Abstract
Introduction
The aim of this study was to examine ranibizumab treatment patterns in “real-world” practice and clinical settings, as well as to assess quality of life outcomes over a 24-month period.
Materials and methods
This was a prospective, observational, multicenter, open-label study of 0.5 mg of ranibizumab administered intravitreally. Patients were followed over 24 ± 3 months with intermediate data points at 6 ± 2 months and 12 ± 2 months, and a limited data point at 2.5 ± 1 month that coincided with the end of the loading phase. Outcomes included visual acuity (Early Treatment Diabetic Retinopathy Study), visual function (National Eye Institute Visual Function Questionnaire-25 [NEI VFQ-25]), quality of life (Health Utilities Index Mark III [HUI3]), and safety.
Results
A total of 267 patients with wet age-related macular degeneration (mean ± standard deviation [SD] age = 78.5 ± 7.3 years; 62.4% were female; 34.5% with dual eye involvement; 74.9% were treatment-naïve) were treated (309 eyes were treated). The mean ± SD Early Treatment Diabetic Retinopathy Study score at baseline was 56.3 ± 14.3 letters. The mean ± SD number of injections over 24 months was 7.6 ± 4.1, including 2.5 ± 0.7 and 5.9 ± 3.6 during the loading and maintenance phases, respectively, with corresponding treatment intervals of 4.8 ± 1.4 weeks and 11.5 ± 9.5 weeks, respectively. Improvements in visual acuity over baseline were reached at 2.5 months and maintained at 6 months (both P < 0.0001). The mean visual acuity increase over baseline at 12 months was not significant (P = 0.08); the decline over baseline at 24 months statistically significant (P = 0.02). Overall, 94.3% of patients showed stable or improved disease at 6 months and 81.5% of patients showed stable or improved disease at 24 months. At 6 months, improvements over baseline were significant for VFQ-25 (P = 0.03) and HUI3 (P = 0.02), but not at 12 months and 24 months. Improvements in VFQ-25 and HUI3 were maintained at 24 months in 38% and 34% of patients, respectively. In total 78 serious adverse events were reported in 40 patients and 77 nonserious adverse events in 34 patients. Nine serious adverse events and nine nonserious adverse events in 14 patients were suspected to be related to ranibizumab treatment.
Conclusion
The “real-world” clinical effectiveness of ranibizumab was evidenced by the initial improvements over baseline in visual acuity and quality of life, as well as the maintenance of these outcomes at baseline levels at 24 months, and this was observed under variable treatment conditions. The findings underscore the need for individualized treatment with regular monitoring to achieve optimal vision and quality of life outcomes.
Introduction
Age-related macular degeneration (AMD), particularly with secondary choroidal neovascularization (CNV), is the leading cause of blindness in individuals older than 50 years in the Western world.Citation1–Citation5 In a population-based cohort study of elderly Caucasian patients in Iceland,Citation6 the prevalence of early AMD was 36.0% in persons aged 85 years and older versus 12.4% in those age 66 years to 74 years. The prevalence of exudative or neovascular AMD, commonly referred to as wet AMD, was 11.4% in those aged 85 years and older (versus 3.3% in the total cohort); the rate for pure geographic atrophy was 7.6% among the 85+ subjects (2.4% in the total cohort). Persons aged 85 years and over had a tenfold higher prevalence of late AMD compared to those between the ages of 70 years and 74 years. New vessel formation and proliferation occurs in a subset of patients and may result in progressive, severe, and irreversible central vision loss,Citation2,Citation3,Citation5 with visual acuity declining to less than 20/200 in over 75% of patients.Citation7 In the Age-Related Eye Disease Study, 35.3% of patients developed CNV in the fellow eye (median of 6.3 years).Citation8 Though often underestimated, AMD significantly affects patients’ mental health, functioning, independence, and health-related quality of life (QoL).Citation9–Citation11
Ranibizumab (Lucentis®; Novartis International AG, Basel, Switzerland) is a recombinant, humanized, monoclonal antibody fragment that inhibits neovascularization by neutralizing all active forms of vascular endothelial growth factor-A, a diffusible cytokine that plays a key role in the formation of CNV lesions through promotion of angiogenesis and vascular permeability. Sustained improvements in visual acuity over 24 months have been demonstrated with monthly intravitreal injections of 0.5 mg of ranibizumab in the ANCHOR (Anti-vascular endothelial growth factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-related Macular Degeneration)Citation12 and MARINACitation13 Phase III trials. However, quarterly maintenance phase injections in the PIER (Phase IIIb, multicenter, randomized, double-masked, sham injection– controlled study of the efficacy and safety of ranibizumab in subjects with subfoveal CNV with or without classic CNV secondary to AMD) trial, which included later conversion to monthly injections, did not show sustained benefits in visual acuity outcomes at 24 months and resulted in a net loss of visual acuity.Citation14 The PrONTO (Prospective OCT, Imaging of Patients with Neovascular AMD Treated with intraOcular Ranibizumab) trial, which evaluated variable “as-needed” treatment and retreatment with monthly monitoring of visual acuity and optical coherence tomography (OCT), revealed sustained improvements in visual acuity at 24 months.Citation15 While the intent of ranibizumab therapy is to improve visual acuity, preventing decline is equally important.
In the clinical setting, treatment decisions may be affected by a range of factors that not only include protocols of registration clinical trials (for example, monthly versus “as-needed” or pro re nata [PRN] dosing), but also include commonly-applied criteria for clinical decision making, as well as the locally-approved product labels.Citation16 All of these factors have the potential to affect patient outcomes. The purpose of the HELIOS study was to (1) describe patterns of ranibizumab treatment in real-world clinical practice; (2) assess the associated clinical and QoL outcomes; while (3) contributing to continued pharmacovigilance on ranibizumab. HELIOS was conducted over an observational period of 24 months.
Materials and methods
Design
HELIOS was a prospective, observational, open-label effectiveness study of 0.5 mg of ranibizumab administered by intravitreal injection in 267 patients with wet AMD seen in 15 eye centers in Belgium and followed over 24 months. The study was designed to assess practice patterns, clinical and QoL effectiveness outcomes, and safety in the “real-world” setting of daily clinical practice. Data collection was intended to be ad hoc at each routine patient visit. Investigators were asked to include follow-up visits at 6 months, 12 months, and 24 months after treatment (baseline). There was also a clinical follow-up visit at 2.5 ± 1 month, coinciding with the end of the loading phase of ranibizumab treatment. Only data available from routine clinical practice were recorded. There were no mandatory assessments, laboratory analyses, or other investigations. All patients treated with ranibizumab at baseline were followed regardless of interruption or discontinuation of treatment.
Patients included were those for whom the treating physician decided, in his/her best clinical judgment, to prescribe ranibizumab in accordance with the product label and Belgian reimbursement criteria.Citation17 Ranibizumab treatment decisions were left to the discretion of the prescribing physician. The treatment recommendations in the product label at the time of this study were to deliver ranibizumab as three monthly 0.5 mg intravitreal loading injections, followed by a maintenance phase comprised of monthly visual acuity monitoring, with retreatment if visual acuity declined by more than five Early Treatment Diabetic Retinopathy Study (ETDRS) letters or by one Snellen line equivalent. The label stipulates a minimum of 1 month between injections. No medication was provided to patients.
Sampling
Eligible patients were consenting male and female adults with either a new or prior diagnosis of wet AMD who were prescribed treatment with ranibizumab. Patients with a prior diagnosis had to show evidence of recent disease progression, but they may or may not have received prior therapy. Excluded patients were those concurrently participating in a controlled or observational clinical trial of other investigational drugs. Patients may have been receiving other medications or undergoing other medical or surgical treatments during the study, which may have been discontinued or continued during the study period, as determined by the treating physician. Patients were screened, recruited, and consented by their physician.
Assessments
Visual acuity
Visual acuity was assessed by means of the ETDRS chart. However, if the ETDRS method was not available at the study site, the Snellen chart was used instead and scores were converted using an ETDRS and Snellen equivalency chart. For patients being treated in both eyes, data from the eye with the poorer results were used in the analysis.
Visual function
Visual function, a patient-reported outcome, was evaluated with the National Eye Institute Visual Function Questionnaire-25 (VFQ-25; French and Dutch versions).Citation18–Citation20 This scale has been validated in AMD.Citation21,Citation22 Based on wet-AMD Phase III trials, changes of 4.34 points in VFQ-25 composite scores are clinically important, as are 6.06 points for the “Near activities” subscale score, 5.38 points for the “Distance activities” subscale score, and 4.98 points for the “Vision-specific dependency” subscale score.Citation22
Quality of life
QoL, which is also a patient-reported outcome, was evaluated by the Health Utilities Index Mark III (HUI3; French and Dutch versions), a generic multiattribute measure of health status and health-related QoL.Citation23 A change of >0.03 in the composite score and >0.05 on the visual subscore are considered clinically important changes in QoL for AMD patients.Citation24
Safety
Safety was monitored by the physician investigators asking patients to provide details of any adverse events (AEs), serious (SAEs) or otherwise, at each visit. This information complemented clinician observations in the course of the clinical encounter, as well as data from patients’ medical records. All AEs and SAEs were recorded on the study materials, and they were also reported in accordance with regulations and laws governing AE reporting in Belgium.
Statistical analysis
Descriptive statistics of frequency, central tendency, and dispersion were used under consideration of applicable levels of measurement of the variables in the analysis. Testing for significance of changes in visual acuity, visual function, and QoL was done using mixed regression analyses involving generalized estimation equations. Contingency table analysis methods were used to determine whether efficacy rates observed in the lesion subtypes were statistically similar to prior estimates within each lesion subtype and between lesion subtypes. To examine differences in visual acuity scores between lesion subtypes, contrasts were used to compare changes in scores between lesion subtypes. Correlational/associative analyses were performed if descriptive analyses suggested the presence of associations between variables (for instance, but not limited to, Pearson’s r, Spearman’s rho, χ2/Fisher’s exact tests, and so on). The binomial test was used to test the respective null hypotheses regarding the proportions of patients responding to treatment with ranibizumab. The level of statistical significance was set at 0.05. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Human subjects
This study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki as subsequently amended. The protocol was reviewed and approved by the medical ethics committee at each participating center. Patients were only entered into the study if they or their legal guardian had provided written informed consent.
Results
Patient disposition and populations
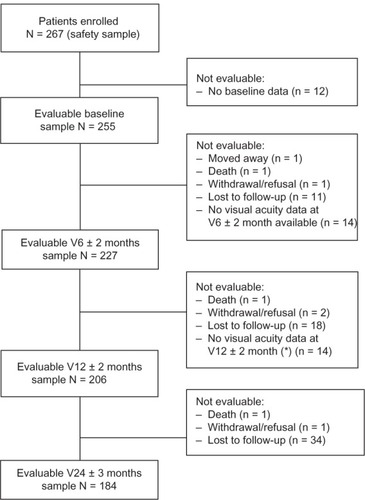
presents the flowchart of patient disposition. In total, 267 patients from 15 retinal centers received ranibizumab treatment in a total of 309 eyes, and these patients constitute the safety population. Of these, 255 patients comprised the evaluable sample at baseline. Evaluable samples included 227 patients at 6 months, 206 patients at 12 months, and 184 patients at 24 months. Among the 71 patients who were evaluable at baseline but not at 24 months, loss to follow-up was the most common cause of drop-out (n = 63). There were no relevant differences at baseline between evaluable and nonevaluable patients in terms of demographics and clinical status. Treatment was discontinued in 37 patients, sometimes for more than one reason per patient. In descending order, reasons included vision decline to <20/200 (21/49 stated reasons); other (15/49; for example, patient request, pigment epithelial tear, subjective link to hypertension, cost); unsatisfactory therapeutic effect (8/49); fibrosis (4/49); and side effects (1/49). Treatment discontinuation did not imply that these patients dropped out of the study, only that treatment was stopped. Follow-up data on these patients were recorded as available and included in the analysis, as appropriate.
Sample
summarizes the patient characteristics at baseline. Summarized, the mean ± standard deviation (SD) age was 78.5 ± 7.3 years, the majority of patients were female (62.4%), and all but one was Caucasian. As to time of onset of disease, a quarter (25.9%) had been diagnosed at baseline, and half (53.3%) within the past 2 months. Mean visual acuity was 56.3 ± 14.3 letters, and the majority had a Snellen status ≥20/200. Occult wet AMD was the most frequent lesion (60.4%), and subfoveal was the most common location (86.3%). One-third (34.5%) of patients had CNV involvement in both eyes. Three-quarters of the sample (74.9%) were treatment-naïve for wet AMD. Among the 64 patients with at least one prior treatment, 28 had received bevacizumab, 25 had received photodynamic therapy, and 13 had received ranibizumab, among other treatments. Thirty-six (14.1%) patients had a family history of AMD and 111 (43.6%) were current or former smokers. Rates for cardiovascular disease or risk factors were low.
Table 1 Patient demographic and clinical characteristics at baseline
Treatment patterns and patient exposure
Of the 255 patients treated at baseline, 242 (94.9%) received treatment in one eye and 13 (5.1%) in two eyes. Over the course of the study, 309 eyes were treated. Patients received an average of 2.5 ± 0.7 injections during the loading phase from baseline to 2.5 months (±2 weeks) and 5.9 ± 3.6 injections during the maintenance phase from months 2.5 through 24. The mean number of ranibizumab injections over 24 months was 7.6 ± 4.1, with mean values of 5.0 ± 2.1 in year 1 and 3.7 ± 2.1 in year 2. Patients with the minimally classic subtype received slightly more injections over the 24-month study period (8.2 ± 4.5) than patients with predominantly classic (7.6 ± 3.3) or occult lesions (7.5 ± 4.3). Only half of patients (52.6%) received the three injections during the loading phase, as was recommended in the product label during the time of the study, with 39.2% of patients receiving only two injections and 8.2% of patients receiving only one. The mean interval between injections was 4.8 ± 1.4 weeks during the loading phase and 11.5 ± 9.5 weeks during the maintenance phase. The mean number of injections during this phase was 5.9 ± 3.6. Ranibizumab treatment in the maintenance phase was dependent on follow-up monitoring and assessment of wet AMD status. In addition to visual acuity monitoring, after the loading phase, ophthalmological exams commonly performed to monitor clinical status included fundus examination (87.0% of visits), OCT (82.7%), and fluorescein angiography (32.1%). CNV activity, including an increase in lesion size, new growth of CNV, fluid, leakage, or hemorrhage, was cited as a reason for retreatment in 33.9% of patients at the 6-month, 33.3% at the 12-month, and 26.5% at the 24-month visit. The average time between visual acuity assessments during the maintenance phase was 8.4 ± 5.7 weeks – approximately double the monthly frequency recommended in the product label.
Outcomes
Visual acuity
From a mean of 56.3 ± 14.3 ETDRS letters read at baseline, the mean number of ETDRS letters read increased significantly by 2.5 months and 6 months, as did the mean change in ETDRS letters read from baseline to 2.5 months and 6 months (all P < 0.0001) (). By 12 months and 24 months, visual acuity reached levels statistically similar to baseline. There were no differences in visual acuity when stratified by lesion subtype and by prior treatment status (though caution is indicated given the small sample sizes in some cells). Baseline visual acuity accounted for 25% of the variability in mean changes in letters read at later time points.
Table 2 Changes in visual acuity (ETDRS letters read; mean ± SD) and percentages of patients with attained changes in disease status
Observed differences in mean changes from baseline between patients with adequate versus suboptimal treatment loading were significant at 6 months and 12 months (both P < 0.0001), but not at 24 months. There was no statistically significant association between the frequency of visits (and thus monitoring) during the maintenance phase and visual acuity.
In terms of the association of disease status with treatment, 69.6% of patients showed improvement at 6 months and 81.5% showed improvement at 24 months (). When adding in the quarter of patients with stable disease at each of these time points, 94.3% of patients showed stable or improved disease at 6 months and 81.5% showed improvement at 24 months. The proportion of patients with significant improvement (a gain of ≥15 letters) did not change significantly from 6 months to 12 months to 24 months, nor did the proportion of patients showing stable disease (a loss of <15 letters). There was a significant increase over time in the proportion of patients with disease progression (a loss of ≥15 letters).
Patient-reported outcomes
Mean composite visual function (VFQ-25) scores were significantly higher at 6 months compared to baseline (P = 0.03) (). Differences between baseline and 12 months and 24 months were statistically nonsignificant. Clinical improvements were maintained at 24 months in 38% of patients. Differences from baseline to 6 months, 12 months, and 24 months were statistically significant for the subscore of patient-appraised vision (all P < 0.0001), but they were nonsignificant for the subscores of “ocular pain”, “distance activities”, “vision-specific social functioning”, “vision-specific role difficulties”, “driving”, and “peripheral vision.” The “near activities” (P = 0.02), “vision-specific mental health” (P < 0.0001), and “vision-specific dependency” (P < 0.0001) subscores were significantly better at 6 months but not at later time points. Unchanged from baseline to 6 months and 12 months, patient-reported “general health” (P = 0.03) and “color vision” (P = 0.02) scores had declined by 24 months. The proportions of patients with clinically important improvements in the composite score at 6 months, 12 months, and 24 months were statistically similar; and this was also the case for each of the wet AMD-relevant subscores of “near activities”, “distance activities”, and “vision-specific dependency”.
Table 3 Changes in visual function (VFQ-25; 0–100 scale) and proportions of patients with clinically important improvements in wet AMD-relevant scores
Compared to baseline, mean composite QoL (HUI3) scores were significantly higher at 6 months (P = 0.02), but not at 12 months and 24 months (). Clinical improvements were maintained at 24 months in 34% of patients. The “vision” subscore improved from baseline to 6 months (P = 0.03) and 12 months (P = 0.02); at 24 months, it was not significantly different from baseline. The “emotion” subscore at 6 months was significantly better than at baseline (P = 0.01), but not at 12 months and 24 months. The “pain” subscore was statistically the same at baseline, 6 months, and 12 months, but worse at 24 months (P = 0.03). The proportions of patients with clinically important improvements in the HUI3 composite score at 6 months, 12 months, and 24 months were statistically similar. There were proportionately more patients with clinically important improvements in the “vision” subscore at 12 months than at 6 months and 24 months.
Table 4 Changes in quality of life parameters (HUI3, mean ± SD) and proportions of patients with clinically important improvements in wet AMD-relevant scores
Safety
Of the 267 patients in the safety population, 78 SAEs were reported in 40 patients and 77 nonserious AEs in 34 patients, for a total of 155 events in 74 patients. In total, 18 AEs, including nine SAEs and nine AEs, of a total of 14 patients were suspected to be related to ranibizumab treatment. Nine of 13 events classified as eye disorders are known and listed in the Summary of Product Characteristics.Citation25 Four of these events were suspected to be related to ranibizumab treatment, and two of the systemic events were attributed to ranibizumab treatment. Not listed in the Summary of Product Characteristics were none of the 24 cardiovascular events, one of the six systemic events, and one of the nine gastrointestinal events. Five of the 78 SAEs were fatal, but none were related to ranibizumab. One death was attributed to very old age (96 years), two to pneumonia, and one each to lung edema and metastatic small cell bronchus carcinoma.
Discussion
A major objective in the management of progressive disorders like wet AMD is to halt or delay disease progression and, if possible, improve disease status. The HELIOS study of clinical practice with ranibizumab in the management of neovascular AMD revealed a consistent pattern of improvements in clinical and patient-reported outcomes from baseline to 6 months, and a subsequent return to baseline levels. Thus, in general, injections of 0.5 mg of ranibizumab on schedules determined by patients’ treating physicians per their best clinical judgment is associated with short-term improvements in visual acuity, visual function, and QoL, as well as with a delay of disease progression up to 24 months.
The “real-world” variable injection regimens in both the treatment loading and maintenance phases in HELIOS are in contrast to both the MARINACitation13 and ANCHOR trials,Citation12 which included scheduled monthly treatments throughout both phases, as well as to the PIER trial,Citation14 which had scheduled monthly loading phase treatments followed by quarterly injections, and a later return to monthly treatment. Further, while in the PrONTO trialCitation15 retreatments were on a PRN basis, but patients were monitored monthly; in HELIOS there was significant variability in the frequency of monitoring and treatment patterns during the loading and maintenance phases. Only half of patients (52.6%) received monthly treatments during the loading phase. During the maintenance phase, from months 2.5 through 24 ± 3 months, patients received an average of 5.9 injections. Treatment during this phase was dependent on the frequency of monitoring, but the average interval was 11.5 weeks, which was more than double the recommended interval.
As a result, HELIOS patients did not experience the sustained improvements from baseline visual acuity (mean ETDRS letters read) at 24 months that patients in MARINACitation13 or ANCHORCitation12 did. Visual acuity outcomes were more similar to PIER,Citation14 where disease stabilization at 12 months was followed by a decline by 24 months even with monthly injections between months 19 and 24. At 24 months, HELIOS patients showed an average loss from baseline of −2.4 ETDRS letters read, which was similar to patients in the PIER trial (−2.3 letters),Citation14 less than the gains of 6.6 letters in MARINA,Citation13 and 10.7 letters in ANCHOR,Citation12 but much better than the losses of −14.9 letters in the MARINACitation13 and −21.4 letters in the PIERCitation14 sham arms.
The “real-world” treatment patterns in HELIOS mitigated expected disease progression, but they did not result in the improvements observed with scheduled monthly dosing. The extended interval in HELIOS signifies missed opportunities to identify potential disease progression and the need for retreatment. The suboptimal loading and maintenance phase follow-up in HELIOS and the impact on visual acuity, QoL, and visual function outcomes may have played an important role in the discontinuation of ranibizumab treatment in the 22 patients with vision <20/200 or the eight patients with unsatisfactory therapeutic effects. The sustained improvement in visual acuity at 24 months in PrONTOCitation15 (+11.1 letters) underscores the importance of close monitoring and strict criteria to guide PRN retreatment, particularly given the highly variable progression of wet AMD disease among patients.
Several more recent PRN studies have used more qualitative OCT retreatment criteria resulting in the maintenance of initial visual gains and reducing the extent of irreversible photoreceptor damage. The variable treatment arm in the CATT (Comparison of Age-Related Macular Degeneration Treatments Trials) trial, using monthly monitoring and any evidence of disease progression in the retreatment criteria, demonstrated improvements of +6.8 letters at 12 months.Citation26 This was less than the +8.5 letters observed in the monthly treatment group, but was still considered to be statistically noninferior. In the HARBOR study (Phase III, double-masked, multicenter, randomized, active treatment-controlled study of the efficacy and safety of 0.5 mg and 2.0 mg ranibizumab administered monthly or on an as-needed basis (PRN) in patients with subfoveal neovascular age-related macular degeneration),Citation27 the visual acuity curve remained horizontal over 9 months following the loading dose, suggesting that PRN dosing was able to maintain initial visual acuity gains. The new stability-driven posology for ranibizumab recommends an individualized treatment initiation to maximum visual acuity, instead of just three doses as applied in the HELIOS study. This posology also advises that therapy be resumed if monthly monitoring indicates a loss of visual acuity, and to continue treatment until stable visual acuity is regained for three consecutive monthly assessments (implying a minimum of two injections). This posology possibly allows for more initial treatments and results in greater visual acuity gains. During the first year of treatment, we have learned that it is of crucial importance to follow patients closely with regular monthly monitoring visits, if possible, and to apply an individualized treatment approach to learn and observe if patients need further intensive treatment or not.
Ranibizumab therapy preserved patient-reported visual function (VFQ-25) and QoL (HUI3) at 24 months on the overall scores as well as on scores of clinically important subscales. Patients in HELIOS did not show the improvements in mean VFQ-25 composite scores that were observed in MARINACitation13 (+4.5) and ANCHORCitation12 (+5.8); trends were also observed with the “near activity”, “distance activity”, and “vision-specific dependency” subscales. However, the composite and subscore improvements noted in HELIOS patients are in contrast with the losses observed in visual function, as seen in the MARINA sham patients at 24 months.Citation13 This indicates that ranibizumab treatment at least stabilizes patient-reported visual function over 24 months.
Physician investigators were instructed to base their retreatment decisions on the approved label recommendations; however investigators also cited various other reasons for their decision to re-treat. This offers insight into the clinical evidence that clinicians use to decide on retreatment in routine clinical practice with a (at that time) novel treatment.
Few (18/155) of the safety events were suspected to be related to the study medication. Nineteen percent of patients reported ocular AEs. Safety was comparable to findings from Phase III trials and the product label listings.
The proportion of nonevaluable patients increased as the study progressed. This too may provide initial insights into the management of wet AMD with ranibizumab in the “real-world.” Note also that 43.6% of patients were current or former smokers; while this may appear high, an estimated 51% of the Belgian population is classified as current or former smokers.Citation28
The HELIOS study enrolled the first patients in early 2008 shortly after the commercial availability of ranibizumab in Belgium in November 2007. The short time span between commercial approval and the start of the study may reflect the limited experience on the part of physician investigators with ranibizumab therapy and, perhaps, explain the observed variability in the treatment patterns. Consequently, the HELIOS results reflect Belgian clinical practice in the time period soon after approval. Future studies are needed to assess more recent treatment patterns and outcomes, and to reconcile these outcomes with the emerging evidence on alternate intervention schedules. Currently, real-world data on the use of ranibizumab are being collected within the multicountry, prospective LUMINOUS study, funded by Novartis.Citation29 This may shed more light on the barriers to optimal treatment in Belgium, as well as in other countries. Future studies are also needed to determine whether the label- and possibly guideline-congruent practice results in higher effectiveness rates and, in turn, translates into more sustained changes in QoL and vision functioning outcomes. Also needed are large multicenter, international, randomized trials to test the hypothesis that retreatment based on morphological changes, as observed by spectral-domain OCT, leads to earlier intervention in the case of recurring disease activity. Variable OCT-guided treatment regimens aiming to maintain an exudation-free macula with the fewest number of injections and office visits may improve clinical outcomes, but this cannot be evaluated from our data and should be the subject of future studies.
In conclusion, this “real-world” prospective observational study of 255 evaluable patients with wet-AMD treated with intravitreal injections of ranibizumab treatment demonstrated disease stabilization or improvement in 81.5% of patients, including disease improvement in 52.7% of patients and significant disease improvement for 14% of patients at 24 months. These effectiveness rates confirm that ranibizumab is effective under conditions of greater heterogeneity of patients, clinicians, and centers. Patient-reported visual function and QoL were maintained at 24 months. The study also provides evidence for the need for compliant regular monitoring and individualized retreatment timing as determinants of visual acuity, visual function, and QoL outcomes.
Disclosure
The HELIOS study was sponsored by Novartis Pharma. J-M Rakic and A Leys have served as advisors to Novartis. K Denhaerynck, C Pacheco, K MacDonald, and I Abraham are employees of Matrix45, which was under contract with Novartis to consult on study design, study protocol, statistical analysis, results reporting, and dissemination. Company policy prohibits employees from owning equity in client organizations of Matrix45 (except for independently administered collective funds). Matrix45 performs similar studies for other pharmaceutical companies on a non-exclusivity basis. H Brié, S Vancayzeele, and C Hermans are employees of Novartis. The authors report no other conflicts of interest in this work.
References
- AugoodCFletcherABenthamGMethods for a population-based study of the prevalence of and risk factors for age-related maculopathy and macular degeneration in elderly European populations: the EUREYE studyOphthalmic Epidemiol200411211712915255027
- BresslerNMAge-related macular degeneration is the leading cause of blindnessJAMA2004291151900190115108691
- FriedmanDSO’ColmainBJMuñozBEye Diseases Prevalence Research GroupPrevalence of age-related macular degeneration in the United StatesArch Ophthalmol2004122456457215078675
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ2004821184485115640920
- FolkJCStoneEMRanibizumab therapy for neovascular age-related macular degenerationN Engl J Med2010363171648165520961248
- JonassonFArnarssonAEiríksdottirGPrevalence of age-related macular degeneration in old persons: age, gene/environment Susceptibility Reykjavik StudyOphthalmology2011118582583021126770
- WongTYWongTChakravarthyUThe natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysisOphthalmology2008115111612617675159
- ClemonsTEMiltonRCKleinRSeddonJMFerrisFLAge-Related Eye Disease Study Research GroupRisk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no 19Ophthalmology2005112453353915808240
- SteinJDBrownMMBrownGCHollandsHSharmaSQuality of life with macular degeneration: perceptions of patients, clinicians, and community membersBr J Ophthalmol200387181212488253
- RovnerBWCastenRJMassofRWLeibyBETasmanWSWills Eye AMD StudyPsychological and cognitive determinants of vision function in age-related macular degenerationArch Ophthalmol2011129788589021746979
- StevensonMRHartPMMontgomeryAMMcCullochDWChakravarthyUReduced vision in older adults with age related macular degeneration interferes with ability to care for self and impairs role as carerBr J Ophthalmol20048891125113015317701
- BrownDMMichelsMKaiserPKHeierJSSyJPIanchulevTANCHOR Study GroupRanibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR studyOphthalmology200911615765. e519118696
- RosenfeldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- AbrahamPYueHWilsonLRandomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2Am J Ophthalmol20101503315324. e120598667
- LalwaniGARosenfeldPJFungAEA variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO StudyAm J Ophthalmol200914814358. e119376495
- PauleikhoffDKirchhofBRetreatment criteria in anti-VEGF therapy of exudative AMD: critical analysis of present regimes and new morphological definition of “lesion activity”Graefes Arch Clin Exp Ophthalmol2011249563163221380538
- Rijksinstituut voor Ziekte-en InvaliditeitsverzekeringVergoedbaarheid van de specialiteit Lucentis. [Eligibility of the specialty Lucentis] Available at: http://www.riziv.fgov.be/inami_prd/ssp/cns2/pages/MinisterialDecisionDet.asp?qs_SpcCod=00643937&qs_EffDat&=20071101&qs_MdId&=4852Accessed on 22 July 2013 German
- MangioneCMLeePPPittsJGutierrezPBerrySHaysRDPsychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test InvestigatorsArch Ophthalmol199811611149615049823352
- MangioneCMLeePPGutierrezPRSpritzerKBerrySHaysRDNational Eye Institute Visual Function Questionnaire Field Test InvestigatorsDevelopment of the 25-item National Eye Institute Visual Function QuestionnaireArch Ophthalmol200111971050105811448327
- ClemonsTEChewEYBresslerSBMcBeeWAge-Related Eye Disease Study Research GroupNational Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS): AREDS Report No 10Arch Ophthalmol2003121221121712583787
- OrrPRentzAMMargolisMKValidation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in age-related macular degenerationInvest Ophthalmol Vis Sci20115263354335921282568
- SuñerIJKokameGTYuEWardJDolanCBresslerNMResponsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trialsInvest Ophthalmol Vis Sci20095083629363519255158
- HorsmanJFurlongWFeenyDTorranceGThe Health Utilities Index (HUI): concepts, measurement properties and applicationsHealth Qual Life Outcomes200315414613568
- SharmaSOliver-FernandezAAge-related macular degeneration and quality of life: how to interpret a research paper in health-related quality of lifeCurr Opin Ophthalmol200415322723115118510
- European Medicines AgencyEuropean Public Assessment Report Lucentis Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdfAccessed July 22, 2013
- CATT Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- BusbeeBGHoAcBrownDMHeierJSSUnerIJLiZRubioRGLaiPHARBOR Study GroupTwelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degenerationOpthalmology20131205104656
- TNS Opinion and SocialSpecial Eurobarometer 332TobaccoBrussels, BelgiumEuropean Commission2010 Available from: http://ec.europa.eu/health/tobacco/docs/ebs332_en.pdfAccessed June 20, 2013
- LUMINOUS: Study to Observe the Effectiveness and Safety of Ranibizumab Through Individualized Patient Treatment and Associated Outcomes Available from: http://clinicaltrials.gov/show/NCT01318941Accessed 22 July 2013