Abstract
Glaucoma is the second leading cause of blindness worldwide and intraocular pressure (IOP) is currently its only modifiable risk factor. Peak IOP has for a long time been considered as a major contributor to glaucoma progression, but its effects may depend not only on its magnitude, but also on its time course. The IOP is nowadays considered to be a dynamic parameter with a circadian rhythm and spontaneous changes. The current practice of punctual measuring the IOP during office hours is therefore a suboptimal approach, which does not take into account the natural fluctuation of IOP. Because of its static nature a single IOP measurement in sitting position fails to document the true range of an individual’s IOP, peak IOP, or variation throughout the day. Phasing means monitoring a patient’s IOP during the daytime or over a 24-hour period. This can provide additional information in the management of glaucoma patients. This review focuses on the current insight of non-invasive IOP monitoring as a method of obtaining more complete IOP profiles. Invasive techniques using an implantable sensor are beyond the scope of this review.
Introduction
Glaucoma is a chronic optic neuropathy characterized by the excavation of the optic disc due to the atrophy of the retinal ganglion cells. A progression of such atrophy leads to further reduction of the visual field and eventually to the loss of sight at the end-stage of the disease. Glaucoma is the second leading cause of blindness worldwide and intraocular pressure (IOP) is currently its only modifiable risk factor.Citation1 Peak IOP has for a long time been considered as a major contributor to glaucoma progression, but its effects may depend not only on its magnitude, but also on its time course.Citation2–Citation4 The IOP is nowadays considered to be a dynamic parameter with a circadian rhythm and spontaneous changes.Citation5,Citation6 The current practice of punctual measuring of the IOP during office hours is therefore a suboptimal approach, which does not take into account the natural fluctuation of IOP. Because of its static nature, a single IOP measurement in sitting position fails to document the true range of an individual’s IOP, peak IOP, or variation throughout the day.Citation7–Citation9 Phasing means monitoring a patient’s IOP during the daytime or over a 24-hour period. This can provide additional information in the management of glaucoma patients. This review focuses on the current insight of noninvasive IOP monitoring as a method of obtaining more complete IOP profiles. Invasive techniques using an implantable sensor are beyond the scope of this review.
Timing of diurnal fluctuation in IOP
Diurnal fluctuations in IOP of up to 5 mmHg have been reported in healthy subjects, and in glaucoma patients these variations are even higher.Citation5,Citation6 Initial studies suggested that the peak IOP occurred in the early morning, while other studies found a drop in IOP during the nocturnal period.Citation10–Citation12 However, if changes in body position that occur during sleep are taken into account by measuring the IOP in the physiologic positions (sitting while awake and supine while asleep), IOP is higher during the nocturnal/sleep period than during the diurnal period.Citation6,Citation13,Citation14 Other sleep-laboratory studies have confirmed that IOP in most glaucoma patients is at its highest value during the nocturnal/sleep period with the patient in the supine body position.Citation8,Citation15 Furthermore, like IOP, ocular perfusion pressure is believed to be a dynamic parameter with a circadian rhythm with dips at night or in the early morning.Citation16 The timing of fluctuations in both IOP and ocular perfusion pressure may compromise the optic nerve head perfusion in susceptible individuals, worsening glaucoma in both functional and structural tests.Citation4,Citation17 Effects of the circadian IOP peaks may therefore depend on their time course as well as their magnitude.Citation4 Animal-model research suggested that acceleration of the arterial blood pressure resulted in surges in IOP and this induced IOP variability may be more important in ocular hypertensive eyes.Citation18,Citation19
Diurnal IOP fluctuation and glaucoma progression
Variation in IOP has been increasingly considered to be important, with several studies suggesting that its fluctuations are an independent risk factor for glaucoma progression.Citation4,Citation17,Citation20–Citation27 Other studies, however, suggested that it is the absolute IOP itself rather than its fluctuation that had the most significant effect on glaucoma progression, but differences in study design, or failure in obtaining multiple IOP measurements on the same day, may explain these discrepancies.Citation4,Citation28,Citation29 Apart from circadian variation, IOP can fluctuate randomly long-term, but its significance on glaucoma progression is unclear.Citation23,Citation30 Confusion regarding the definition of such long-term IOP fluctuations may contribute to this discrepancy. If the IOP measurements are taken at different times of the day on different visits, these fluctuations are not clearly distinguished from circadian variations. IOP measurements fluctuating over years on the other hand might represent true disease progression or change in therapy.Citation31
Benefits of IOP monitoring
There are several benefits in documenting circadian IOP curves. Phasing allows detecting inherent IOP profiles that predispose to glaucoma development and progression, independently of absolute value of IOP. Patients with ocular hypertension who subsequently developed glaucoma had inherently different IOP profiles compared to non-converters.Citation32 Particularly in patients with progressive visual field loss that is disproportionate to measured IOP, monitoring of the IOP provides additional information.Citation7,Citation33 It has been shown that analyzing IOP curves allows a more accurate detection of the IOP fluctuations and peak values, which occur outside office hours in 52%–69% of patients.Citation7,Citation34,Citation35 Such 24-hour IOP monitoring led to immediate treatment change in over one-third of patients.Citation7 In 11%–20% of the suspected cases of normal-tension glaucoma, IOP monitoring can register IOP values above 21 mmHg during daytime or nighttime, and therefore it also allows identification of false-positive normal-tension glaucoma cases.Citation36 Furthermore, the effect of various antiglaucoma drops on IOP over a 24-hour period can be compared.Citation37–Citation39 Identification of the chronobiology of a patient’s IOP may help in selecting the appropriate IOP-lowering treatment with the right timing of application of drops for the individual patient. In addition to simple IOP reduction, this IOP “modulation” may help in reducing glaucoma progression.Citation27 Another advantage is that the patient’s understanding of glaucoma and adherence with glaucoma drops can be improved by visualizing 24-hour IOP profiles and the impact of therapy on them. It also allows identification of favorable and adverse behavioral and occupational patterns on IOP.Citation9
Strategies in IOP monitoring
Phasing may be undertaken either only during clinic hours or covering a full 24-hour period. Monitoring of IOP limited to daytime pressure curves typically will represent only a portion of the 24-hour IOP pattern. It seems not to predict the nocturnal rhythm, suggesting that a full 24-hour circadian IOP profile should be evaluated in clinical practice.Citation40 Although a retrospective study found no statistically significant increase in the mean peak IOP or mean IOP during the nighttime phasing period compared to the clinic or daytime phasing period, this study was prone to methodological weaknesses.Citation33,Citation41 Nevertheless, a major disadvantage of 24-hour monitoring compared to phasing during office hours is the need for sleep-laboratory settings which are costly and inconvenient for the patient. A 24-hour (nyctohemeral) IOP cycle is characterized by an acrophase, ie, the time period in this cycle during which the IOP curve peaks, and the bathyphase, which represents the part of the cycle during which the IOP curve is low.
Phasing using Goldmann applanation tonometry
There are several ways of documenting the 24-hour IOP cycle. Usually, 24-hour IOP rhythms are documented using Goldmann applanation tonometry (GAT). GAT is considered the “gold standard” for measuring IOP. The cornea is flattened over a defined area (3.06 mm in diameter) and the required applanation pressure serves to estimate IOP. This technique is based on the Imbert-Fick law and assumes that the eyeball is a perfect sphere and the cornea a perfectly thin, elastic, and flexible membrane. GAT is therefore influenced by several ocular factors, such as central corneal thickness, corneal biomechanical properties, and scleral rigidity. Apart from these biomechanical factors of bias, GAT also suffers from operator bias.Citation42–Citation44 It implies sitting in front of a slit lamp, which is far from the physiologic position during the nocturnal/sleep period. Even if the Perkins applanation or Tono-pen® (Reichert Technologies, Munich, Germany) are used in the supine position at night, awakening the patient remains necessary, potentially introducing stress-related artifacts, disturbing sleep organization, and ignoring dynamic changes related to daily-life physical activities.Citation45 Reproducibility of inter-day IOP measurements with GAT has been reported to be only moderate at best in healthy volunteers and glaucoma patients.Citation4,Citation46–Citation48
Self-tonometry
Another strategy in attempting to document round-the-clock IOP measurements is self-tonometry. Self-tonometry is an elegant alternative avoiding cumbersome hospitalization, and is a valuable adjunct to office measurements. In the practice of medicine today, patients with diabetes, asthma, and arterial hypertension participate actively in monitoring their diseases by frequent measurements of the blood glucose, peak flow, and blood pressure at home. Similarly, self-tonometry has the potential to empower the patient to take regular measurements of their IOP through the day and overnight in a familiar environment without stress, enhancing their compliance to therapy. Apart from discovering any fluctuations, self-tonometry may reduce the number of routine visits, but also expedite emergency evaluation of an acute angle closure attack. It opens up the perspective of the telemedicine application and can enlarge our understanding of glaucoma pathophysiology in ocular hypertension and normal-tension glaucoma. Several methods are available.
The Proview Eye Pressure Monitor (Bausch & Lomb Incorporated, Bridgewater, NJ, USA) was invented by Fresco in 1997.Citation49 It is a spring-compression device with a 3.06 mm diameter circular tip that is applied to the superonasal orbit over the upper eyelid while the eye is directed inferotemporally. As increasing pressure is applied to the eye, a visual sensation that has been variously described as like a solar eclipse or a dark circle surrounded by a bright halo is produced, and the measured IOP is read off the scale. Although the Proview tonometer is easy to use, there is no consensus on the effectiveness of the Proview tonometer.Citation50 In a randomized prospective clinical trial involving glaucoma patients, the absolute mean difference between Proview Eye Pressure Monitor and GAT readings was 3.5±2.9 mmHg.Citation51 In patients with IOP less than 10 mmHg or greater than 20 mmHg, this discrepancy with GAT was even higher (6.6±3.6 mmHg) with an overestimation in lower IOP and overestimation in higher IOPs.Citation51,Citation52 The intraclass correlation coefficients between IOPs obtained with GAT and Proview were not strong (0.2), whereas for the GAT and the Tono-pen correlated fairly well (0.78) among glaucoma patients.Citation53 In a prospective study, Proview detected a high IOP (defined as a Goldmann pressure of >21 mmHg by GAT) in only 18% (4/22) of patients.Citation54 The Ocuton-S applanation self-tonometer is a hand-held electronic automatic applanation tonometer based on the same principles as the GAT, requiring anesthetic drops. This dependency on anesthetic drops and the fact that the device contacts the cornea in a “home” environment are of concern, because of the risks of corneal abrasion, ulceration, and infection. Furthermore, there is a lack of reproducibility compared to the results of the GAT-measurements.Citation50
The Tiolat iCare tonometer is a hand-held unit which measures the deceleration of a magnetized probe in an electromagnetic field on the rebound from the cornea. It has the advantages of a noncontact unit, not requiring anesthetic drops.Citation55 Its use as a self-tonometer has been reported and its readings correlate well with GAT.Citation56 However, the iCare readings are influenced by the measuring position, with central IOP significantly greater than peripheral measurements by approximately 3–4 mmHg.Citation57 When measurements are taken by patients on their own eyes in a home setting, these may not always be consistently obtained in the central position, hence questioning the accuracy and reproducibility of the IOP measurements. Self-tonometry techniques are therefore prone to technical difficulties for many older glaucoma patients. Last but not least, viable self-tonometry does not address the crucial issue of IOP behavior during undisturbed sleep.Citation31
Contact lens sensor
Temporary IOP monitoring systems using a contact lens had been previously tried. Greene and Gilman embedded two strain gauges in a soft contact lens that could measure angular changes at the meridian angle of the corneoscleral junction secondary to IOP variations.Citation58 In order to detect small changes occurring at the meridian angle, the contact lenses needed to be custom molded for each eye, which made this prototype economically not viable. Twa et al integrated a piezo-resistive sensor tip of the dynamic contour tonometer in a hard contact lens, and their system provided reliable IOP measurements in a group of healthy volunteers for only up to 100 seconds.Citation59
Concept of the contact lens sensor
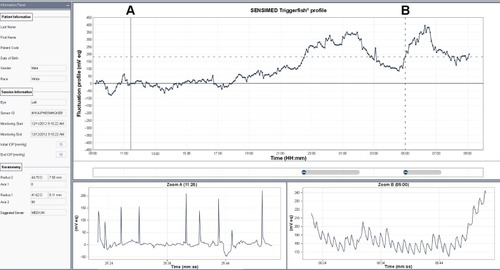
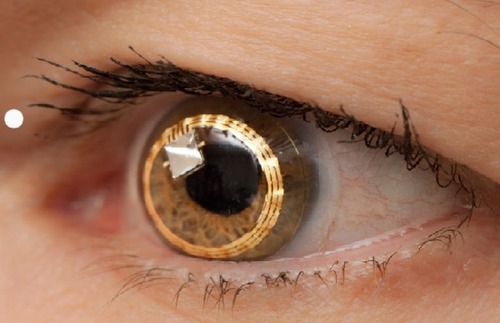
An alternative technique is a disposable contact lens sensor (CLS) (SENSIMED Triggerfish®; Sensimed AG, Lausanne, Switzerland) that has been developed to register corneal curvature changes ().Citation58 The corneal curvature in human eyes correlates well with IOP, in which an IOP change of 1 mmHg causes a change of central corneal radius of curvature of approximately 3 µm.Citation60,Citation61 In vitro studies using a sensor with an embedded micro-fabricated strain gauge on porcine eyes have demonstrated a very good correlation between the IOP variations and the changes in the corneal curvature measured in the corneoscleral area, where it is believed IOP changes induce maximum corneal deformation.Citation62,Citation63 Any ocular dimensional changes in the corneoscleral area are transmitted to the recorder expressed as an equivalent of the electric voltage (mV equivalents) (). Recorded profiles are visual-ized graphically on a computer interface with approximately 300 data points acquired during a 30-second period, every 5 minutes, resulting in a total of 288 measurements over a 24-hour period. As IOP fluctuates synchronically with heart rate, this 24-hour registration of IOP rhythm can be inspected for ocular pulsation, including systolic and diastolic peaks and ocular pulsation frequency (). The noise could be significantly allowing the detection an artificially induced IOP change over a 10-minute interval in four human volunteers, correlating well with the expected GAT-values.Citation64 IOP can be measured by GAT before and after CLS wear, but GAT is not possible during the CLS wear, making simultaneous validation of the registered spikes difficult.Citation65
Figure 1 Contact lens sensor (SENSIMED Triggerfish®) on the eye. Reproduced with permission from Sensimed AG © 2014.
Functionality and tolerability of the CLS
Studies in healthy subjects and glaucoma patients confirmed the functionality and tolerability of the CLS.Citation66–Citation68 The most common adverse effects were blurred vision, conjunctival hyperemia, and superficial punctate keratitis, all of which resolved spontaneously within 48 hours. Using the CLS, a moderate agreement between 24-hour IOP profiles in both healthy subjects and glaucoma patients was found when monitoring was repeated.Citation69,Citation70 The disposable CLS exists in three different base curves in order to ensure a good fit of the CLS, which is necessary for accurate measurements of small changes in the cornea and avoidance of movement artifacts of the strain gauges. Adapting a modified cosinor-rhythmometry method, this CLS allows accurate and reproducible registration of IOP rhythm with repeatable nocturnal acrophases seen in 62.9% of glaucoma patients.Citation71
Clinical applications of the CLS
Nocturnal acrophases showed a higher peak and a prolonged duration in patients with glaucoma (70%) than in healthy subjects (33.3%).Citation40 A study evaluating the effect of selective laser trabeculoplasty demonstrated a reduction of the mean acrophase amplitude of the fitted cosinor function (global variability) by 24.6% after selective laser trabeculoplasty in the success group.Citation72 The CLS was found to be sensitive to individual IOP rhythms and to differences in such rhythms induced by anti-glaucomatous drug therapy.Citation73 Some studies suggested that the CLS is a more sensitive method than the noncontact tonometry in documenting characteristic acrophases and bathyphases of a 24-hour IOP rhythm in healthy participants.Citation69 Others, however, found 24-hour GAT IOP measurements far superior in detecting a treatment-induced significant IOP decrease than the CLS system.Citation74 These authors were concerned that a trend for increasing measurement values along with increasing time of CLS wear during the 24-hour measurement period may mask some of the true IOP-related alterations induced by treatment or related to postural changes.Citation74 Another study reported that an increased IOP induced by a ring pressed against the eye did not result in increased recording of the CLS, while GAT did detect the increased IOP values, suggesting that the relationship between IOP and corneal curvature also depends on thickness and elasticity of cornea and sclera, and variable corneal hydration.Citation75 This discrepancy, however, could be due to improper use of the CLS, since pressing with a ring prevents the globe to expand freely and naturally, and hence prevents the capture of ocular dimensional changes in the corneoscleral area related to IOP.Citation76
Advantages and drawbacks of the CLS
Currently, the CLS is the only commercially available device that has the potential to record the IOP-related profile over a prolonged period using a minimally invasive technique, regardless of the position of the patient and his activities, thus opening up new perspectives for the management of glaucoma. Furthermore, the CLS output also gives information on the effect of eye blinks and eye movements on IOP and documents the ocular pulsation continuously (). One major drawback of the CLS is that its values are expressed in mV equivalents (instead of mmHg), making the clinical interpretation challenging. Algorithms need to be developed to translate these units to a more clinically relevant form. With the current commercial price of around 500 Euros per eye per 24 hours, affordability of the technology is also another important issue, but this needs to be put into perspective of the high costs of running a sleep laboratory.
Conclusion
There is mounting evidence that IOP fluctuations play an important role in the development and progression of glaucoma. Despite numerous efforts, temporary monitoring of IOP over a 24-hour period in humans remains a technical challenge. Currently, the CLS is the only commercially available device that is promising in providing 24-hour information on IOP fluctuations. Further development of this technology will be a critical step in understanding and managing IOP variations in glaucoma.
Acknowledgments
The author thanks Sensimed AG for the illustrations used in this work.
Disclosure
The author reports no conflicts of interest in this work.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ2004821184485115640920
- ZeimerRCWilenskyJTGieserDKVianaMAAssociation between intraocular pressure peaks and progression of visual field lossOphthalmology199198164692023735
- KonstasAGQuarantaLMikropoulosDGPeak intraocular pressure and glaucomatous progression in primary open-angle glaucomaJ Ocul Pharmacol Ther2012281263222004074
- MansouriKWeinrebRNMedeirosFAIs 24-hour intraocular pressure monitoring necessary in glaucoma?Semin Ophthalmol201328315716423697618
- LiuJHKripkeDFTwaMDTwenty-four-hour pattern of intraocular pressure in the aging populationInvest Ophthalmol Vis Sci1999402912291710549652
- LiuJHZhangXKripkeDFWeinrebRNTwenty-four-hour intraocu-lar pressure pattern associated with early glaucomatous changesInvest Ophthalmol Vis Sci20034441586159012657596
- BarkanaYAnisSLiebmannJTelloCRitchRClinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucomaArch Ophthalmol2006124679379716769832
- MosaedSLiuJHWeinrebRNCorrelation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patientsAm J Ophthalmol2005139232032415733994
- MansouriKThe road ahead to continuous 24-hour intraocular pressure monitoring in glaucomaJ Ophthalmic Vis Res20149226026825279129
- DranceSMThe significance of the diurnal tension variations in normal and glaucomatous eyesArch Ophthalmol19606449450113724271
- KitazawaYHorieTDiurnal variation of intraocular pressure in primary open-angle glaucomaAm J Ophthalmol19757945575661168023
- KonstasAGMantzirisDACateEAStewartWCEffect of timolol on the diurnal intraocular pressure in exfoliation and primary open-angle glaucomaArch Ophthalmol199711589759799258218
- LiuJHKripkeDFHoffmanRENocturnal elevation of intraocu-lar pressure in young adultsInvest Ophthalmol Vis Sci19983913270727129856781
- LiuJHKripkeDFHoffmannREElevation of human intraocular pressure at night under moderate illuminationInvest Ophthalmol Vis Sci1999402439244210476816
- HaraTHaraTTsuruTIncrease of peak intraocular pressure during sleep in reproduced diurnal changes by postureArch Ophthalmol2006124216516816476884
- JürgensCGrossjohannRTostFHRelationship of systemic blood pressure with ocular perfusion pressure and intraocular pressure of glaucoma patients in telemedical home monitoringMed Sci Monit20121811MT85MT8923111749
- ChoiJKimKHJeongJChoHSLeeCHKookMSCircadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucomaInvest Ophthalmol Vis Sci200748110411117197523
- LiJCGuptaVKYouYNgKWGrahamSLThe dynamic response of intraocular pressure and ocular pulse amplitude to acute hemodynamic changes in normal and glaucomatous eyesInvest Ophthalmol Vis Sci201354106960696724045988
- VaajanenAMervaalaEOksalaOVapaataloHIs there a relationship between blood pressure and intraocular pressure? An experimental study in hypertensive ratsCurr Eye Res200833432533218398707
- MansouriKOrguelSMermoudAQuality of diurnal intraocular pressure control in primary open-angle patients treated with latanoprost compared with surgically treated glaucoma patients: a prospective trialBr J Ophthalmol200892333233618211927
- MansouriKMedeirosFAWeinrebRNLetter to the editor: 24-hour versus daytime intraocular pressure phasing in the management of patients with treated glaucomaBr J Ophthalmol201195459459521270436
- AsraniSZeimerRWilenskyJGieserDVitaleSLindenmuthKLarge diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucomaJ Glaucoma20009213414210782622
- Nouri-MahdaviKHoffmannDColemanALPredictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention StudyOphthalmology200411191627163515350314
- BergeåBBodinLSvedberghBImpact of intraocular pressure regulation on visual fields in open-angle glaucomaOphthalmology199910659971004 discussion 1004–100510328403
- HongSSeongGJHongYJLong-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedureArch Ophthalmol200712581010101317698746
- CollaerNZeyenTCaprioliJSequential office pressure measurements in the management of glaucomaJ Glaucoma200514319620015870600
- CaprioliJVarmaRIntraocular pressure: modulation as treatment for glaucomaAm J Ophthalmol2011152334034421855671
- JonasJBBuddeWMStrouxAOberacher-VeltenIMJünemannADiurnal intraocular pressure profiles and progression of chronic open-angle glaucomaEye (Lond)200721794895116601737
- Ocular Hypertension Treatment Study GroupEuropean Glaucoma Prevention Study GroupGordonMOValidated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertensionOphthalmology20071141101917095090
- BengtssonBLeskeMHymanLHeijlAEarly Manifest Glaucoma Trial GroupFluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trialOphthalmology200711420520917097736
- SitAJContinuous monitoring of intraocular pressure: rationale and progress toward a clinical deviceJ Glaucoma200918427227919365190
- GrippoTMLiuJHZebardastNArnoldTBMooreGHWeinrebRNTwenty-four-hour pattern of intraocular pressure in untreated patients with ocular hypertensionInvest Ophthalmol Vis Sci201354151251723211815
- MoodieJWildeCRotchfordAPVernonSAKingAJ24-Hour versus daytime intraocular pressure phasing in the management of patients with treated glaucomaBr J Ophthalmol2010948999100219965831
- SaccàSCRolandoMMarlettaAMacríACerquetiPCiurloGFluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjectsOphthalmologica19982121151199486551
- HughesESpryPDiamondJ24-hour monitoring of intraocular pressure in glaucoma management: a retrospective reviewJ Glaucoma20031223223612782841
- RenardEPalombiKGronfierCTwenty-four hour (Nyctohe-meral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucomaInvest Ophthalmol Vis Sci201051288288919684006
- QuarantaLGandolfoFTuranoREffects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucomaInvest Ophthalmol Vis Sci20064772917292316799034
- KonstasAGHollóGMikropoulosDG24-hour efficacy of the bimatoprost-timolol fixed combination versus latanoprost as first choice therapy in subjects with high-pressure exfoliation syndrome and glaucomaBr J Ophthalmol201397785786123686322
- SeiboldLKKahookMYThe diurnal and nocturnal effects of travoprost in normal-tension glaucomaClin Ophthalmol201482189219325382969
- AgnifiliLMastropasquaRFrezzottiPCircadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensorActa Ophthalmol2015931e14e2124720477
- SchieferUMeisnerCZiemssenF24-Hour intraocular pressure phasing remains an important tool in glaucoma diagnosticsBr J Ophthalmol201195459421258077
- EhlersNBramsenTSperlingSApplanation tonometry and central corneal thicknessActa Ophthalmol (Copenh)197553134431172910
- LiuJRobertsCJInfluence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysisJ Cataract Refract Surg200531114615515721707
- WhitacreMMSteinRSources of error with use of Goldmann-type tonometersSurv Ophthalmol19933811308235993
- LiuJHWeinrebRNMonitoring intraocular pressure for 24 hBr J Ophthalmol201195559960021330554
- WilenskyJTGieserDKDietscheMLMoriMTZeimerRIndividual variability in the diurnal intraocular pressure curveOphthalmology199310069409448510909
- RealiniTWeinrebRNWisniewskiSRDiurnal intraocular pressure patterns are not repeatable in the short term in healthy individualsOphthalmology201011791700170420557945
- RealiniTWeinrebRNWisniewskiSShort-term repeatability of diurnal intraocular pressure patterns in glaucomatous individualsOphthalmology20111181475120709404
- FrescoBBA new tonometer – the pressure phosphene tonometer: clinical comparison with Goldman tonometryOphthalmology199810511212321269818616
- LiangSYLeeGAShieldsDSelf-tonometry in glaucoma management – past, present and futureSurv Ophthalmol200954445046219539833
- RaiSMosterMRKesenMLevel of disagreement between Proview phosphene tonometer and Goldmann applanation tonometer intraocular pressure readingsJ Glaucoma200514212012315741812
- TaiMCChenPLWuJNLuDWClinical evaluation of the intraocular pressure in patients with glaucoma or ocular hypertension by a self-assessable tonometerJ Ocul Pharmacol Ther2005211556115718828
- LiJHerndonLWAsraniSGStinnettSAllinghamRRClinical comparison of the Proview eye pressure monitor with the Goldmann appla-nation tonometer and the TonopenArch Ophthalmol200412281117112115302650
- AlvarezTLGollanceSAThomasGAThe Proview phosphene tonometer fails to measure ocular pressure accurately in clinical practiceOphthalmology200411161077108515177956
- KontiolaAIA new induction-based impact method for measuring intraocular pressureActa Ophthalmol Scand200078214214510794245
- RosentreterAJablonskiKSMelleinACGakiSHueberADietleinTSA new rebound tonometer for home monitoring of intraocular pressureGraefes Arch Clin Exp Ophthalmol2011249111713171921842435
- MuttuveluDVBaggesenKEhlersNPrecision and accuracy of the ICare tonometer – Peripheral and central IOP measurements by rebound tonometryActa Ophthalmol201290432232620840218
- GreeneMEGilmanBGIntraocular pressure measurement with instrumented contact lensesInvest Ophthalmol1974132993024818815
- TwaMDRobertsCJKarolHJMahmoudAMWeberPASmallRHEvaluation of a contact lens-embedded sensor for intraocular pressure measurementJ Glaucoma201019638239020051894
- HjortdalJOJensenPKIn vitro measurement of corneal strain, thickness, and curvature using digital image processingActa Ophthalmol Scand1995735117627759
- LamAKDouthwaiteWAThe effect of an artificially elevated intraocu-lar pressure on the central corneal curvatureOphthalmic Physiol Opt19971718249135808
- LeonardiMLeuenbergerPBertrandDBertschARenaudPFirst steps toward noninvasive intraocular pressure monitoring with a sensing contact lensInvest Ophthalmol Vis Sci2004453113311715326128
- LeonardiMPitchonEMBertschARenaudPMermoudAWireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyesActa Ophthalmol20098743343719016660
- PitchonEMLeonardiMRenaudPMermoudAZografosLFirst in vivo human monitoring of intraocular pressure fluctuation and ocular pulsation by wireless soft contact lens sensorPoster presented at: ARVO. 27 April – 1 May 2008, Fort Lauderdale American Glaucoma Society, 18th Annual MeetingMarch 2008Washington2008
- FaschingerCMossböckGContinuous 24 h monitoring of changes in intraocular pressure with the wireless contact lens sensor Triggerfish™. First results in patientsOphthalmologe20101071091892220535482
- De SmedtSMermoudASchnyderC24-hour intraocular pressure fluctuation monitoring using an ocular telemetry Sensor: tolerability and functionality in healthy subjectsJ Glaucoma201221853954421602707
- MansouriKShaarawyTContinuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucomaBr J Ophthalmol201195562762921216796
- LorenzKKorbCHerzogNTolerability of 24-hour intraocular pressure monitoring of a pressure-sensitive contact lensJ Glaucoma201322431131623524857
- MottetBAptelFRomanetJPHubanovaRPépinJLChiquetC24-hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens sensorJAMA Ophthalmol2013131121507151624158696
- MansouriKMedeirosFATafreshiAWeinrebRNContinuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucomaArch Ophthalmol20121301215341539
- MansouriKLiuJHWeinrebRNTafreshiAMedeirosFAAnalysis of continuous 24-hour intraocular pressure patterns in glaucomaInvest Ophthalmol Vis Sci201253138050805623139273
- LeeJWFuLChanJCLaiJSTwenty-four-hour intraocular pressure related changes following adjuvant selective laser trabeculoplasty for normal tension glaucomaMedicine (Baltimore)20149327e23825501089
- PajicBPajic-EggspuchlerBHaefligerIContinuous IOP fluctuation recording in normal tension glaucoma patientsCurr Eye Res201136121129113821978205
- HollóG1KóthyPVarghaPEvaluation of continuous 24-hour intraocular pressure monitoring for assessment of prostaglandin-induced pressure reduction in glaucomaJ Glaucoma2014231e6e1224370812
- Sunaric-MegevandGLeuenbergerPPreußnerPRAssessment of the Triggerfish contact lens sensor for measurement of intraocular pressure variationsActa Ophthalmol2014925414415
- LeonardiMResponse to the letter to the editor entitled ‘Assessment of the Triggerfish® contact lens sensor for measurement of intra-ocular pressure variations’Acta Ophthalmol Epub 2014 Dec 7