Abstract
Objective
To compare the 3-year incidence of de novo ocular hypertension (OHT) after Descemet stripping automated endothelial keratoplasty (DSAEK) and penetrating keratoplasty (PK). For DSAEK, to evaluate predictors for OHT and 2-year outcomes after OHT development.
Methods
This was a review of the prospective Singapore Corneal Transplant Study at a single tertiary referral center. Consecutive DSAEKs and PKs for Fuchs’ endothelial dystrophy (FED) and pseudophakic bullous keratopathy (PBK) in eyes without pre-existing glaucoma were analyzed. OHT incidence after DSAEK and PK were compared using Kaplan–Meier survival analysis, and OHT risk factors identified using Cox proportional regression. OHT was defined: intraocular pressure (IOP) ≥ 24 mmHg or ≥ 10 mmHg from baseline. Secondary outcomes 2 years after OHT development in DSAEK were rates of glaucoma medical therapy failure, IOP success, graft failure and rejection, and best-spectacle corrected visual acuity (BSCVA).
Results
There were 108 (96.4%) DSAEKs and 216 (96%) PKs. The 1-, 2- and 3-year de novo OHT incidence was not significantly different between DSAEK (36.1%, 47.2%, 47.2%, respectively) and PK (35.7%, 44.9%, 45.8%, respectively; P = 0.914). OHT incidence did not differ in subgroup analyses of multiple clinical variables (P > 0.1). OHT predictors after DSAEK were: fellow eye glaucoma (hazard ratio [HR] 3.20, P = 0.004), age <60 years (HR 2.41, P = 0.016), concurrent goniosynechiolysis (HR 3.29, P = 0.021), post-graft complications or procedures (HR 2.85, P = 0.006). Two years after OHT onset, 29.7% of DSAEKs failed glaucoma medical therapy requiring trabeculectomy. Complete and qualified IOP success was achieved in 23.5% and 76.5%, respectively. Graft failure developed in 9.8% and graft rejection in 5.9%. At 6 months, 1, and 2 years from OHT onset, 86.3%, 88.3%, and 92.1% achieved BSCVA 20/40, respectively.
Conclusion
DSAEK and PK have comparable OHT risks. A significant 30% of DSAEK eyes with OHT require filtration surgery. Effective IOP control and good graft and visual outcomes are achieved with treatment.
Introduction
Descemet stripping automated endothelial keratoplasty (DSAEK) has emerged as the standard of care for corneal endothelial dysfunction. Compared to penetrating keratoplasty (PK), DSAEK has earlier and predictable visual recovery, less ocular surface complications, and superior tectonic safety.Citation1–Citation3
Initial expectations were that DSAEK would offer lower risks of post-graft ocular hypertension (OHT) than PK, due to absence of graft-host disparity or suture-induced angle distortion. Post-PK OHT is a significant complication as it leads to graft failure, endothelial cell loss, and poor visual outcomes.Citation4,Citation5 However, the incidence of post-DSAEK OHT is reported to occur between 35% and 43%.Citation6–Citation11
Several areas of uncertainty exist. First, although an earlier report found that 35% of DSAEK eyes with no preexisting glaucoma developed OHT at 1-year (ie, de novo OHT),Citation6 the comparative medium-term (ie, 3-year) OHT incidence after DSAEK and PK is unknown. A prospective randomized trial would be the most ideal comparative method, but this is ethically unjustifiable as DSAEK has clearly superior short-term visual outcomes. An acceptable method would be a single-center analysis, using standardized OHT diagnostic criteria, intraocular pressure (IOP), and corneal thickness measurement techniques, and steroid regimens. Second, predisposing factors for OHT after DSAEK are unknown. Third, studies have largely focused on IOP elevation in eyes with pre-existing glaucoma, and Fuchs’ endothelial dystrophy (FED);Citation6–Citation11 with limited data for de novo OHT, or pseudophakic bullous keratopathy (PBK). Finally, a comprehensive evaluation of intermediate-term IOP and graft outcomes after DSAEK are unknown.
The aims of this study were to: (1) compare the incidence of de novo post-graft OHT between DSAEK and PK within 3 years, (2) evaluate risk factors for OHT after DSAEK, and (3) determine 1-year outcomes (failure of medical therapy, IOP success, visual acuity, and graft survival) after OHT development.
Patients and methods
Inclusion and exclusion criteria
A retrospective review was performed of DSAEK and PK grafts from the Singapore Corneal Transplant Study (SCTS). The SCTS is an ongoing long-term prospective study of corneal grafts at the Singapore National Eye Centre, a tertiary referral center for corneal diseases for >90% of transplants in Singapore.Citation12 This study received Singhealth Institutional Board approval.
We included all cases expected to have 3 years follow-up at review in January 2013; ie, cases performed up to January 2010. Therefore, of 250 consecutive DSAEKs performed from January 2, 2006 to January 3, 2010, we selected eyes with FED and PBK. We also reviewed consecutive PK grafts from March 5, 2005 to January 7, 2010, and identified eyes with FED or PBK. We specifically included recent PK cases to minimize temporal bias, however the slightly earlier eligibility period for PKs was due to DSAEKs being increasingly performed for endothelial dysfunction. We excluded eyes with pre-existing glaucoma or other surgical indications ie, regrafts, laser-induced bullous keratopathy, or iridocorneal endothelial syndrome. The first eye was included in patients with bilateral grafts.
Eyes with no pre-existing glaucoma were defined based on the European Glaucoma Society Guidelines and by Vajaranant et al:Citation6,Citation13 (1) no IOP of >21 mmHg, and no topical or systemic IOP-lowering medication use, (2) no glaucomatous optic neuropathy (GON; cup-disc ratio >0.6 in the presence of a glaucomatous visual field defect, focal neuroretinal rim defects), (3) no previous laser peripheral iridotomy, iridoplasty, cyclodestructive, or filtration procedures, and (4) no documented history of glaucoma.
Preoperative evaluation
All patients underwent a comprehensive ophthalmic examination including best-spectacle corrected (BSCVA) Snellen visual acuity testing, gonioscopy, anterior and dilated posterior segment evaluation including stereoscopic optic disc assessment with a 78-D lens (Volk Optical Inc, Mentor, OH, USA), and IOP measurement while undilated with Goldmann appalanation tonometry (GAT, Haag-Streit, Konig, Switzerland), or Tonopen-XL (Reichert Inc., Depew, NY, USA) in eyes with significant stromal scarring precluding GAT measurements. Central corneal thickness (CCT) was measured with an ultrasound pachymeter (Sonogage, Cleveland, OH, USA). Automated perimetry at baseline was documented (Humphrey visual field analyzer II, SITA 24-2 fast, Carl Zeiss Meditec, Dublin, CA, USA).
Operative technique
Five corneal surgeons (DTT, JSM, and three other surgeons) performed DSAEK according to a previously described standardized technique.Citation14,Citation15 A donor lamellar dissection was achieved with a Moria automated lamellar therapeutic keratoplasty (ALTK) microkeratome (Moria USA, Doylestown, PA, USA), and donor trephination performed using a standard Hanna punch system with trephine sizes between 7.75 mm and 9 mm in 0.25 mm increments. A 5 mm scleral tunnel was created. Descemet’s stripping was performed under airCitation14 and the graft inserted using the Sheets-glide technique. A surgical iridectomy was performed in all cases. Interface fluid was drained through paracentral venting incisions. An air bubble was injected into the anterior chamber to oppose the graft to the host cornea, but partially evacuated to a size approximating the dimensions of the graft before the end of surgery.
PK grafts were performed by nine corneal surgeons, including the aforementioned five surgeons. The Hanna punch trephine was used to cut 0.25 to 0.50 mm oversized donor buttons. A Hanna suction trephine centered on the geometric center of the recipient cornea was used to cut a circular, partial depth incision in the recipient, and the remaining cornea excised. The donor was sutured with either an 8-bite 10-0 nylon double continuous running suture or a combination of single 8-bite 10-0 nylon continuous and eight interrupted sutures.
For DSAEK, concurrent surgeries (eg, cataract extraction, goniosynechiolysis, anterior chamber intraocular lens [ACIOL] exchange, vitrectomy) were performed before donor insertion. ACIOLs were exchanged or scleral-fixated IOLs were implanted. For PK, concurrent surgeries were performed before excision of the recipient cornea.
Postoperative evaluation and management
Follow-up examinations were performed on the 1st and 5th postoperative day, 2nd postoperative week, and 1-, 2-, 3-, 6-, 9-, and 12-, 18-, 24-, and 36-month intervals. Further visits were scheduled where clinically indicated. A postoperative clinical evaluation was performed as for the preoperative visit, including BSCVA, anterior and posterior segment evaluation, undilated IOP measurement with GAT in all cases other than those with high astigmatism or corneal edema, where Tonopen was used, and CCT measurement. All DSAEK and PK grafts received daily 3-hourly topical prednisolone acetate 1% (Pred Forte®, Allergan, Irvine, CA, USA) for the first 7 days, tapering to 4, 3, 2, and 1-times a day by 1, 3, 6, and 12 months, respectively.
DSAEK cases with OHT were managed by two glaucoma fellowship-trained specialists (TTW and CLH). Topical IOP-lowering medications were instituted as the initial treatment for all cases. For OHT cases judged to be at low risk for graft rejection, prednisolone acetate 1% was substituted with fluorometholone 0.25% (FML, Allergan) or loteprednol etabonate 0.5% (Lotemax, Bausch and Lomb, Rochester, NY, USA) at the same frequency. Visual field testing was conducted at 6-monthly intervals from OHT onset.
Definition of post-graft ocular hypertension
Post-graft OHT was defined as IOP ≥ 24 mmHg or ≥ 10 mmHg increase at any postoperative examination, consistent with an earlier study.Citation6
Glaucoma medical therapy failure, IOP success, and graft failure
Outcomes of OHT in DSAEK eyes were assessed at 2 years after OHT onset. The primary outcomes were failure of medical therapy and IOP. A mitomycin-C-augmented trabeculectomy was indicated when IOP was >21 mmHg on two consecutive visits, despite maximum medical therapy. Complete IOP success was defined as IOP < 22 mmHg, and qualified IOP success as IOP < 22 mmHg on medications.
Secondary outcomes were graft failure and rejection, and BSCVA. Graft failure was defined as irreversible corneal edema with increased corneal thickness ≥2 months; graft wrinkles causing reduced VA were not considered graft failures as endothelial reserve was considered healthy. Graft rejection was defined by an epithelial rejection line, subepithelial infiltrates, stromal rejection with Krachmer’s spots, anterior chamber cells, keratic precipitates, or an endothelial rejection line, with or without concomitant corneal edema.
Statistical analysis
Quantitative variables were compared with two-sample Student t-tests, and categorical variables using chi-square tests. The proportions of DSAEK and PK grafts developing OHT through 3 years were compared using Kaplan–Meier survival analysis and the log-rank test. To address potential systematic errors of different IOP measurement techniques and corneal edema, the survival analysis was repeated only in eyes with pre- or post-graft GAT measurements and clear post-graft corneas. As OHT risk could depend on various clinical characteristics (), Cox proportional regression was used to systematically assess individual associations between OHT and the interaction term of each factor with graft type (DSAEK/PK).
Table 1 Demographic, pre-, intra-, and postoperative characteristics of Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty eyes
Cox regression was also performed to analyze ten potential post-DSAEK OHT risk factors (). Statistically significant variables in the univariate analysis were included in multivariate models. The fit of the multivariate model was evaluated using the R2 statistic, indicating the proportion of variance in 3-year OHT incidence explained by the statistically significant factors. All tests were two-tailed, and P < 0.05 was considered statistically significant. Statistical analyses were performed with STATA 12.1 (StataCorp LP, College Station, TX, USA).
Table 2 Ocular hypertension following Descemet stripping automated endothelial keratoplasty: unadjusted Cox proportional hazard risk by demographics, diagnosis, and clinical characteristics
Results
Characteristics
There were 112 DSAEKs and 225 PKs. After excluding 4 DSAEKs and 9 PKs with <3 years of follow-up, 108 DSAEKs and 216 PKs were analyzed. DSAEK and PK cohorts were comparable for age (66.2 ± 12.1 versus 67.1 ± 12.3 years), gender (male, 47.2% versus 46.3%), surgical indication (FED, 47.2% versus 47.2%), phakic status (pseudophakic, 79.6% versus 70.8%), postoperative procedures and complications (89.8% versus 84.7%), pre- (687.7 ± 92.1 versus 667.8 ± 140.3 μm) and post-graft CCT (674 ± 125.3 versus 658 ± 102.0 μm; P > 0.05 for all; ). However, the DSAEK group had less Chinese (67.6% versus 79.2%; P = 0.023), lower pre-graft IOP (12.9 versus 14.2 mmHg; P = 0.002), and more stand-alone grafts (73.2% versus 49.5%, P < 0.001). Mean DSAEK graft diameter was 8.67 ± 0.44 mm; donor and recipient PK diameters were 7.88 ± 0.35 and 7.54 ± 0.87 mm, respectively. Details of concurrent procedures and postoperative procedures and complications are appended in .
Incidence of post-graft OHT
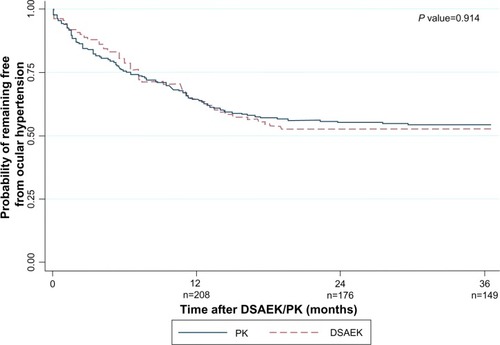
There were no significant differences in the cumulative probabilities of post-DSAEK and post-PK OHT at 1-year (36.1% versus 35.7%), 2 years (47.2% versus 44.9%), and 3 years (47.2% versus 45.8%; log-rank P = 0.914; ). The mean survival time to OHT onset was 7.7 ± 5.4 months after DSAEK and 7.3 ± 6.4 months after PK. Analyzing 93 DSAEK and 174 PK eyes with only pre- and post-graft IOP measurements by GAT and clear grafts showed comparable OHT incidence at all time points (P = 0.214). Ethnicity, pre-graft IOP, and concurrent procedures differed between DSAEK and PK groups, but their interactions with graft type were not associated with OHT in Cox regression (P = 0.865, P = 0.162, and P = 0.607, respectively). In similar regression models, OHT risk was not associated with all interaction terms of graft type and each variable in (P > 0.05).
DSAEK OHT risk factors
In univariate analyses, post-DSAEK OHT was associated with glaucoma in the fellow eye (P = 0.011), DSAEK grafts with goniosynechiolysis (with or without ACIOL exchange and anterior vitrectomy; P = 0.042), and postoperative procedures and complications (P = 0.011), and age <60 years (P = 0.03; ). Other factors, including surgical indication, were not associated with OHT risk (P > 0.05). These four factors remained significant in the multivariate analysis ( and –): glaucoma in the fellow eye (hazard ratio [HR] 3.20; 95% confidence interval [CI], 1.46,7.05; P = 0.004), post-operative procedures and complications (HR 2.85; 95% CI, 1.35,6.04; P = 0.006), goniosynechiolysis (HR 3.29; 95% CI; 1.20,9.01; P = 0.021), and age <60 years (HR 2.41; 95% CI, 1.17,4.93; P = 0.016). The cumulative OHT risk at each interval is indicated in –. These four factors explained 23% of variability in OHT risk in the multivariate model.
Figure 2 Kaplan–Meier survival curve showing probability of remaining ocular hypertension-free following Descemet stripping automated endothelial keratoplasty: (A) by fellow eye glaucoma status; (B) by concurrent procedures; (C) by postoperative complications and procedures; and (D) by age.
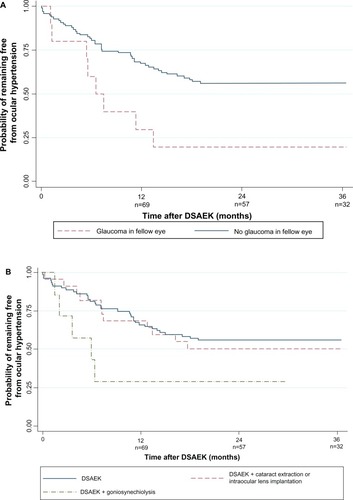
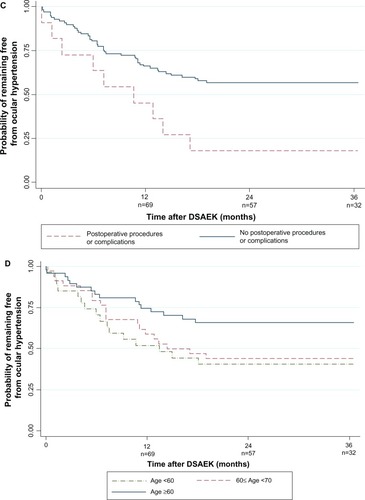
Table 3 Ocular hypertension following Descemet stripping automated endothelial keratoplasty: multivariate-adjusted Cox proportional hazard risk for recipient age, fellow eye glaucoma status, concurrent procedures, and postoperative procedures or complications
IOP and graft outcomes 2 years after OHT in DSAEK
Data on 2-year outcomes after OHT development were available for all 51 DSAEK eyes with OHT (). Switch to low-potency steroids was made in 7 DSAEK eyes (5.6%). Failure of medical therapy requiring trabeculectomy was observed in 15 eyes (29.4%). Complete IOP success at 2 years was achieved in 12 eyes (23.5%; all requiring trabeculectomy), and qualified IOP success in 39 eyes (76.5%; 3 requiring trabeculectomy). The mean IOP at 2 years was 17.8 mmHg. Proportion achieving BSCVA of 20/40 or better was 86.3% at 6 months, 88.3% at 1-year, and 92.1% at 2 years. Endothelial failure occurred in 9.8%, and graft rejection in 5.9%.
Table 4 Two year outcomes after onset of de novo post-Descemet stripping automated endothelial keratoplasty ocular hypertension: glaucoma filtration surgery requirement, IOP success, graft failure, and BSCVA
Three post-hoc multivariate analyses were conducted to determine the impact of OHT on risk of trabeculectomy requirement, graft failure and rejection at 3 years for 108 DSAEK eyes (Supplementary Table S1). Relevant variables were included in the respective models as indicated. OHT was not associated with BSCVA, graft failure, or graft rejection outcomes (P = 0.951, P = 0.489, P = 0.300, respectively).
Discussion
To date, comparing glaucoma or OHT rates between DSAEK and PK has been difficult because of heterogeneity in case definitions, follow-up duration, steroid regime, and inclusion criteria. This is the first study comparing intermediate-term incidence of post-DSAEK and post-PK OHT. At 3 years, 47.2% of DSAEK grafts developed OHT, which was not statistically different from 46.3% of PK grafts managed on the same steroid regimen. Systematic analysis of the impact of various clinical characteristics (eg, differences in corneal thickness) did not reveal differences in OHT rates between the two graft cohorts. This study is also the first to show that OHT development plateaus out in the second year, with none to minimal cases in the third year. Several clinical factors were linked with OHT after DSAEK, which could assist in risk prognostication. Although 29.7% failed glaucoma medical therapy, 100% achieved either qualified or complete IOP success with glaucoma medications and/or surgery. OHT did not compromise graft outcomes, as 92.1% achieved BSCVA ≥ 20/40, graft survival was 90.2%, and 94.1% remained rejection-free. Collectively, these findings still support DSAEK as the preferred surgical strategy for endothelial decompensation, taking into consideration the need for close IOP monitoring and treatment.
OHT is a significant DSAEK complication. Wandling et al reported comparable rates of “glaucoma therapy escalation” using a different definition in a smaller cohort (34 DSAEKs, 20 PKs, 41.2% versus 35%), but did not adjust for differences in multiple clinical variables, including surgical indication, pre-existing glaucoma status, pachymetry, and follow-up duration.Citation16 Our findings add to evidence of DSAEK/PK comparability in key graft outcomes, ie, graft failure,Citation17–Citation19 3- and 5-year endothelial cell loss,Citation17,Citation18 although DSAEK is clearly superior for visual and tectonic outcomes. Indeed, OHT is the most frequent DSAEK complication, higher than graft dislocation (2% to 31%),Citation20–Citation22 rejection (6% to 7.6% at 1-year, and 12% to 14% at 2 years),Citation23,Citation24 and endothelial failure (24% for PBK and 5% for FED at 5 years).Citation18 In our study, the 1-year OHT incidence was 36.2%, comparable with 35% reported by Vajaranant et al using the same definition of preexisting glaucoma and OHT.Citation6 Allen et al reported a 51% OHT incidence, but included cases with and without pre-existing glaucoma.Citation7 An earlier report from the American Academy of Ophthalmology (AAO) summarizing the DSAEK literature through 2009, concluded that “glaucoma” following DSAEK varied between 0% to 15% from 23 studies with 3 to 18 months of follow-up.Citation25 The “glaucoma” definition was solely elevated intraocular pressure, ie, OHT. The lower OHT rates were likely due to shorter follow-up.
Understanding of post-DSAEK OHT has evolved over the last 5 years. Initial studies attributed IOP elevations to pupil-block by air migration behind the iris;Citation3,Citation25 however this has been eliminated with routine iridectomies. Vajaranant et al proposed steroid response as a causative factor, based on 30% having IOP elevation >10 mmHg above baseline, and IOP peaking at 3 months.Citation6 The current study provides further evidence, based on leveling off in OHT incidence in the second year (consistent with once-daily steroid dosing by the first post-DSAEK year), and increased risk in younger recipients. The clinical implications are twofold. First, the dose and potency of steroids that allows appropriate trade-offs between OHT risk and rejection needs to be considered by ophthalmologists. Second, Descemet membrane endothelial keratoplasty (DMEK) may be increasingly important, because its lower risk of graft rejection may allow less reliance on post-graft steroids,Citation26 possibly conferring a significantly lower risk of OHT.
Although steroid response is the main cause for OHT, four additional factors predict OHT risk, furthering our understanding of OHT after DSAEK. Peripheral anterior synechia, PBK, tight and long sutures, relatively undersized donor grafts, smaller recipient corneas, concurrent surgeries, and pre-existing glaucoma, are well-recognized risk factors for post-PK OHT.Citation4,Citation27,Citation28 One study found that pre-existing glaucoma predicts IOP elevation after DSAEK,Citation7 however other risk factors are unknown. In our study, OHT risk was increased 3.3, 2.9, 2.4, and 3.2 times in eyes with goniosynechiolysis, postoperative procedures and complications, age <60 years, and fellow eye glaucoma, respectively. Requirement for goniosynechiolysis may reflect more severe angle closure and compromised trabecular outflow with a propensity for OHT development. Postoperative procedures and complications (eg, ocular infections, retinal detachment, yttrium aluminum garnet [YAG] capsulotomies) expectedly result in increased intraocular inflammation, which could reduce trabecular function over time, as has been observed for PK.Citation27–Citation29 The tendency towards steroid-responsiveness in younger adults is consistent with the well-recognized risk of IOP elevation in this age group with topical or intravitreal steroids.Citation30–Citation32 It is not clear how glaucoma in the fellow eye influences OHT risk, but this may be a surrogate for trabecular function in the grafted eye. OHT risk after DSAEK was not influenced by surgical indication, unlike in PK.Citation27,Citation28 These four factors contributed a modest 23% to variability in OHT risk, with 77% possibly accounted for by differential steroid response susceptibility. Thus, IOP monitoring could be indicated for all eyes at 1, 3, and 6 months, and 6-monthly thereafter within the first three post-graft years in average-risk eyes, in line with recommendations for corticosteroid therapy.Citation33 For eyes with high-risk characteristics, 3-monthly follow-up up to 3 years may be required.
Over 2 years from OHT onset, almost 30% of DSAEK eyes failed medical therapy and required trabeculectomy. In contrast, only 8.6% required glaucoma surgery in an earlier study.Citation6 This difference was likely due to continuation of high-potency steroids in 94.2% (compared to 62.8%) instead of switching to lower-potency agents. This practice was based on evidence that graft rejection is a major cause for graft failure in eyes at our center,Citation12 and steroid reduction results in endothelial rejection.Citation19,Citation23,Citation24 The high rate of glaucoma surgical escalation in this study approaches figures reported for intravitreal steroid implants (ie, >35%).Citation34 Nevertheless, glaucoma therapy was effective, such that all eyes achieved qualified or complete IOP success <21 mmHg at 2 years. These findings are consistent with an earlier report on the efficacy of filtration surgery in DSAEK in eyes with or without glaucoma, which could have a comparative advantage for DSAEK versus PK eyes.Citation35
DSAEK eyes with OHT had comparable BSCVA, graft failure, and graft rejection as other published reports. Post-hoc analyses showed that OHT was not associated with these outcomes. In our study, 92.1% of DSAEK eyes with OHT achieved BSCVA of 20/40 or better. Using similar DSAEK techniques, BSCVA ranged between 20/34 to 20/66 at about 9 months (range 3–21 months);Citation25 other authors reported BSCVA 20/40 or better in 92% at 6 monthsCitation36 and 98.1% at 3 years.Citation37 OHT rarely leads to compromised short-term visual outcomes, if IOP control has been achieved and central visual field damage has not developed. Graft rejection occurred in only 5.9% at 2 years post-OHT, which is within the range of reported rejection rates, if not more favorable, than in other recent cohorts (11%–14% at 2 years).Citation23,Citation24 No studies have concluded that OHT or steroid response independently lead to DSAEK rejection, but steroid taper regardless of indication, is the major cause for rejection.Citation23,Citation24 Likewise, endothelial failure rate of 9.8% in this study is compatible with 4% to 14% 3-yearCitation17 and 7% to 27% 5-yearCitation18 rates for FED and PBK. Interestingly, Anshu et al reported that glaucoma surgery, in particular drainage implants, increases the 5-year risk for graft failure, however this was not observed in our study. This could be due to shorter follow-up, and that all eyes received trabeculectomy instead of drainage implants, resulting in less endothelial attrition.Citation38
Strengths include the prospective data in SCTS, >96% who completed 3-year follow-up, and standardization of steroid regime and indication for glaucoma surgery. The conclusions on comparable OHT risk are valid as major clinical variables (importantly pre- and post-graft pachymetry) were comparable, and possible interactions between all variables with graft type were assessed and found to be absent. The analysis was repeated in eyes with clear grafts and GAT-measured IOPs to ensure uniformity of IOP measurements. Nevertheless, IOP differences in DSAEK and PK corneas based on different instruments or corneal thickness are generally not clinically significant.Citation39–Citation42
A limitation is the non-randomized design; however randomization would be unethical. The slightly earlier inclusion period for PK could have introduced temporal bias, although this is inherent in any DSAEK/PK comparative study,Citation17,Citation18 and grafts from largely comparable time periods were included. As graft failures were censored in the survival analysis, OHT development could not be observed after graft failure. However, such cases comprised the minority; >90% DSAEK and PK eyes completed 3-year follow-up without censure.
In summary, this study of 324 DSAEK and PK procedures found no statistical difference in 3-year post-graft OHT incidence. Although a significant 30% of DSAEK eyes with OHT required filtration surgery, acceptable IOP and graft outcomes were achieved in these eyes. Age <60 years, fellow eye glaucoma, concurrent goniosynechiolysis, and postoperative complications and procedures prognosticate higher OHT risk and may be important considerations in post-graft management. Longer-term prospective studies are warranted to confirm these findings.
Supplementary table
Table S1 Multivariate analysis of OHT as a risk factor for BSCVA, graft failure, and graft rejection outcomes in 108 DSAEK eyes at 3 years post-graft
Disclosure
The authors report no conflicts of interest in this work.
References
- PriceMOPriceWPDescemet’s stripping endothelial keratoplastyCurr Opin Ophthalmol200718429029417568204
- BaharIKaisermanIMcAllumPSlomovicARootmanDComparison of posterior lamellar keratoplasty techniques to penetrating keratoplastyOphthalmology200811591525153318440638
- KoenigSBCovertDJEarly results of small-incision Descemet’s stripping and automated endothelial keratoplastyOphthalmology2007114222122617156845
- BrandtJDLimMCO’DayDGGlaucoma After Penetrating KeratoplastyKrachmerJHMannisMJHollandEJCornea2nd ed2PhiladelphiaElsevier Mosby200515751579
- PriceMOThompsonRWPriceFWRisk factors for various causes of failure in initial corneal graftsArch Ophthalmol200312181087109212912684
- VajaranantTSPriceMOPriceFWGaoWWilenskyJTEdwardDPVisual acuity and intraocular pressure after Descemet’s stripping endothelial keratoplasty in eyes with and without preexisting glaucomaOphthalmology200911691644165019643499
- AllenMBLieuPMoothaVVRisk factors for intraocular pressure elevation after descemet stripping automated endothelial keratoplastyEye Contact Lens201036422322720539235
- WiauxCBaghdasaryanELeeOLOutcomes after Descemet stripping endothelial keratoplasty in glaucoma patients with previous trabeculectomy and tube shunt implantationCornea201130121304131121963858
- PhillipsPMTerryMAShamieNDescemet stripping automated endothelial keratoplasty in eyes with previous trabeculectomy and tube shunt procedures: intraoperative and early postoperative complicationsCornea201029553454020299975
- KimPAmiranMDLichtingerAYeungSNSlomovicARRootmanDSOutcomes of Descemet stripping automated endothelial keratoplasty in patients with previous glaucoma drainage device insertionCornea201231217217522146552
- QuekDTWongTTanDMehtaJSCorneal graft survival and intraocular pressure control after descemet stripping automated endothelial keratoplasty in eyes with pre-existing glaucomaAm J Ophthalmol201115214854e221570672
- TanDTJanardhananPZhouHPenetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant StudyOphthalmology20081156975982e118061267
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edCityDogma2008
- MehtaJSHanteraMMTanDTModified air-assisted descemetorhexis for Descemet-stripping automated endothelial keratoplastyJ Cataract Refract Surg200834688989118498990
- MehtaJSPorYMPohRBeuermanRWTanDComparison of donor insertion techniques for descemet stripping automated endothelial keratoplastyArch Ophthalmol2008126101383138818852416
- WandlingGRRauenMPGoinsKMGlaucoma therapy escalation in eyes with pseudophakic corneal edema after penetrating keratoplasty and Descemet’s stripping automated endothelial keratoplastyInt Ophthalmol201232191422246622
- AngMMehtaJSLimFBoseSHtoonHMTanDEndothelial cell loss and graft survival after Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplastyOphthalmology2012119112239224422885122
- PriceMOGorovoyMPriceFWBenetzBAMenegayHJLassJHDescemet’s stripping automated endothelial keratoplasty: three-year graft and endothelial cell survival compared with penetrating keratoplastyOphthalmology2013120224625123107581
- PriceMOFairchildKMPriceDAPriceFWDescemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell lossOphthalmology2011118472572921035862
- GosheJMTerryMALiJYStraikoMDDavis-BoozerDGraft dislocation and hypotony after Descemet’s stripping automated endothelial keratoplasty in patients with previous glaucoma surgeryOphthalmology201211961130113322385970
- JangiAARitterbandDCWuEIMehtaVVKoplinRSSeedorJADescemet stripping automated endothelial keratoplasty after failed penetrating keratoplastyCornea201231101148115322357384
- ClementsJLBouchardCSLeeWBRetrospective review of graft dislocation rate associated with descemet stripping automated endothelial keratoplasty after primary failed penetrating keratoplastyCornea201130441441821099405
- PriceMOJordanCSMooreGPriceFWGraft rejection episodes after Descemet stripping with endothelial keratoplasty: part two: the statistical analysis of probability and risk factorsBr J Ophthalmol200993339139519019938
- WuEIRitterbandDCYuGShieldsRASeedorJAGraft rejection following descemet stripping automated endothelial keratoplasty: features, risk factors, and outcomesAm J Ophthalmol20121535949957e122265142
- LeeWBJacobsDSMuschDCKaufmanSCReinhartWJShteinRMDescemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of OphthalmologyOphthalmology200911691818183019643492
- AnshuAPriceMOPriceFWRisk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplastyOphthalmology2012119353654022218143
- KaradagOKuguSErdoganGKandemirBEraslan OzdilSDoganOKIncidence of and risk factors for increased intraocular pressure after penetrating keratoplastyCornea201029327828220118781
- Al-MohaimeedMAl-ShahwanSAl-TorbakAWagonerMDEscalation of glaucoma therapy after penetrating keratoplastyOphthalmology2007114122281228618054642
- OruçogluFBlumenthalEZFrucht-PeryJSolomonARisk Factors and Incidence of Ocular Hypertension After Penetrating KeratoplastyJ Glaucoma2013
- ChangDFTanJJTripodisYRisk factors for steroid response among cataract patientsJ Cataract Refract Surg201137467568121420592
- JonasJBDegenringRFKreissigIAkkoyunIKamppeterBAIntraocular pressure elevation after intravitreal triamcinolone acetonide injectionOphthalmology2005112459359815808249
- ShuklaDVidhyaNPrasadNMMahalakshmiRKolluruCKrishnadasREvaluation of patient age as a risk factor for intraocular pressure elevation after intravitreal triamcinoloneAm J Ophthalmol2007144345345417765428
- KerseyJPBroadwayDCCorticosteroid-induced glaucoma: a review of the literatureEye (Lond)200620440741615877093
- BollingerKKimJLowderCYKaiserPKSmithSDIntraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitisOphthalmology2011118101927193121652079
- BoeyPYMehtaJSHoCLTanDTWongTTOutcomes of trabeculectomy after descemet stripping automated endothelial keratoplasty: a comparison with penetrating keratoplastyAm J Ophthalmol2012153610911098e222397954
- TerryMAShamieNChenESPhillipsPMHoarKLFriendDJPrecut tissue for Descemet’s stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survivalOphthalmology2009116224825619091414
- LiJYTerryMAGosheJDavis-BoozerDShamieNThree-year visual acuity outcomes after Descemet’s stripping automated endothelial keratoplastyOphthalmology201211961126112922364863
- AnshuAPriceMOPriceFWDescemet’s stripping endothelial keratoplasty: long-term graft survival and risk factors for failure in eyes with preexisting glaucomaOphthalmology2012119101982198722705345
- ChouCYJordanCAMcGheeCNPatelDVComparison of intraocular pressure measurement using 4 different instruments following penetrating keratoplastyAm J Ophthalmol2012153341241822000702
- ChangDTPantchevaMBNoeckerRJCorneal thickness and intraocular pressure in edematous corneas before and after Descemet stripping with automated endothelial keratoplastyCornea201029101125113020548237
- VajaranantTSPriceMOPriceFWWilenskyJTEdwardDPIntraocular pressure measurements following Descemet stripping endothelial keratoplastyAm J Ophthalmol2008145578078618329627
- MawatariYKobayashiAYokogawaHSugiyamaKIntraocular pressure after Descemet’s stripping and non-Descemet’s stripping automated endothelial keratoplastyJpn J Ophthalmol20115529810221400052