR which improved to 0.66 ± 0.42 logMAR at the last examination. This increase in visual acuity was statistically significant (P = 0.031). At the end of follow-up, nine patients (60%) had increased visual acuity compared with baseline. While there was no change in visual acuity in three patients (20%), a further three patients (20%) had decreased visual acuity. However, the patients with no change or a decrease in visual acuity had reduced central macular thickness. All patients with increased visual acuity also had significantly decreased central macular thickness.
The main problem with using intravitreal bevacizumab is maintenance of the central macular thickness and visual acuity levels initially achieved. Reinjections and/or combined laser treatments seem to be the most appropriate way to overcome this problem. In a study by Hanada et al in which intravitreal bevacizumab was injected a total of 95 times in 37 eyes with BRVO, the mean number of injections required to treat macular edema was four.Citation10
In our study, three patients (20%) experienced a rebound increase in central macular thickness at 1 month. But with repetitive injections of bevacizumab, decrease in central macular thickness occurred at 2 months in controls. Bevacizumab injection was administered on average 2.73 (1–7) times in our patients.
In the other studies analyzed, no systemic side effects or serious ocular complications, like development of cataracts, glaucoma, inflammation, endophthalmitis, retinal tear, retinal detachment, and vitreous hemorrhage, were reported. Likewise, no systemic or ocular side effects or complications occurred in our study. The most common side effects were localized hyperemia and subconjunctival hemorrhage at the injection site.
The results of intravitreal bevacizumab injection after an average follow-up of 11 months showed significant improvement in macular edema secondary to BRVO by optical coherence tomography, with a significant increase in visual acuity. Ongoing benefits are achieved by repeated injections.
Disclosure
The authors have no proprietary interest in this work and no financial support was received.
References
- OzmenMCOzdekS[Recent Treatment Modalities For Macular Edema Secondary to Retinal Vein Occlusions.] Retina Ven Tıkanıklıklarına Bagli Gelisen Maküla Ödeminde Güncel Tedavi YöntemleriRetina-Vitreus J20081618 Turkish
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol200624430931516133018
- RehakJRehakMBranch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalitiesCurr Eye Res20083311113118293182
- BoydSRZacharyIChakravarthyUCorrelation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central retinal vein occlusionArch Ophthalmol20021201644164512470137
- Avastin (Product monograph)Mississauga, ONGardiner-Caldwell Communications, Hoffmann-La Roche Ltd2005
- FunatsuHYamashitaHNomaHAqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patientsGraefes Arch Clin Exp Ophthalmol20052433815258777
- NgEWAdamisAPTargeting angiogenesis, the underlying disorder in neovascular age-related macular degenerationCan J Ophthalmol20054035236815947805
- WuLArevaldoJFRocaJAComparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of Follow-UpRetina20082821221918301025
- KangHMChungEJKimYMSpectral-domain optical coherence tomography (SD-OCT) patterns and response to intravitreal bevacizumabtherapy in macular edema associated with branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol201325150150822653439
- HanadaNIijimaHSakuradaYRecurrence of macular edema associated with branch retinal vein occlusion after intravitreal bevacizumabJpn J Ophthalmol20125616517422183139