Abstract
Background
The purpose of this paper is to report our experience of Descemet’s stripping and non-Descemet’s stripping automated endothelial keratoplasty (DSAEK/nDSAEK) for microcorneas using 6.0 mm donor grafts.
Methods
Three eyes of two patients (a 56-year-old woman and a 59-year-old woman) with microcornea and suffering from bullous keratopathy were treated with either DSAEK or nDSAEK. A small donor graft (6.0 mm) was inserted into the anterior chamber using a double glide (Busin glide and intraocular lens sheet glide) donor insertion technique. Both patients were followed for at least 12 months. Clinical outcomes, including intraoperative and postoperative complications, visual acuity, and endothelial cell density were evaluated.
Results
In all three cases (100%), no intraoperative complications were noted. In one case with a flat keratometry value (32.13 D), a partial donor detachment was noted one day postoperatively, but it was reattached by rebubbling. In another case, rejection was noted 8 months postoperatively, but treatment with systemic corticosteroids was successful. A clear cornea remained in all three cases (100%), with best-corrected visual acuity greater than 20/100 (mean 20/50) at 12 months. Mean postoperative endothelial cell counts were 2,603 ± 18 cells/mm2 at 6 months (7.4% decrease from preoperative donor cell counts) and 1,799 ± 556 cells/mm2 at 12 months (36.5% decrease).
Conclusion
We report for the first time the successful use of a small donor graft (6.0 mm) for DSAEK/nDSAEK in cases of microcornea. Additional stud ies using a large number of patients are required to evaluate fully the potential advantages and drawbacks of small diameter donor grafts for microcornea.
Introduction
Descemet’s stripping automated endothelial keratoplasty (DSAEK) has been widely adopted for the treatment of corneal endothelial dysfunction.Citation1–Citation5 DSAEK has many advantages over standard penetrating keratoplasty, including fast visual recovery with minimal refractive change, structural integrity of the cornea, and reduction of ocular surface complications.Citation6,Citation7 Further modification to eliminate Descemet’s membrane stripping was also shown to be quite effective for non-Fuchs dystrophy type bullous keratopathies, with rapid visual recovery and minimal induced astigmatism. This procedure was called non-Descemet’s stripping automated endothelial keratoplasty (nDSAEK).Citation8–Citation10 Advantages of nDSAEK are simplifying the surgery, and reducing the surgical time and invasion.Citation8–Citation10
However, DSAEK/nDSAEK are not panaceas to treat bullous keratopathies because challenges still exist, such as significant stromal scarring, iris abnormalities, lens abnormalities, aniridic aphakic eyes, anterior chamber intraocular lens, iridocorneal endothelial syndrome, and “small eye” including pediatric eye and microcornea.Citation11–Citation17
Toshida et al recently reported a successful experience of DSAEK using a small diameter donor graft (7.5 mm) for treatment of microcornea.Citation17 Herein, we report our experience and clinical outcomes of DSAEK/nDSAEK for microcorneas using 6.0 mm donor grafts.
Materials and methods
This retrospective study was approved by the ethical committee of Kanazawa University Graduate School of Medical Science and followed the tenets of the Declaration of Helsinki. All patients read and signed an informed consent document prior to enrolment.
Three eyes of two patients (a 56-year-old woman and a 59-year-old woman) with microcorneas and suffering from bullous keratopathies were treated with either DSAEK or nDSAEK. Corneal horizontal and vertical diameters (in mm) were 9.0/8.0, 9.0/7.5, and 9.0/7.5 in cases 1, 2, and 3, respectively (mean 8.3 mm, ). The axial lengths of each case were 24.93 mm, 23.97 mm, and 24.27 mm, respectively (mean 24.39 mm), indicating that none of the eyes were microphthalmic. The causes of bullous keratopathy were iridocorneal endothelial syndrome (case 1) and secondary to argon laser iridotomy (cases 2 and 3, see ). For case 1, Descemet’s membrane removal was necessary to remove peripheral anterior synechia and pathological endothelium. For cases 2 and 3, Descemet’s membrane was not removed during endothelial keratoplasty (nDSAEK) since the cases had no guttae and good visual acuity was expected without any Descemet’s membrane removal.Citation8–Citation10
Table 1 Demographic data and clinical outcomes of two patients (three eyes) with microcornea
All DSAEK/nDSAEK procedures were performed as previously described ().Citation8,Citation9,Citation11 In brief, DSAEK/nDSAEK donor grafts, already precut by eyebank technicians, were obtained through SightLife™ (Seattle, WA, USA). After rinsing with balanced salt solution for several minutes, the donor graft was transferred to a punching system and cut with a donor punch (Barron® donor corneal punch, Katena Products, Inc., Denville, NJ, USA). A 6.0 mm donor punch was used in all cases because the host corneal diameter was extremely small (average 8.3 mm). For all cases, a phacoemulsification and a single-piece acrylic intraocular lens insertion procedure was performed from a 3 mm limbal incision just prior to DSAEK/nDSAEK. This had the added benefit of creating more space in the anterior chamber to safely position the graft. In all cases, four corneal fenestrations were performed to drain the interface fluid. A small inferior iridectomy at the 6 o’clock position was then routinely created using a 25-gauge vitreous cutter (MID Labs, San Leandro, CA, USA). Continuous irrigation from a 25-gauge anterior chamber maintainer (25-gauge DSAEK Chamber Maintainer, Catalog AE-7802, ASICO, Westmont, IL, USA) was used. An ophthalmic viscosurgical device (Viscoat®, Alcon, Fort Worth, TX, USA) was applied to the endothelial surface of the graft and the donor graft was inserted using both a Busin glide and an intraocular lens sheet glide (double glide technique) through a 4.0 mm clear corneal incision.Citation11 After insertion of the donor graft, the wound was secured with interrupted 10-0 nylon sutures. Air was injected into the anterior chamber to press the donor graft against the recipient cornea. Corneal massage was performed to adjust donor graft centering and to eliminate residual fluid at the donor graft-recipient interface. Also, residual interface fluid was drained through the corneal venting incisions. Air was left in the anterior chamber and the patient was instructed to lie on her back for at least one hour.
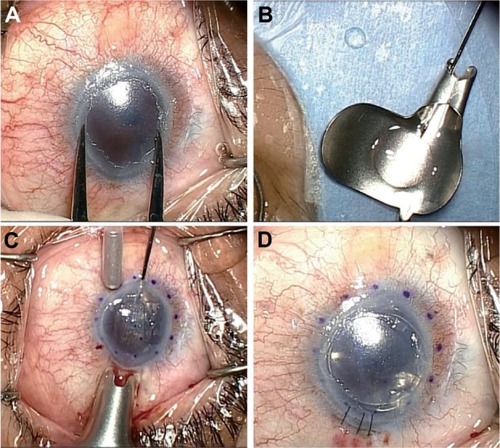
Figure 1 Surgical technique of non-Descemet’s stripping automated endothelial keratoplasty in case 2. (A) Under the surgical microscope, the recipient corneal size was horizontal 9.0 mm × vertical 7.5 mm, and the donor diameter was determined as 6.0 mm. (B) A 6.0 mm diameter donor lamella, in which the endothelial surface was coated with viscoelastic material, was loaded into the Busin glide. (C) Without recipient descemetorhexis, the donor lamella was easily inserted into the anterior chamber with a pull-through technique using the sheets glide and the Busin glide. Note the violet dot marking was 8.0 mm in diameter. (D) after securing the wound with an interrupted 10-0 nylon suture, air was injected to press the donor tissue against the recipient cornea.
Postoperatively, the patients were followed for at least 12 months. Clinical outcomes, such as intraoperative and postoperative complications, visual acuity, and endothelial cell density, were evaluated. Central endothelial cell density was measured by noncontact specular microscopy (Noncon-robo, Konan Medical Inc., Hyogo, Japan), using the center method as outlined by the manufacturer’s software at 6 and 12 months.
Results
summarizes the clinical outcomes of the three cases enrolled in this study. In all cases (100%), no difficulties during donor insertion and no intraoperative complications were noted. In case 3, partial donor detachment was noted at one day postoperatively, but the donor was attached by rebubbling (). In case 2, rejection was noted 8 months postoperatively while being on topical fluorometholone 0.1%, but treatment with additional intensive topical betamethasone 0.1% and systemic corticosteroids was successful. All cases (100%) achieved clear cornea at 12 months postoperatively, and best-corrected visual acuity was greater than 20/100 (mean 20/50) at 12 months (). Mean postoperative endothelial cell counts were 2,603 ± 18 cells/mm2 at 6 months and 1,799 ± 556 cells/mm2 at 12 months. Mean endothelial cell loss rate was 7.4% (95% confidence interval [CI] −5.7% to 20.6%) at 6 months and 36.5% (95% CI −9.1% to 82.1%) at 12 months compared with donor cell counts (2,820 ± 181 cells/mm2).
Figure 2 Slit-lamp photographs and anterior segment optical coherent tomography images in case 3. (A and B) Preoperatively, mild stromal edema and shallow anterior chamber were noted, although best-corrected visual acuity was 20/20. A flat corneal section was noted by anterior segment optical coherent tomography. (C and D) At one day postoperatively, partial donor detachment (arrow) was noted, and rebubbling was performed immediately. (E and F) At 2 months postoperatively, total donor attachment was noted.
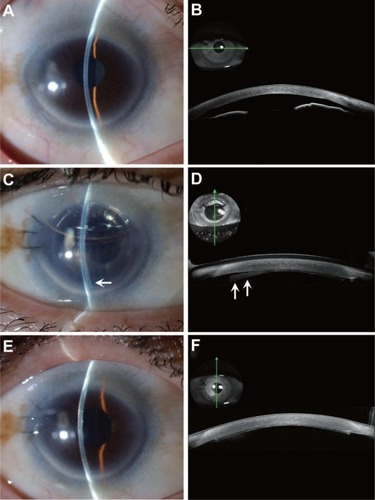
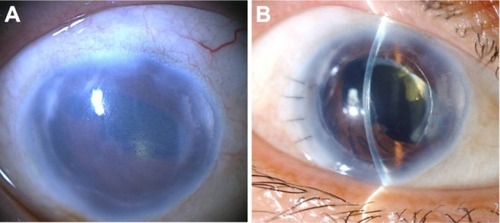
Figure 3 Slit-lamp photographs in case 1 with microcornea and iridocorneal endothelial syndrome. (A) Preoperatively, severe corneal edema, pupil deformity, and near 360° peripheral anterior synechia were noted. (B) Postoperatively, the cornea became clear. Best-corrected visual acuity improved from 20/1000 to 20/100.
Discussion
Microcornea is defined as a cornea that has a horizontal diameter ≤10 mm in an otherwise normal-sized globe and is distinct from nanophthalmus.Citation18 The condition may be unilateral or bilateral. Most cases are inherited in an autosomal dominant or recessive fashion. Bullous keratopathy of microcornea may be a rare condition, but once it occurs, it is a technical challenge for corneal surgeons.
Previously, Kobayashi et al reported six case series of bullous keratopathies with small cornea (9.0 mm or less in diameter) successfully treated with DSAEK using a special donor inserter (Neusidl Corneal Inserter®, Fischer Surgical Inc, Imperial, MO, USA).Citation19 The donor diameters used in their series were 7.5 mm for three cases and 8.0 mm for the remaining three cases. Generally speaking, the standard graft diameter is 8.0–9.0 mm for a normal cornea.Citation20,Citation21 In previous reports, a 7.0 mm diameter donor is a minimum size.Citation20 On the other hand, Unterlauft et al reported a case of a buphthalmic eye treated with DSAEK using a maximum size (10.0 mm) donor.Citation22
Subsequently, Toshida et al reported two cases of bullous keratopathies with microcornea (8.8 mm and 9.3 mm in diameter) successfully treated with DSAEK.Citation17 In this series, the donor diameters used were 7.5 mm in both cases. Herein, we report a successful DSAEK/nDSAEK case series using 6.0 mm donors for microcornea with a mean corneal diameter of 8.3 mm, which indicates that a 6.0 mm graft was able to clear the host cornea. The advantage of using the 6.00 mm graft instead of the 8.0–9.0 mm graft is that it required less manipulation of the donor, such as pushing and pulling with the needle, which contacts the donor endothelial side, in a small anterior chamber of microcornea. Manipulation and centering of the standard size graft for a microcornea is quite difficult, and too much manipulation may result in a reduction of the endothelial cell count. We were unable to find any previous reports about DSAEK/nDSAEK for small corneas using ≤6.0 mm donor grafts in a PubMed database search performed in May, 2013.
In this study, we experienced partial donor detachment in one case. In this case, the keratometry was quite flat (29.0 to 33.0 D). Usually, the microcornea is considered to be flatter than the normal cornea.Citation18 This could be one reason for the postoperative partial donor detachment in this particular case, although there are several factors that affect graft adhesion.
Theoretically, small donor grafts may not be able to provide sufficient endothelial cells compared with a larger graft; a 6.0 mm graft has approximately 56.3% of the endothelial surface area of an 8.0 mm graft. Ang et al advocated use of larger donor grafts because they showed that primary graft failure was less when using a >8.0 mm donor.Citation23 Meanwhile, in a 2-year follow-up study by Terry et al, no difference was found in terms of endothelial cell density between the 8 mm and 8.5 mm grafts in Fuchs dystrophy eyes.Citation20 A 5-year follow-up study by Anshu et al also found no significant difference in endothelial cell density when comparing 8.5 mm grafts with 9 mm grafts in Fuchs dystrophy.Citation21 In penetrating keratoplasty, a graft diameter <7.0 mm and >9.0 mm are known to be associated with the greatest risk of graft failure.Citation24,Citation25 In small penetrating keratoplasty grafts, the lower endothelial cell reservoir may result in reduced endothelial cell density over time, whereas larger grafts are exposed to the recipient’s immune system, which also may be deleterious for endothelial cell density.
Previously, we have reported outcomes of DSAEK/nDSAEK using several different types of donor insertion device, eg, the Busin glide with sheet-glide, Tan EndoGlide (Network Medical Products, North Wales, PA, USA), Neusidl Corneal Inserter, or intraocular lens cartridge, and mean cell loss rate after 6 months was 22.0%–22.9%, and 24.6%–29.0% after 12 months (n = 5–19).Citation26,Citation27 Price et al analyzed a 5-year follow-up of their initial series of DSAEK with the forceps donor insertion technique. They reported endothelial loss was 37% after one year and 53% after 5 years, and the 5-year graft survival rates for DSAEK were similar to those reported for penetrating keratoplasty.Citation28 Busin et al reported that the endothelial cell loss rate after DSAEK using the Busin glide was 20% at 6 months and 24% at 12 months (n = 10).Citation29 Khor et al also reported excellent results of DSAEK with EndoGlide for Asian eyes. The cell loss rate after 6 months was 13.1% (n = 20) and 15.6% after 12 months (n = 11).Citation30 In the current small case series, over a short period, the two eyes excluding the eye with graft rejection demonstrated probably more endothelial cell loss from 4.1% and 13.6% in 6 months to 22.6% and 29.5% in 12 months, comparable with previous other reports which showed a slight cell decrease over the same interval. Citation26,Citation27,Citation29,Citation30
In a large case series, the cumulative probability of a rejection episode at one year post-DSAEK was 6.0%–8.0%.Citation31,Citation32 In our study, allograft rejection was noted in case 2 (33.3%), which resulted in significant endothelial cell loss of 57.3% after 12 months. Considering the small number of patients in this study (n = 3), it is difficult to draw conclusions about the relationship between microcornea and predisposition of allograft rejection.
In conclusion, DSAEK/nDSAEK using a 6.0 mm diameter donor was useful in cases with microcornea; however, the number of en rolled patients in this study is limited. Additional stud ies using a large number of patients are required to evaluate fully the potential advantages and drawbacks of small diameter donor grafts for microcornea.
Acknowledgment
This study was supported by a Grant-in-Aid for Scientific Research© (KAKENHI), Japan (22591934, 23890072). The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure
No authors have any financial/conflicting interests to disclose in this work.
References
- MellesGREgginkFALanderFA surgical technique for posterior lamellar keratoplastyCornea1998176186269820943
- GorovoyMSDescemet’s stripping automated endothelial keratoplastyCornea20062588688917102661
- PriceFWJrPriceMODescemet’s stripping with endothelial keratoplasty in 200 eyes: early challenges and techniques to enhance donor adherenceJ Cataract Refract Surg20063241141816631048
- TerryMAChenESShamieNHoarKLFriendDJEndothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective seriesOphthalmology200811548849618164063
- PriceMOPriceFWJrDescemet’s stripping with endothelial keratoplasty comparative outcomes with microkeratome-dissected and manually dissected donor tissueOphthalmology20061131936194216935344
- PriceMOGorovoyMBenetzBADescemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor StudyOphthalmology201011743844420031230
- BaharIKaisermanILevingerERetrospective contralateral study comparing Descemet’s stripping and automated endothelial keratoplasty with penetrating keratoplastyCornea20092848548819421053
- KobayashiAYokogawaHSugiyamaKNon-Descemet stripping automated endothelial keratoplasty for endothelial dysfunction secondary to argon laser iridotomyAm J Ophthalmol200814654354918614137
- KobayashiAYokogawaHSugiyamaKIn vivo laser confocal microscopy after non-Descemet’s stripping automated endothelial keratoplastyOphthalmology20091161306131319576498
- ChaurasiaSRamappaMMurthySIGargPSangwanVSEndothelial keratoplasty without stripping the Descemet’s membraneBr J Ophthalmol2011951473147421602477
- KobayashiAYokogawaHSugiyamaKDescemet stripping with automated endothelial keratoplasty for bullous keratopathies secondary to argon laser iridotomy – preliminary results and usefulness of double-glide donor insertion techniqueCornea200827Suppl 1S62S6918813077
- YokogawaHKobayashiASaitoYYamazakiNMasakiTSugiyamaKRationale for performing penetrating keratoplasty rather than DSAEK in patients with bullous keratopathy in JapanOphthalmic Surg Lasers Imaging20124344645122869384
- PriceMOPriceFWJrTrespalaciosREndothelial keratoplasty technique for aniridic aphakic eyesJ Cataract Refract Surg20073337637917321384
- EsquenaziSSchechterBAEsquenaziKEndothelial survival after Descemet-stripping automated endothelial keratoplasty in eyes with retained anterior chamber intraocular lenses: two-year follow-upJ Cataract Refract Surg20113771471921420597
- PriceMOPriceFWJrDescemet stripping with endothelial keratoplasty for treatment of iridocorneal endothelial syndromeCornea20072649349717457204
- RamappaMAsharJVaddavalliPKChaurasiaSMurthySIEndothelial keratoplasty in children: surgical challenges and early outcomesBr J Ophthalmol2012961149115122539750
- ToshidaHOhtaTMurakamiAKobayashiASugiyamaKDescemet stripping automated endothelial keratoplasty for microcorneaJpn J Ophthalmol20125643644022772816
- GuptaPKKimTDevelopmental corneal anomalies of size and shapeKrachmerJHMannisMJHollandEJCorneaPhiladelphia, PAElsevier Mosby2011
- KobayashiAYokogawaHSugiyamaKClinical results of the Neusidl Corneal Inserter(®), a new donor inserter for Descemet’s stripping automated endothelial keratoplasty, for small Asian eyesOphthalmic Surg Lasers Imaging20124331131822589337
- TerryMALiJGosheJDavis-BoozerDEndothelial keratoplasty: the relationship between donor tissue size and donor endothelial survivalOphthalmology20111181944194921652077
- AnshuAPriceMOPriceFWJrDescemet stripping automated endothelial keratoplasty for Fuchs endothelial dystrophy-influence of graft diameter on endothelial cell lossCornea2013325822710494
- UnterlauftJDWellerKGeerlingGA 10.0-mm posterior lamellar graft for bullous keratopathy in a buphthalmic eyeCornea2010291195119820595891
- AngMHtoonHMCajucom-UyHYTanDMehtaJSDonor and surgical risk factors for primary graft failure following Descemet’s stripping automated endothelial keratoplasty in Asian eyesClin Ophthalmol201151503150822069353
- TanDTJanardhananPZhouHPenetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant StudyOphthalmology200811597598218061267
- WilliamsKALoweMBartlettCKellyTLCosterDJAll ContributorsRisk factors for human corneal graft failure within the Australian corneal graft registryTransplantation2008861720172419104411
- MasakiTKobayashiAYokogawaHSaitoYSugiyamaKClinical evaluation of non-Descemet stripping automated endothelial keratoplasty (nDSAEK)Jpn J Ophthalmol20125620320722350384
- YokogawaHKobayashiASugiyamaKClinical evaluation of a new donor graft inserter for Descemet’s stripping automated endothelial keratoplastyOphthalmic Surg Lasers Imaging201243505622251845
- PriceMOFairchildKMPriceDAPriceFWJrDescemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell lossOphthalmology201011872572921035862
- BusinMBhattPRScorciaVA modified technique for Descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell lossArch Ophthalmol20081261133113718695109
- KhorWBMehtaJSTanDTDescemet stripping automated endothelial keratoplasty with a graft insertion device: surgical technique and early clinical resultsAm J Ophthalmol201115122323221168813
- WuEIRitterbandDCYuGShieldsRASeedorJAGraft rejection following Descemet stripping automated endothelial keratoplasty: features, risk factors, and outcomesAm J Ophthalmol201215394995722265142
- AnshuAPriceMOPriceFWJrRisk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplastyOphthalmology201211953654022218143