Abstract
Purpose
To compare the aqueous humor (AH) and iris-ciliary body (ICB) concentration of bimatoprost in rabbit eyes treated with ISV-215 (0.03% bimatoprost formulated in DuraSite) with the marketed product bimatoprost 0.03% ophthalmic solution.
Methods
The left eye of rabbits received a single topical instillation of either ISV-215 (n = 32 eyes) or bimatoprost 0.03% (n = 32 eyes). At predetermined time points, levels of bimatoprost and bimatoprost acid in the AH and the ICB were quantified by HPLC-MS/MS.
Results
Both bimatoprost and bimatoprost acid were detected in the AH and the ICB within 15 minutes of dosing. Bimatoprost acid concentrations in both compartments were markedly higher than bimatoprost. There was a statistically significant (P < 0.01) increase in the concentration of the prodrug in the AH and its acid form in the ICB in animals treated with ISV-215 compared to bimatoprost 0.03%. In the ISV-215-treated rabbit eyes, the highest concentrations of bimatoprost and bimatoprost acid were in the ICB and AH, respectively, while in the bimatoprost 0.03%-treated eyes, no differences in the drug content of the selected ocular tissues were observed.
Conclusions
Bimatoprost 0.03% formulated in DuraSite has superior ocular distribution and area under the curve compared to bimatoprost 0.03% in rabbit eyes. This improvement in the pharmacokinetic parameters of ISV-215 may provide us with a better platform to optimize a bimatoprost formulation that offers the same degree of efficacy in lowering intraocular pressure and improved therapeutic index in glaucomatous patients by lessening the ocular side effects associated with long-term use of topical prostaglandin F2α analogs.
Introduction
Effective intraocular pressure (IOP) lowering appears to be the most common way to slow retinal ganglion cell apoptosis and optic nerve atrophy in patients with primary open-angle glaucoma (POAG).Citation1 IOP lowering is achieved by one or more parameters of aqueous humor (AH) dynamics, including reducing AH production or increasing AH outflow through the trabecular meshwork (TM) or through the uveoscleral pathway. Prostaglandin (PG)-F2α analogs are considered the most effective agents to enhance uveoscleral outflow, and are more efficacious in lowering IOP compared to other classes of ocular hypotensive drugs.Citation2–Citation6 Bimatoprost, latanoprost, travoprost, and tafluprost are the only approved PGF2α analogs in the US. These hypotensive agents are formulated for ophthalmic use as ester or amide prodrugs in order to improve their ocular penetration across lipophilic corneal epithelial cells, and to improve the stability and side-effect profile of the active PGF2α.Citation7–Citation9 Prodrugs are rapidly hydrolyzed into their free acid forms by the esterases and aminopeptidases present in the eye, which then allows the hydrolyzed active form to bind PG receptors (FP receptors) present in the iris-ciliary body (ICB) and TM cells to facilitate outflow.Citation10–Citation13
Bimatoprost is an ethyl amide prodrug of 17-phenyl-trinor PGF2α, and is one of the most frequently prescribed therapeutics for the treatment of ocular hypertension. Clinical trials have shown bimatoprost to be better than or equal to latanoprost or travoprost in lowering IOP; this may be due to the higher affinity of bimatoprost acid for the FP receptor than latanoprost acid or travoprost acid.Citation5,Citation14–Citation17 However, like other PGF2α analogs, bimatoprost has been associated with side effects, such as superficial irritation, hyperpigmentation of iris, periorbital tissue, and eyelashes, eyelash growth, and conjunctival hyperemia.Citation18,Citation19
Bimatoprost is marketed as Lumigan (Allergan, Irvine, CA, USA) at concentrations of 0.01% and 0.03% in the US. In a 12-month, double-masked, multicenter, randomized, controlled clinical trial comparing different concentrations of bimatoprost (0.01% vs 0.03%) for efficacy and tolerability, the 0.01% concentration was clinically equivalent to 0.03% bimatoprost with significantly improved tolerability, including less frequent or severe conjunctival hyperemia.Citation20 Achieving equivalent IOP reduction for bimatoprost 0.01% formulation necessitated improved ocular penetration. This was achieved by increasing the concentrations of benzalkonium chloride (BAC) by fourfold compared to bimatoprost 0.03% (0.005% vs 0.02%). In this situation, BAC is acting both as a preservative and as an excipient to elevate bimatoprost transcorneal penetration.Citation20,Citation21 However, BAC has also been associated with a variety of side effects in the eye, including induction of inflammation, dry eye, and corneal epithelial changes.Citation22 Drug-delivery systems such as DuraSite (InSite Vision, Alameda, CA, USA) have the potential to improve ocular pharmacokinetics of an active ingredient by prolonging the residence time on the ocular surface without the need to increase BAC levels in the formulation.
DuraSite is a polycarbophil-based ophthalmic delivery system that forms a stable mucoadhesive matrix to maintain contact with various ocular tissues by increasing formulation residence time, especially in the conjunctiva. Currently, there are two approved ophthalmic products marketed in the US with DuraSite as the bioadhesive support matrix: AzaSite (1% azithromycin) and Besivance (0.6% besifloxacin). In clinical trials, AzaSite resolved bacterial conjunctivitis within 5 days; the dosing regimen included drug administration twice daily for the first 2 days, followed by once daily for 3 days, providing a more convenient therapeutic course than either aminoglycoside or fluoroquinolone in their current ophthalmic formulations.Citation23 In clinical trials in adults and children, Besivance was efficacious in treating bacterial conjunctivitis when administered twice daily for only 3 days compared to the vehicle.Citation24,Citation25
In this study, we evaluated ISV-215 ocular pharmacokinetics compared to the commercially available bimatoprost 0.03%, and hypothesized the former would be superior in terms of drug penetration into the ocular tissues. Lower concentrations of active ingredients and BAC content are possible with the DuraSite technology. Taken together, the novel formulation of ISV-215 may have the properties necessary to improve both the efficacy and tolerability of bimatoprost/bimatoprost acid in POAG patients.
Materials and methods
DuraSite formulations were prepared using polycarbophil USP obtained from Lubrizol (Wickliffe, OH, USA); bimatoprost was purchased from Flavine North America (Closter, NJ, USA). Lumigan 0.03% was purchased as a commercial product.
Animals
All animals were treated according to the European Convention and the Association for Research in Vision and Ophthalmology Statement for the Use for Animals in Ophthalmic and Visual Research.
Male and female pigmented rabbits (HY79b), approximately 3 months old and weighing 2.33–3.61 kg, were obtained from Hypharm (Roussay, France). All animals were kept in individual cages in a room maintained at 22°C ± 2°C, with relative humidity at 55% ± 10% and a 12-hour light/dark cycle. The animals were fed a standard pellet diet and tap water ad libitum. Macroscopic examinations, including the presence of conjunctival hyperemia of the rabbit eyes, were performed at baseline and prior to euthanasia. Only healthy animals with normal eyes were selected for the study.
Experimental design and sample collection
Male (n = 32) and female (n = 32) pigmented rabbits were randomized into two groups based on body weights, using the randomization function in Microsoft Excel software. Utilizing a micropipette, the left eye (n = 32 eyes/group) of conscious rabbits received one topical instillation (35 μL) of either ISV-215 (0.03% bimatoprost formulated in DuraSite) or commercially available bimatoprost 0.03%. At predetermined time points (0.25, 0.5, 1, 2, 4, 6, 12, and 24 hours postdose), four rabbits (two from each sex)/group were first anesthetized with xylazine (5 mg/kg) and ketamine (35 mg/kg) for blood/plasma collection (samples were not analyzed), and then they were euthanized by cardiac injection of pentobarbital. At least 50 μL of AH was aspirated using a 26-gauge needle attached to a 1-mL syringe, placed in a preweighed labeled centrifuge tube, reweighed and immediately placed in liquid nitrogen. Eyes were enucleated ocular tissues including ICB were dissected and particular attention was paid to avoid tissue cross-contamination. Samples were weighed and processed as aforementioned and stored frozen until analysis.
Bioanalytical analysis
AH and ICB were assayed by HPLC-MS/MS methods. In brief, bimatoprost, bimatoprost acid and internal standards tetradeuterated bimatoprost-d4 (Favine), and bimatoprost free acid-d4 (USP, Rockville, MD, USA) were extracted from the rabbits’ ICB and AH (50 μL) with acidified acetonitrile. Extractions were evaporated to dryness with nitrogen at 40°C, and reconstituted in the mobile phase. Bimatoprost and bimatoprost acid were separated on a C-18 column in an liquid chromatography-mass spectrometry (LCMS) system. The lower limits of quantification for ICB bimatoprost and bimatoprost acid were 0.1 ng/mL, and for AH bimatoprost and bimatoprost acid were 0.2 ng/mL and 0.5 ng/mL, respectively. Mass spectrometry was performed on the HPLC fractions using a Sciex API 5000 mass spectrometer (AB/SCIEX, Concord Ontario, Canada). The working ranges for bimatoprost and bimatoprost acid were 0.2–100 ng/mL for AH and 0.05–100 ng/mL for ICB samples.
Data analysis
The mean AH and ICB concentrations of bimatoprost and bimatoprost acid were determined using data from four rabbits at each time point. The following pharmacokinetic parameters were determined for AH and ICB: maximum mean concentration (Cmax), time to achieve Cmax (Tmax), and area under the AH/ICB concentration–time curve from time 0.25 to 24 hours (AUC0.25–24 h). The mean concentration for all values at given time points were used for AUC0.25–24 h calculations using the trapezoidal method. Calculations were performed using Microsoft Excel.
One-way analysis of variance followed by Bonferroni posttest statistical analysis was performed by Prism (version 5.0) statistical software (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
Results
Both bimatoprost and bimatoprost acid were detectable in both the AH and ICB of rabbit eyes at 0.25 hour postdosing of 35 μL of ISV-215 or bimatoprost 0.03%. lists the ocular pharmacokinetic parameters of bimatoprost for AH and ICB after a single instillation; lists the ocular pharmacokinetic parameters of bimatoprost acid in AH and ICB after a single instillation. In rabbit eyes receiving ISV-215, bimatoprost distributed into the eye rapidly achieved AH concentrations of 26.57 ± 19.16 ng/mL (Cmax) at 0.5 hour postdose (Tmax), which was significantly (P < 0.05) higher than Cmax values of bimatoprost 0.03%. Bimatoprost was quantifiable in all the AH samples (4/4) between the 0.25-and 1-hour time points, in three eyes (75%) at 2 hours and in one eye (25%) at 4 hours. Bimatoprost concentrations were below quantifiable levels between the 6- and 24-hour time points. In AH, bimatoprost hydrolyzed rapidly to its free acid, reaching concentrations fourfold higher than the parent amide (103.4 ± 42.36 ng/mL) at 2 hours postdose (Tmax). Bimatoprost acid was quantifiable in all the AH samples (4/4) up to 6 hours postdose, in one eye (25%) at the 12-hour time point, and in no eyes at the 24-hour time point. In the eyes treated with bimatoprost 0.03%, the AH concentrations of bimatoprost and bimatoprost acid were significantly (P < 0.0001) lower than in eyes treated with ISV-215, with Cmax values of 12.11 ± 21.72 ng/mL and 29.58 ± 26.37 ng/mL at 1 hour postdose (Tmax), respectively. In bimatoprost 0.03%-treated eyes, bimatoprost was quantifiable in all (4/4) the AH samples at 0.25 and 0.5 hour, in three eyes (75%) at 1 hour postdose, in no eyes at 2 hours postdose, and in one eye (25%) at 4 hours postdose, with undetectable AH levels at the 12- and 24-hour time points. Bimatoprost acid was detected in four of four rabbit eyes at the 0.25- and 0.5-hour time points, in three eyes (75%) between the 1- and 6-hour time points and was undetectable at the 12- and 24-hour time points. AH drug exposure (AUC0.25–24 h) was two- and 3.1-fold higher for bimatoprost (24.29 ng/mL·h versus 12.31 ng/mL·h) and bimatoprost acid (302.6 ng/mL·h versus 98.79 ng/mL·h), respectively, in the ISV-215 group compared to the bimatoprost 0.03% group ().
Figure 1 Aqueous humor (AH) concentrations of bimatoprost (A) and bimatoprost acid (B) after a single topical instillation of ISV-215 or bimatoprost 0.03% in pigmented rabbits. Results are means ± standard deviation, n = 4 rabbits per time point. Two-way analysis of variance followed by Bonferroni multiple comparisons.
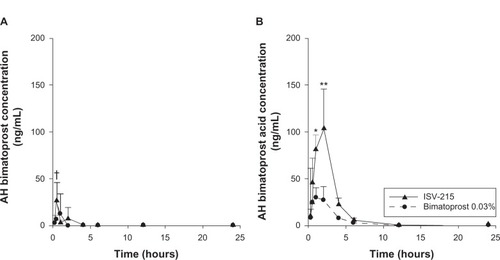
Table 1 Pharmacokinetic parameters of bimatoprost in aqueous humor (AH) and iris-ciliary body (ICB) for rabbit eyes receiving a single instillation of ISV-215 (0.03% bimatoprost formulated in DuraSite) or bimatoprost 0.03% ophthalmic solution
Table 2 Pharmacokinetic parameters of bimatoprost acid in aqueous humor (AH) and iris-ciliary body (ICB) for rabbit eyes receiving a single instillation of ISV-215 (0.03% bimatoprost formulated in DuraSite) or bimatoprost 0.03% ophthalmic solution
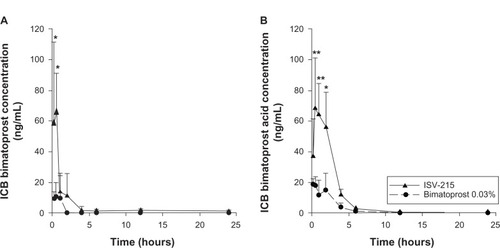
The concentrations of both bimatoprost and bimatoprost acid in ICB were higher than the concentrations of both bimatoprost and bimatoprost acid in AH in the ISV-215-treated eyes. ISV-215-treated eyes had Cmax values of 65.05 ± 26.20 ng/mL and 68.21 ± 33.00 ng/mL for bimatoprost and bimatoprost acid, respectively, at 0.5 hour postdose (Tmax). Bimatoprost concentrations were significantly higher at the 0.25- and 0.5-hour time points (P < 0.001) compared to bimatoprost 0.03%, and bimatoprost acid concentrations were higher at the 0.5- and 1-hour time points (P < 0.0001) and the 2-hour time point (P < 0.001) compared to bimatoprost 0.03%. Bimatoprost was quantifiable in all ICB samples (4/4) between the 0.25- and 2-hour time points, in two eyes (50%) at the 4- and 24-hour time points and in three eyes (75%) at the 6- and 12-hour time points. Bimatoprost acid was detectable in all the ICB samples (4/4) between the 0.25- and 6-hour time points, in two eyes (50%) at the 12-hour time point, and in one eye (25%) at the 24-hour time point. The ICB concentrations of bimatoprost and bimatoprost acid were 6.1- and 3.7-fold lower in the bimatoprost 0.03%-treated eyes compared to ISV-215-treated eyes. The highest bimatoprost and bimatoprost acid concentrations in ICB for this treatment were 10.73 ± 9.01 ng/mL and 18.38 ± 4.05 ng/mL, respectively, at 0.5 hour postdose. Bimatoprost was quantifiable in all ICB samples (4/4) up to 0.5 hour and in three eyes (75%) at 1 hour postdose, in none of the eyes at the 2-, 4-, 12-, and 24-hour time points, and in one eye (25%) at the 6-hour time point. Bimatoprost acid was quantifiable in all ICB samples up to 0.5 hour, in three samples between 1 and 6 hours postdose, in none of the eyes at the 12-hour time point, and in one eye at the 24-hour time point. ICB drug exposure (AUC0.25–24 h) was 6.6- and 3.7-fold higher for bimatoprost (95.14 ng/g·h versus 14.44 ng/g·h) and bimatoprost acid (207.7 ng/g·h versus 56.76 ng/g·h), respectively, in the ISV-215-treated eyes compared to bimatoprost 0.03%-treated eyes (). Macroscopic examination of the treated and untreated eyes showed no signs of conjunctival hyperemia, ocular irritation, or tolerability after single instillation of test articles at any of the time points studied.
Figure 2 Iris/ciliary body (ICB) concentrations of bimatoprost (A) and bimatoprost acid (B) after a single topical instillation of ISV-215 or bimatoprost 0.03% in pigmented rabbits. Results are means ± standard deviation, n = 4 rabbits per time point. Two-way analysis of variance followed by Bonferroni multiple comparisons.
Discussion
PGF2α analogs are the most effective class of pharmaceutical agents to date for lowering IOP and slowing retinal ganglion cell death. Their mechanism of action appears to be through the prostanoid FP receptor. FP receptors have been detected in normal human ocular tissues, including corneal epithelial cells; on TM endothelial cells in the outer portions of the meshwork; along Schlemm’s canal and collector channels; and in individual cells in the iris stroma, iris-sphincter muscle, and ciliary body.Citation12,Citation13,Citation26 Engaging these FP receptors leads to a reduction in collagen synthesis,Citation7,Citation27 an increase in matrix metalloproteinases,Citation7,Citation27–Citation31 and to alterations in the shape of ciliary muscle cells.Citation32
The mechanism of action behind the ability of bimatoprost to lower IOP is not completely understood; however, in some preclinical and clinical pharmacokinetic studies, bimatoprost was shown to remain mostly unchanged with minimal conversion to its free acid form.Citation14,Citation33,Citation34 These investigators showed that unlike latanoprost (another PG analog) that rapidly and almost completely hydrolyzed to its free acid form in the AH, bimatoprost was detected in levels well above its half-maximal effective concentration (EC50) values in the AH and ICB, with very low levels of bimatoprost acid that did not reach EC50 values high enough to be therapeutic. These observations have led researchers to question whether the IOP-lowering properties of this particular PG are through a yet-to-be-identified prostamide receptor(s). Other investigators have demonstrated significant hydrolysis of bimatoprost to its free acid form in the eye, suggesting a typical PGF2α analog prodrug IOP-lowering effect through the FP-receptor activation as a more plausible mechanism of action.Citation11,Citation35
In our study, bimatoprost rapidly hydrolyzed to bimatoprost acid in the AH and ICB of rabbit eyes, reaching concentrations well above the EC50 values (103.4 ng/mL or 248.8 nM 2 hours postdose, and 68.21 ng/g or 164.4 nM 0.5 hour postdose, respectively), which is consistent with other published preclinical and clinical data supporting the role of bimatoprost acid for lowering IOP.Citation11,Citation35,Citation36 ISV-215 formulation also increased bimatoprost levels in the AH and ICB (26.57 ng/mL or 64.02 nM and 65.05 ng/mL or 156.8 nM 0.5 hour postdose, respectively), reaching reported EC50 values for human ciliary smooth-muscle cells, compared to results with just bimatoprost 0.03% alone. However, a wide range of EC50 values have been reported for bimatoprost by various groups: 140 nM in cat iris-sphincter smooth-muscle cells to 1.7 nM in human ciliary smooth-muscle cells.Citation37,Citation38 Therefore, in bimatoprost 0.03%-treated eyes, bimatoprost concentrations in the AH and ICB (12.11 ng/mL or 29.18 nM 1 hour postdose, and 10.73 ng/mL or 25.86 nM 0.5 hour postdose, respectively) may not be sufficient to activate the compound’s receptors based on the data reported by Sharif and colleagues.Citation37 Further work by independent laboratories is required to investigate the reported discrepancies in the EC50 values for bimatoprost.
The bimatoprost AH and ICB exposure was increased in the ISV-215 formulation by 2.2- and 6.1-fold, respectively, and for bimatoprost acid by 3.5- and 3.7-fold, respectively, compared with bimatoprost 0.03%. These data clearly demonstrate the superiority of the DuraSite formulation in delivering high levels of bimatoprost to the eye. Therefore, whether bimatoprost is classified as an active drug or a prodrug, the ISV-215 formulation in DuraSite has the ability to prolong the residence time of bimatoprost on the ocular surface, which in turn leads to high levels of both bimatoprost and/or bimatoprost acid activating their respective receptors. In this scenario, bimatoprost and its acid form can potentially have an additive or a synergistic effect in lowering IOP in patients with ocular hypertension.
In clinical trial studies, bimatoprost 0.03% in its current marketed formulations has been shown to be better or equal in lowering IOP compared to latanoprost.Citation19,Citation39 However, high incidences of conjunctival hyperemia (37.4%) in patients being treated with bimatoprost 0.03% may lead to noncompliance in some cases.Citation20 The DuraSite formulation significantly (P < 0.05) improved the drug distribution of bimatoprost and its acid form in the AH and ICB, potentially providing a platform to lower the bimatoprost concentration while maintaining adequate efficacy paired with a lower side-effect profile. Indeed, in a clinical trial, lower-concentration bimatoprost 0.01% formulation was shown to be as efficacious as bimatoprost 0.03%, with significantly reduced incidences of moderate-to-severe conjunctival hyperemia, skin hyperpigmentation, and eye pruritus.Citation20,Citation40 To achieve better ocular penetration of the drug and maintain efficacy, the concentration of BAC in bimatoprost 0.01% (Lumigan 0.01%) was quadrupled from 0.005% to 0.02%. In vitro studies have shown that BAC improves drug penetration through the corneal epithelial layer by making tight junctions more porous.Citation21,Citation41 However, in a recent preclinical pharmacokinetic study comparing bimatoprost 0.01% to bimatoprost 0.03% in rabbit eyes, no improvements in bimatoprost acid ocular penetration were observed with bimatoprost 0.01% levels 3.3- and −2.2-fold lower than bimatoprost 0.03% at 30 and 90 minutes, respectively, following topical administration (bimatoprost levels were not reported).Citation42 To date, no comparative clinical pharmacokinetic study has been reported with the two bimatoprost concentrations to clarify the role of increasing BAC in improving ocular distribution in healthy volunteers. ISV-215 achieved superior ocular pharmacokinetics with BAC concentrations of 0.001%, fivefold less than bimatoprost 0.03% and 20-fold less than bimatoprost 0.01% ophthalmic solutions. Lowering BAC concentration will have the added advantage of reduced ocular surface findings. Pellinen and colleaguesCitation43 demonstrated the cytotoxicity of PG analogs with BAC concentrations of >0.001% in human corneal epithelial cells and human conjunctival cells. BAC also has been shown to induce dry eye in preclinical in vivo models and ocular inflammation in patients being treated with ophthalmic formulations containing the preservative, and therefore serious consideration must be given to its concentration in formulations in ophthalmic products.Citation44–Citation46
This study design allowed us to show significant (P < 0.05) differences in AH and ICB drug distribution between DuraSite and Lumigan formulations of bimatoprost (0.03%). However, the addition of lower concentrations of bimatoprost formulated in DuraSite to the study design would have given us a better indication on the lower limit of drug levels for potential clinical use. Further pharmacodynamic and pharmacokinetic studies are needed for a complete optimization of ISV-215.
In summary, ISV-215 achieves higher ocular penetration than bimatoprost 0.03% in AH and in ICB. Based on the EC50 for bimatoprost and bimatoprost acid, the drug exposure would be large enough for ISV-215 to lower IOP in POAG patients. It is not known whether increasing the tissue concentration of bimatoprost and/or its free acid form would improve the IOP-lowering effect of the drug, but in one phase III clinical study it was reported that lowering the drug concentration significantly reduced the most serious bimatoprost-associated side effects, conjunctival hyperemia, and improved patient tolerance and compliance.Citation20 Clinical trials comparing different formulations of ISV-215 versus marketed products would conclusively address these questions.
Disclosure
The authors are employees of InSite Vision Incorporated. The authors declare no other conflicts of interest.
References
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670171312049574
- BrandtJDVanDenburghAMChenKWhitcupSMComparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trialOphthalmology200110861023103111382623
- EinarsonTRKulinNATingeyDIskedjianMMeta-analysis of the effect of latanoprost and brimonidine on intraocular pressure in the treatment of glaucomaClin Ther200022121502151511192141
- FungATReidSEJonesMPHealeyPRMcCluskeyPJCraigJCMeta-analysis of randomised controlled trials comparing latanoprost with brimonidine in the treatment of open-angle glaucoma, ocular hypertension or normal-tension glaucomaBr J Ophthalmol2007911626816956912
- NoeckerRSDirksMSChoplinNTBernsteinPBatoosinghALWhitcupSMA six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucomaAm J Ophthalmol20031351556312504698
- van der ValkRWebersCASchoutenJSZeegersMPHendrikseFPrinsMHIntraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trialsOphthalmology200511271177118515921747
- StjernschantzJSelénGSjöquistBResulBPreclinical pharmacology of latanoprost, a phenyl-substituted PGF2 alpha analogueAdv Prostaglandin Thromboxane Leukot Res1995235135187732899
- WhitsonJTTravoprost – a new prostaglandin analogue for the treatment of glaucomaExpert Opin Pharmacother20023796597712083996
- WoodwardDFKraussAHChenJReplacement of the carboxylic acid group of prostaglandin f(2alpha) with a hydroxyl or methoxy substituent provides biologically unique compoundsBr J Pharmacol200013081933194310952685
- AnthonyTLPierceKLStamerWDReganJWProstaglandin F2 alpha receptors in the human trabecular meshworkInvest Ophthalmol Vis Sci19983923153219477988
- HellbergMRKeTLHaggardKKlimkoPGDeanTRGraffGThe hydrolysis of the prostaglandin analog prodrug bimatoprost to 17-phenyl-trinor PGF2alpha by human and rabbit ocular tissueJ Ocul Pharmacol Ther20031929710312804054
- MatsuoTCynaderMSLocalisation of prostaglandin F2 alpha and E2 binding sites in the human eyeBr J Ophthalmol19927642102131327095
- MukhopadhyayPBianLYinHBhattacherjeePPatersonCLocalization of EP(1) and FP receptors in human ocular tissues by in situ hybridizationInvest Ophthalmol Vis Sci200142242442811157877
- CantorLBHoopJWudunnDLevels of bimatoprost acid in the aqueous humour after bimatoprost treatment of patients with cataractBr J Ophthalmol200791562963217135335
- SharifNAWilliamsGWKellyCRBimatoprost and its free acid are prostaglandin FP receptor agonistsEur J Pharmacol20014322–321121311740958
- SimmonsSTDirksMSNoeckerRJBimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallel-group comparison trialsAdv Ther200421424726215605619
- WoodwardDFKraussAHChenJThe pharmacology of bimatoprost (Lumigan)Surv Ophthalmol200145Suppl 4S337S34511434936
- DayDGSharpeEDAtkinsonMJStewartJAStewartWCThe clinical validity of the treatment satisfaction survey for intraocular pressure in ocular hypertensive and glaucoma patientsEye (Lond)200620558359015933751
- KonstasAGKatsimbrisJMLallosNBoukarasGPJenkinsJNStewartWCLatanoprost 0.005% versus bimatoprost 0.03% in primary open-angle glaucoma patientsOphthalmology2005112226226615691561
- KatzLJCohenJSBatoosinghALFelixCShuVSchiffimanRMTwelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertensionAm J Ophthalmol2010149466167120346780
- NakamuraTTeshimaMKitaharaTSensitive and real-time method for evaluating corneal barrier considering tear flowBiol Pharm Bull201033110711020045945
- LiangHPaulyARianchoLBaudouinCBrignole-BaudouinFToxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium systemBr J Ophthalmol201195686987521429894
- AbelsonMBHellerWShapiroAMSiEHsuPBowmanLMClinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trialAm J Ophthalmol2008145695996518374301
- DeLeonJSilversteinBEAllaireCBesifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis in adults and childrenClin Drug Investig2012325303317
- SilversteinBEAllaireCBatemanKMGearingerLSMorrisTWComstockTLEfficacy and tolerability of besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study in adults and childrenClin Ther2011331132621397770
- Schlotzer-SchrehardtUZenkelMNusingRMExpression and localization of FP and EP prostanoid receptor subtypes in human ocular tissuesInvest Ophthalmol Vis Sci20024351475148711980863
- OcklindAEffect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sectionsExp Eye Res19986721791919733584
- LindseyJDKashiwagiKBoyleDKashiwagiFFiresteinGSWeinrebRNProstaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cellsCurr Eye Res19961588698758921230
- OoiYHOhDJRheeDJEffect of bimatoprost, latanoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human ciliary body smooth muscle cellsInvest Ophthalmol Vis Sci200950115259526519443729
- WeinrebRNLindseyJDMetalloproteinase gene transcription in human ciliary muscle cells with latanoprostInvest Ophthalmol Vis Sci200243371672211867589
- WeinrebRNKashiwagiKKashiwagiFTsukaharaSLindseyJDProstaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cellsInvest Ophthalmol Vis Sci19973813277227809418730
- StjernschantzJSelénGOcklindAResulBEffects of latanoprost and related prostaglandin analoguesAlmAWeinrebRNUveoscleral Outflow: Biology and Clinical AspectsLondonMosby19985772
- IchhpujaniPKatzLJHolloGComparison of human ocular distribution of bimatoprost and latanoprostJ Ocul Pharmacol Ther201228213414522136089
- KraussAHWoodwardDFUpdate on the mechanism of action of bimatoprost: a review and discussion of new evidenceSurv Ophthalmol200449Suppl 1S5S1115016556
- DaviesSSJuWKNeufeldAHAbranDChemtobSRobertsLJHydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitroJ Ocul Pharmacol Ther2003191455412648303
- FaulknerRSharifNAOrrSAqueous humor concentrations of bimatoprost free acid, bimatoprost and travoprost free acid in cataract surgical patients administered multiple topical ocular doses of Lumigan or TravatanJ Ocul Pharmacol Ther201026214715620307216
- SharifNAKaddour-DjebbarIAbdel-LatifAACat iris sphincter smooth-muscle contraction: comparison of FP-class prostaglandin analog agonist activitiesJ Ocul Pharmacol Ther200824215216318355130
- StamerWDPiwnicaDJolasTCellular basis for bimatoprost effects on human conventional outflowInvest Ophthalmol Vis Sci201051105176518120435598
- AptelFCucheratMDenisPEfficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trialsJ Glaucoma200817866767319092464
- CravenERLiuCCBatoosinghASchiffmanRMWhitcupSMA randomized, controlled comparison of macroscopic conjunctival hyperemia in patients treated with bimatoprost 0.01% or vehicle who were previously controlled on latanoprostClin Ophthalmol201041433144021188155
- MajumdarSHippalgaonkarKRepkaMAEffect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit corneaInt J Pharm20083481–217517817897799
- OgundeleABJasekMCAqueous humor penetration of topical bimatoprost 0.01% and bimatoprost 0.03% in rabbitsClin Ophthalmol201041447145021188157
- PellinenPHuhtalaATolonenALokkilaJMaenpaaJUusitaloHThe cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivoCurr Eye Res201237214515422049909
- XiaoXHeHLinZTherapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse modelInvest Ophthalmol Vis Sci201253119119722159022
- XiongCChenDLiuJA rabbit dry eye model induced by topical medication of a preservative benzalkonium chlorideInvest Ophthalmol Vis Sci20084951850185618436819
- StevensAMKestelynPADeBDKestelynPGBenzalkonium chloride induces anterior chamber inflammation in previously untreated patients with ocular hypertension as measured by fare meter: a randomized clinical trialActa Ophthalmol2012903e221e22422489894