Abstract
Pterygium, a sun-related eye disease, presents as wing-shaped ocular surface lesions that extend from the bulbar conjunctiva onto the cornea, most commonly on the nasal side. Pterygia show characteristic histological features that suggest that inflammation plays a prominent role in their initial pathogenesis and recurrence. Appropriate surgery is the key to successful treatment of pterygia, but there is also a rationale for the use of anti-inflammatory agents to reduce the rate of recurrence following surgery. Multiple surgical techniques have been developed over the last two millennia, but these initially had little success, due to high rates of recurrence. Current management strategies, associated with lower recurrence rates, include bare sclera excision and various types of grafts using tissue glues. Adjunctive therapies include mitomycin C and 5-fluorouracil, as well as the topical ocular steroid loteprednol etabonate, which has been shown to have a lower risk of elevated intraocular pressure than have the other topical ocular steroids. Here, the surgical management of pterygium is presented from a historical perspective, and current management techniques, including the appropriate use of various adjunctive therapies, are reviewed, along with an illustrative case presentation and a discussion of the conjunctival forceps designed to facilitate surgical management. Despite thousands of years of experience with this condition, there remains a need for a more thorough understanding of pterygium and interventions to reduce both its incidence and postsurgical recurrence. Until that time, the immediate goal is to optimize surgical practices to ensure the best possible outcomes. Loteprednol etabonate, especially the ointment formulation, appears to be a safe and effective component of the perioperative regimen for this complex ocular condition, although confirmatory prospective studies are needed.
Introduction
The term “pterygium” is a Latinized version of the Greek term “pterygion” meaning “small wing”;Citation1,Citation2 it is the name for the clinical condition consisting of wing-shaped ocular surface lesions extending from the bulbar conjunctiva onto the cornea, most commonly on the nasal side, that potentially result in blindness. On histological examination, the lesions show several characteristic features: inflammatory cells, neovascularization; remodeling of the extracellular matrix; and a leading edge (head) of altered limbal epithelial cells, followed by squamous metaplastic epithelium showing hyperplasia of the goblet cells, and an underlying stroma of activated, proliferating fibroblasts. These histological features allow pterygia to be classified into three types: proliferative, fibromatous, and atrophic sclerotic.Citation3 Its clinical evaluation can be quantified by size, including invasion of the cornea and width at the base, as well as morphologic features.Citation4
While there is no clear understanding of the pathogenesis of pterygium, it is probably multifactorial (including inherited factors and environmental triggers) in origin. Current evidence strongly suggests that ultraviolet (UV) light is probably the single most important factor.Citation3,Citation5–Citation7 Several molecular mechanisms are activated by UV exposure, including oxidative stress and growth factor (GF) receptor signaling; these lead to the production of various factors, such as proinflammatory cytokines, GFs, and matrix metalloproteinases (MMPs), that appear to promote pterygium growth.Citation3 In fact, both connective tissue growth factor and vascular endothelial growth factor (VEGF) are present in the epithelium of pterygia.Citation8
Despite an extensive literature presence that goes back two millennia, pterygium remains somewhat of an enigma. Ancient writers who described its medical and surgical management included Hippocrates, Celsus, Pallus, Sushruta, and Aetius. They all recognized that treatment was difficult and that recurrences were almost inevitable. The first author to document a surgical approach appears to have been Celsus in 25 AD.Citation2 However, little progress in preventing recurrences was described by later writers, even up to the early 20th century. In fact, some success in treating this condition and preventing recurrences has only recently been achieved.Citation2
In recent years, there has been an increased awareness of the role of inflammation in both the pathogenesis of pterygium and in recovery following surgery.Citation9,Citation10 This has prompted the incorporation of topical corticosteroids, such as loteprednol etabonate (LE), into pterygium management protocols. The purpose of this article is to review the current strategies and the authors’ personal experience managing this difficult condition, with added focus on the potential role of LE. We begin with a brief summary of LE.
Loteprednol etabonate
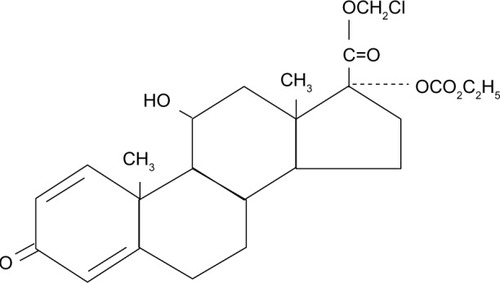
Compared with other corticosteroids, loteprednol etabonate (Lotemax; Bausch & Lomb, Rochester, NY, USA) has a unique structure – the carbon 20 ketone group in the prednisolone core is replaced by an ester ().Citation11 LE is highly lipophilic, which facilitates its easy penetration through cell membranes,Citation12 and it has been shown to have a therapeutic index more than 20-fold higher than that of other corticosteroids.Citation13 LE was developed using “retrometabolic” engineering, to reduce its potential for adverse effects;Citation14 it binds avidly to the glucocorticoid receptor, while the unbound drug is rapidly converted to inactive metabolites, minimizing the potential for unwanted effects, such as intraocular pressure (IOP) elevation and cataractogenesis.Citation11,Citation13,Citation15,Citation16 Studies have confirmed a lower risk of IOP elevations with LE compared with other ocular steroid compounds.Citation11,Citation17–Citation21
LE has three formulations that have been tested clinically for postoperative inflammation management in patients undergoing cataract surgery with intraocular lens implantation: a suspension,Citation22,Citation23 an ointment,Citation24 and most recently, a gelCitation25,Citation26 (). Compared with the suspension formulation, the ointment and gel formulations of LE are expected to have some advantages, including better homogeneity and no need for resuspension by shaking, resulting in more consistent dosing. In addition, there is no preservative in the ointment formulation. Regardless of the formulation, LE significantly reduced postoperative anterior chamber inflammation (cells and flare) and pain compared with vehicle, in cataract surgery patients.Citation11,Citation22,Citation24–Citation26 Further, the proportion of patients requiring rescue medication was lower (approximately half) in the LE-treated group compared with the vehicle-treated group. Overall, there were no safety concerns noted with LE in these trials. Regardless of formulation, the mean IOP decreased (1–2 mmHg) with LE treatment, and few LE-treated patients experienced an IOP increase >10 mmHg.
Table 1 Clinical studies comparing LE with vehicle, in the treatment of postoperative pain and inflammation following cataract surgeryTable Footnote*
There are currently no published controlled studies reporting clinical outcomes following the treatment of pterygium patients with LE, despite its apparent widespread use for this condition and well-documented efficacy in other postoperative indications. In a pilot study, the treatment of pterygium patients with LE twice daily (BID) for 14 days led to an approximate 50% increase in glucocorticoid receptor migration to the cell nucleus in the pterygium head tissue, as demonstrated using Western blot analysis of biopsied pterygium tissue, suggesting that perioperative treatment with topical LE could result in favorable clinical outcomes in pterygium removal.Citation27,Citation28
Current concepts in pterygium management
Historical techniques
The first reported surgical approach to pterygium management was described by Celsus, in which a needle and thread were passed under the pterygium. The thread was then elevated, the pterygium was completely lifted from the cornea to the canthus using a sawing motion, and the pterygium was excised. Unfortunately, this approach was often associated with pain, as well as blindness. Later, in the 16th century, it was noted that this operation should rarely be performed due to its dangers and the risk of recurrence.Citation2 From the 1800s to the 1930s, various techniques were developed, but none proved particularly successful. Though excision with simple conjunctival closure had good short-term results and few complications, the recurrence rates were high.Citation2 In the first half of the 20th century, transplantation of the pterygium head away from the cornea, or redirection, was the most commonly used approach. However, though a relatively simple procedure with few complications, it failed to solve the major problem, that of recurrence, and was eventually replaced.Citation2
Current management techniques
Bare sclera excision
The bare sclera technique heralded a major change in the surgical approach to pterygium, in the 1940s. This technique originally involved the complete excision of the pterygium head and removal of some of the adjacent normal nasal bulbar conjunctiva along with excision of the underlying Tenon’s capsule tissue, which then resulted in a bare sclera. Following suture of the surrounding normal bulbar conjunctiva to the sclera, several millimeters of the sclera next to the limbus were left bare. The corneal epithelium then healed before it was possible for the conjunctival epithelium to reach the limbus. Thus the bare sclera technique compromised limbal stem cells. This technique has been subsequently refined, but when used alone, the recurrence rate is unacceptably high (30%–80%).Citation29 Therefore, some surgeons today use this approach in combination with various adjunctive therapies, to reduce the recurrence rate.Citation2,Citation29,Citation30
Another approach to pterygium surgery involves grafting. This strategy was first described in 1876, starting with mucous membrane grafting and then, conjunctival autografting as well as limbal conjunctival autografting. Conjunctival autografting is still widely used and considered the procedure of choice for primary and recurrent pterygium, due to its convenience and low recurrence rates (2%–39%).Citation2,Citation29 A recent meta-analysis suggested that limbal conjunctival autografting has lower recurrence rates than do the bare sclera technique and conjunctival autografting, with no difference between it and amniotic membrane grafts.Citation31 Large-scale, randomized, controlled studies are needed to better compare the recurrence rates among the various techniques.
Amniotic membrane grafts
The use of amniotic membrane grafts has been considerably refined subsequent to its original description in 1947.Citation2 The amniotic membrane, the placenta’s inner lining, is composed of three layers: a single epithelial layer; a thick basement membrane; and an avascular stroma.Citation32 Several characteristics of amniotic membrane appear to make it suitable for treating pterygium, including its anti-inflammatory, anti-scarring, and anti-angiogenic properties.Citation29 Once harvested, amniotic membrane can be used fresh or preserved (either freeze-dried or cryopreserved). Unfortunately, fresh amniotic membrane is usually not used in developed countries, due to the need to exclude infections, such as human immunodeficiency virus (HIV) and hepatitis.Citation29 While some clinical trials of amniotic membrane grafts for the treatment of pterygium reported an unacceptably high recurrence rate compared with conjunctival autograft, it may provide a superior alternative for selected cases.Citation29,Citation30,Citation33,Citation34 Others have suggested that amniotic membrane may be combined with conjunctival autografting techniques, with the amnion used in the subconjunctival space as a natural inhibitor of fibrosis. Recurrence rates as low as 0.7% have been reported with this technique.Citation35
Role of tissue glue
Fibrin glue can replace or augment sutures when attaching conjunctival grafts or amniotic membrane, significantly shortening operating times and decreasing postoperative discomfort, as well as decreasing recurrence rates.Citation29 In general, fibrin glue consists of two main components: a sealer protein concentrate containing fibrinogen and a fibrinolysis inhibitor, and a thrombin and calcium chloride solution. When mixed at the time of application, the two solutions interact and mimic the clotting cascade, creating adhesion, while allowing the surgeon from 10–60 seconds to complete membrane alignment and orientation. The clot then dissolves after 1–2 weeks, providing adequate time for healing.Citation36 Three products are currently available for use in the USA: Tisseel and Artiss, produced by Baxter International, Inc. (Deerfield, IL, USA); and Evicel®, produced by Ethicon, Inc. (Somerville, NJ, USA).Citation36 The storage requirements and specific usage instructions differ among the available formulations.
Since fibrin adhesives are prepared from pooled donor sources, there is a small but finite risk of infection, such as hepatitis and HIV, as well as of anaphylactic reaction.Citation36 The infection risk is reduced by testing donors for viral markers, at the time of donation and 6 months later, as well as by sterilization of the products by gamma irradiation and treatment with detergents or solvents.Citation36 Recombinant biotechnology could provide an ideal source of fibrinogen and thrombin, thus completely bypassing the viral contamination issues. Interestingly, a recent study suggested autologous fibrin in blood as a useful alternative to fibrin glueCitation37 for pterygium surgery, though preparation can be laborious, requiring special equipment not available to most surgeons.
Adjunctive therapies
Various adjunctive therapies have been used with pterygium surgery, in an attempt to reduce the risk of postoperative recurrence. Two of these, mitomycin C and 5-fluorouracil (5-FU), are commonly employed by contemporary pterygium and glaucoma surgeons.
Mitomycin C
Mitomycin C is a potent alkylating agent that interferes with deoxyribonucleic acid (DNA) replication, particularly in cells that are growing actively. It also interferes with ribonucleic acid (RNA) and protein synthesis. The use of topical mitomycin C as an adjunct to pterygium surgery to inhibit the growth of fibroblasts was first described in 1963 and actively investigated in the late 1980s.Citation38 Mitomycin C treatment combined with conjunctival grafting techniques significantly reduces the recurrence of both primary and recurrent pterygia compared with the bare sclera technique alone.Citation29,Citation39,Citation40 After almost 3 years of follow up, patients treated for recurrent pterygium with intraoperative mitomycin C (0.02%) had a significantly lower rate of recurrence compared with those managed with the bare sclera technique alone (12.5% vs 35.6%) (P=0.027).Citation40
Two approaches have been developed for applying mitomycin C: postoperative use of topical eye drops and intraoperative use of mitomycin C–soaked sponges. Both have similar recurrence rates. Adjunctive mitomycin C treatment is not without risk and has been associated with rare but severe long-term complications, including persistent epithelial defects of both the cornea and sclera, endophthalmitis, infectious scleritis, perforation, and scleral necrosis.Citation29,Citation41
A recent prospective, randomized study that included follow up on 78 eyes (78 patients) showed that conjunctival autografting and conjunctival–limbal autografting followed intraoperatively by a 3-minute application of 0.02% mitomycin C was associated with no significant difference in the pterygium recurrence rate, at 1-year follow up, between the two procedures.Citation42 In another trial involving 41 eyes (37 patients) undergoing pterygium surgery, intraoperative mitomycin C (0.02%) was associated with a similar recurrence rate at 36 months mean follow up compared with conjunctival autograft alone (14.3% vs 5.0%) (P=0.317) in patients with primary pterygium.Citation43 Further studies of adjuvant mitomycin C treatment are necessary.Citation30
5-Fluorouracil
5-FU, a fluorinated pyrimidine, also inhibits the proliferation of fibroblasts, but through different mechanisms involving the inhibition of thymidylate synthetase and other enzymes related to nucleic acid biosynthesis.Citation38,Citation44 Though the use of 5-FU as an adjunct to pterygium surgery has so far been associated with only minor and transient complications, there have been only few trials of its use in this way. On the other hand, serious complications have been reported when 5-FU has been used for glaucoma, especially ocular surface complications leading to severe epitheliopathy and corneal infections.Citation38 The use of 5-FU as an adjuvant to conjunctival autograft post pterygium excision had a lower but not statistically different rate of recurrence compared with low-dose mitomycin-C (0.01%), at 35 weeks mean follow up (8.7% vs 11.8%) (P=0.7).Citation45 Further long-term trials are needed to adequately evaluate the long-term safety and efficacy of 5-FU and other adjunctive treatments.Citation29
Surgical outcomes
Pterygium surgery has four principal goals: restoration of an uninterrupted refractive ocular surface; a low recurrence rate; minimization of complications; and a satisfactory cosmetic outcome.
Immediate complications
Appropriate postoperative care helps reduce complications, but immediate complications can include excessive bleeding, conjunctival chemosis, graft edema, hematoma below the graft, a localized epithelial defect, loose sutures, conjunctival wound dehiscence, infection, and corneal scarring. While some complications may resolve without intervention, such as a localized epithelial defect or conjunctival chemosis, others require specific management.Citation46
Late complications
The major late complication following pterygium surgery is recurrence. Recurrence rates vary by procedure, with bare sclera excision having the highest rate, followed by excision with amniotic membrane grafting, and by conjunctival autografting.Citation46 One study reported a 0.7% rate of recurrence in primary pterygium, using a combination of conjunctival autograft and placement of amniotic membrane in the subconjunctival space.Citation35 The risk factors for recurrence include male sex and a history of high sun exposure.Citation47 Nontranslucent pterygia have been associated with an increased risk of recurrence,Citation3 as have several biomarkers related to proliferation, inflammation, fibrosis, and angiogenesis. While further evaluation of these important biomarkers is needed, they point out the need to develop treatments that target specific receptors if the goal of eliminating recurrence is to be achieved.Citation3
Other late complications include those related to adjunct therapy (such as mitomycin C), suture-related inflammation, Tenon’s capsule cyst, diplopia and strabismus, scleral complications, corneal perforation, graft inversion or retraction, lens changes, and graft necrosis, as well as more unexpected complications, such as ptosis, entropion, symblepharon, and iris atrophy.Citation46
Long-term results were recently reported for the Pterygium Extended Removal Followed by Extended Conjunctival Transplantation (PERFECT) study, which analyzed 1,000 consecutive pterygium procedures between August 2001 and September 2009, with a mean follow up of 616 days.Citation48 Of these cases, 81% were primary pterygia and 19% were recurrent. There was only one postoperative recurrence (0.1%) in this series. Overall, six patients required further surgery, due to hyperemic graft (three patients), exotropia (one patient), granuloma (one patient), inclusion cyst (one patient), and recurrence (one patient).Citation48 The results of this study involving a single surgeon employing relatively large excisions and grafts suggest an extremely low recurrence rate, an acceptable complication rate, and good cosmetic outcomes.Citation48 However, replication of these findings by surgeons in other centers is needed.
Specific pterygium strategies
With the above as background, the techniques and approaches of two of the authors (AM and JS) will be presented in detail, followed by a case presentation.
Approach and techniques
The surgical procedure selected depends primarily on the clinical presentation. A small but elevated pterygium with surface staining and chronic discomfort and foreign body sensation may require minimal corneal surgery with a rotational flap. On the other hand, a large recurrent pterygium with symblepharon, entropion, and medial rectus restriction may require staged surgery, collaboration with an oculoplastic surgeon, and extensive ocular surface reconstruction, with amniotic membrane or buccal mucosal grafting. Thus, the variety of procedures, enhancements, and modifications can be ranked in terms of complexity ().
Table 2 Surgical techniques for pterygium in order of complexity
Several technical points are keys to success. These issues should all be addressed and a plan formulated prior to surgical intervention.
Preoperative preparation
The surgery is best planned when the patient can avoid irritating environments for at least 3 weeks postoperatively or for 6 weeks for more irritated and inflamed cases or for recurrent cases. If at all possible, surgery should be performed after the fall equinox and prior to the spring equinox in the Northern Hemisphere, to avoid significant seasonal and outdoor activity-driven ultraviolet exposure.
Preoperatively, all ocular surface inflammation should be controlled, including allergy, blepharitis, conjunctivitis, and dry eye; LE is also useful for this purpose, using the suspension or gel formulation four times daily (QID) and the ointment every evening (QHS). Some patients can also use LE ointment during the day, which is strongly recommended for patients with preservative hypersensitivity. The ointment formulation avoids the need for shaking prior to administration and ensures optimal dosing homogeneity. Similarly, LE gel also avoids shaking requirements and minimizes preservative exposure. Some surgeons also use a topical antibiotic preoperatively, for example, besifloxacin BID or three times daily in the affected eye, starting 1 day before surgery, then postoperatively for 7 to 14 days when corneal epithelialization is complete. Unlike cataract surgery, topical nonsteroidal anti-inflammatory drugs (NSAIDs) are not given preoperatively for pterygium surgery. Oral NSAIDs and anticoagulants should be temporarily discontinued.
Surgical procedure
The choice of anesthetic depends on the type of surgery: an injection of retrobulbar or peribulbar anesthetic is appropriate if extensive surgery, lengthy operative time, muscle manipulation, or adjunctive lid procedures are anticipated; topical or light anterior subconjunctival anesthesia should be sufficient for relatively simpler cases in cooperative patients. Light anterior subconjunctival lidocaine with 1:100,000 epinephrine, injected with a 30-gauge needle directly beneath the lesion, should be considered to simplify anesthesia and provide excellent hemostasis.
In addition, topical dilating drops are useful in pterygium excision: the sympathomimetic promotes conjunctival vasoconstriction and hemostasis, while the parasympatholytic provides relief of ciliary spasm pain postoperatively. A central corneal pledget of Murocel® (Bausch & Lomb), segmented Weck-Cel® sponge (Beaver Visitec International, Waltham, MA, USA), or even plastic drape, avoids phototoxicity in addition to preventing corneal epithelial desiccation. Topical brimonidine tartrate (Alphagan® P; Allergan, Inc., Irvine, CA, USA) also provides excellent vasoconstriction.
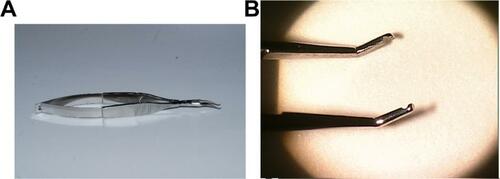
During the procedure itself, it is important to use atraumatic technique throughout, respecting the conjunctival tissues. Specially designed conjunctival forceps (Bausch & Lomb), with a Maumanee handle and Pierse tips, are ideally suited for manipulation of the delicate conjunctiva, thereby reducing the risk of conjunctival tears or button holes (see the section “Discussion of conjunctival forceps design” and Figure S1).
Postoperative care
At the conclusion of the case, a therapeutic bandage soft contact lens (THBL) should be considered because it accelerates reepithelialization and mitigates discomfort. This protection also provides a quantum leap in pain control. The THBL can be used with topical or local anesthesia, allowing the patient to see immediately postoperatively. The THBL can also be used with routine drops and ointment, and a double pressure patch, at the conclusion of retrobulbar or peribulbar block surgery. The patch is used only for the first night, while the THBL remains in place for at least 10–14 days. Some patients experience lens deposits and discomfort fairly early on into the postoperative healing period. The THBL should then be removed but replaced if there are any remaining epithelial defects whatsoever. Limbal sutures, if used, may eventually allow knot tips to protrude anteriorly, a problem for which the THBL is ideally suited. However, many patients tolerate the THBL up to the routine 3-week postoperative visit, at which time the THBL can be removed and the epithelium reassessed. Many patients no longer require a THBL at this 3-week juncture, while many others benefit from an additional 3–4 weeks of THBL therapy.
Topical NSAID drops should be used with some precautions. Older, neurotrophic, dry eye, and very pale hypopigmented patients may experience delayed healing with topical NSAIDs, as may patients with extensive surgery, prolific application of cautery, preexisting scleral or peripheral corneal ectasia, exposed bare sclera, and toxic industrial or outdoor environmental challenges. NSAIDs should be recommended with reservations for all of these patient cohorts, whose pain can alternatively be controlled with topical mydriatics, such as homatropine 5%, Mydriacyl® 1% (Alcon Laboratories, Inc., Fort Worth, TX, USA), or Cyclogel 1% (Alcon Laboratories, Inc.); however, Cyclogel, is particularly likely to cause stinging. Oral NSAIDs also provide excellent analgesia postoperatively, as does the THBL. Younger patients without other ocular conditions may benefit from postoperative NSAID drops, preferably bromfenac (Prolensa™; Bausch & Lomb), nepafenac (Nevanac® and Ilevro®; Alcon Laboratories, Inc.), or generic diclofenac. Topical NSAIDs should absolutely be discontinued and completely avoided if the patient has bare sclera or threatened Dellen formation. LE ointment can provide overnight relief, in a preservative-free preparation, and can be applied even in the presence of a THBL. The ointment formulation provides particular postoperative benefit as it creates a physical “cushion” of sorts, to facilitate epithelial healing.
Strict attention should be paid to concomitant dry eye, in which case ipsilateral punctal occlusion, with a temporary collagen, long-lasting silicone, or even using electrocautery, should be strongly considered. Additional environmental, nutritional, and topical preservative-free tear replacement therapeutic measures to support dry eye therapy are also extremely important. LE suspension, gel, and ointment, are preferred choices for superficial punctate keratitis and dry eye.Citation49
Because pterygia are both actinic and inflammatory in nature, progressive or recurrent degeneration can be avoided with aggressive UV protection and anti-inflammatory therapy. First, a subconjunctival triamcinolone acetonide (Kenalog®; Bristol-Myers Squibb, New York, NY, USA) injection can be given immediately postoperatively in the operating room. Second, the patient must be warned to never expose the eyes to sunlight without wraparound UV sunglasses protection. Baseball-style caps, golf shades, or bonnet-style hats are also very useful. Third, aggressive postoperative application of topical steroids constitutes the mainstay of antirecurrence inflammation control and helps avoid scarring and neovascularization. Topical steroids should continue until all the signs of hypervascularity have vanished. The initial therapy with topical LE suspension or gel up to QID and LE ointment at bedtime is prescribed until the eye is completely “quiet”, all hemorrhage has resolved, full comfort is restored, and all vasculature has returned to normal caliber. This may require up to 3 months in routine cases or much longer in complicated or high-recurrence-risk cases. Once the desired level of anti-inflammatory control has been achieved, the daytime LE treatment can be discontinued and the preservative-free ointment sustained at night (for up to 1 year postoperatively). This ensures compliance and avoids the appearance of limbal vascular tufts that lead to recurrence. For patients who experience difficulty with ointments, the mucoadhesive gel formulation provides sustained exposure to LE throughout the night as well as during waking hours.Citation50
Management of complications and recurrences
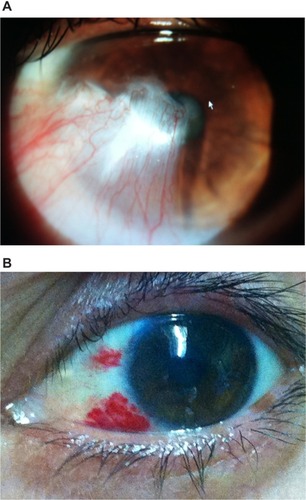
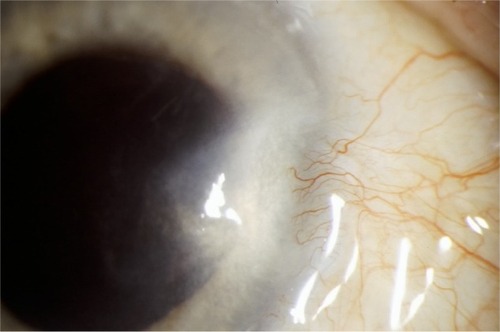
Recurrence is heralded by hypervascularity, leading to limbal violation by abnormal conjunctival neovascularization (). In the event of a recurrence, several courses of action can be taken to avoid repeat surgery, particularly if the recurrence is detected in its earliest stages ().
Figure 2 Untreated pterygium recurrence 3 years following successful surgery.
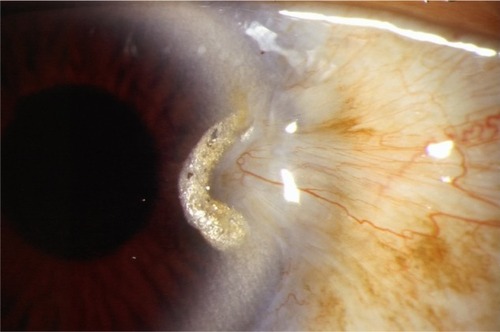
Table 3 Strategies for pterygium recurrence
Topical steroid therapy
Topical steroid therapy prior to and following pterygium surgery is generally a long-term proposition. Patients treated with topical steroids prior to ocular surface surgery invariably fare better, with less immediate postoperative inflammation, fewer complications, a lower risk of recurrence and less extended requirements for inflammation control. Given the chronic nature of topical steroid needs in pterygium management, the potency and safety profile of LE make it an appropriate choice. For routine pterygium patients, LE suspension or gel BID, or LE ointment HS for a month prior to surgery provides sufficient control. Postoperatively, these patients fare well with QID drops or BID ointment for 2 weeks, then half that dose until there are no signs of recurrence and all redness has abated. More complicated cases, involving repeat surgeries and/or high-risk groups, will require more aggressive dosage and duration.
Oral doxycycline
Continuous oral doxycycline can be a useful adjunct due to its beneficial nonantimicrobial effects, including anti-inflammatory, anticollagenase, meibomian lipid viscosity reduction, vascular stabilization, and anti-MMP 9 activities. Doxycycline is preferred over tetracycline or minocycline because of its superior efficacy, more versatile dosing, and a somewhat more favorable side effect profile. Nevertheless, many surgeons and dermatologists prefer minocycline. Fortunately, the nonantimicrobial beneficial effects of doxycycline do not require high dosages. Patients can begin on 100 mg PO BID, eliminating the dinner dose after 10 days and then weaning to 50 mg PO once daily (QD) for a month or so, and then to a sustained maintenance dose of 20 mg PO QD if clinically indicated. Some more severely afflicted or high-body weight patients will require larger maintenance dosages. Patients who require continuous use may benefit from an annual break during the month of June when the sun is strongest in the Northern Hemisphere. The administration instructions for doxycycline and other tetracycline class antibiotics include the directive to “take on an empty stomach”. In most patients, adherence to this recommendation can become difficult if they experience nausea as a result. Concomitant ingestion with dairy products and antacids should be avoided to optimize absorption.
Topical azithromycin
Topical azithromycin (AzaSite®; Akorn, Inc., Lake Forest, IL, USA) has proven useful, not only for conjunctivitis but also for the same menu of nonantimicrobial benefits offered by oral doxycycline. Prolonged therapy can be useful but is often impractical due to cost. Topical azithromycin is particularly useful for patients who are intolerant of oral doxycycline. Many chronic patients benefit from 1 week per month of topical azithromycin HS, year round.
Intraoperative subconjunctival steroid injections
Intraoperative subconjunctival steroid injections provide excellent postoperative inflammation control in addition to topical therapy, allowing a significant reduction in the intensity of the postoperative steroid regimen. Topical 0.04% mitomycin C for intraoperative use is also available from both compounding pharmacies and as a stand-alone US Food and Drug Administration (FDA)-approved medication (Mitosol®; Mobius Therapeutics LLC, St Louis, MO, USA). The potent antifibrotic effects of mitomycin C are essential to reduce the risk of recurrence in virtually all pterygium surgery patients. The dose must be titrated according to age, pigmentation, UV exposure, ocular surface inflammation, neovascularization, concomitant ocular surface disease, pterygium recurrence, previous conjunctival surgery or scarring, keloid formation, dermatologic disease, occupational or avocational environmental irritants, medication compliance, and the best judgment of the operating surgeon. Some surgeons also employ topical 5-FU for the same purpose, although its antifibrotic effect is not as potent as that of mitomycin C. On the other hand, 5-FU can readily be injected subconjunctivally, for postoperative patients with early or threatened recurrence as well as pyogenic granuloma formation or keloids, following surgery.
Postoperative subconjunctival steroid and antifibrosis agent injections
Postoperative subconjunctival steroid and antifibrosis agent injections are also beneficial in some cases of recurrent postoperative pterygium or corneal neovascularization. An excellent postoperative “cocktail” regimen for problematic patients includes 0.1 cc of 5-FU and 0.3 cc of dexamethasone, with 0.1 cc of lidocaine placed into the syringe last so the anesthetic reaches the eye first on the way into the subconjunctival space. This 0.5 cc injection can be given with a bevel-down, 30 gauge needle, tangential to the globe, and adjacent and posterior to the lurking recurrence site. The preparation includes the application of topical tetracaine gel and then, a couple of sterile cotton tip applicators that have been soaked in 4% lidocaine, placed serially into the inferior fornix and resting upon the injection site for several minutes prior to the 5-FU cocktail administration.
Bevacizumab
Bevacizumab (Avastin®; Genentech Inc., South San Francisco, CA, USA) subconjunctival injections provide another strategy to manage neovascularization in both the cornea and the conjunctiva. The surgeon utilizes readily available syringes prepared by a network of reputable compounding pharmacies. This cost-effective anti-VEGF solution minimizes expense to the patient and allows for other subsequent treatments that might otherwise instigate the formation of yet more new unwanted vessels. Subconjunctival bevacizumab can be used alone or in conjunction with argon laser phototherapy, to obliterate specific conjunctival feeder vessels or intrastromal corneal vessels (). The argon laser is typically set to repeat mode (as with a panretinal photocoagulation), 0.1 second duration, 100 micron spot size, and anywhere between 200 and 900 mW. The procedure is comfortable with topical anesthesia, and retinal safety can be enhanced with preoperative pilocarpine miosis. Interestingly, topical sodium fluorescein, routinely used on the ocular surface for clinical diagnostic purposes, is also activated at the 514 nanometer Argon green wavelength incorporated into generations of therapeutic ophthalmic lasers; thus, the simple application of topical fluorescein acts as a photoactivator, significantly enhancing the local effects of the laser, particularly in foci where inflammation may potentiate fluorescein absorption through damaged ocular surface cells and compromised vascular endothelial junctions.
Figure 3 Early pterygium recurrence 2 months after successful surgery.
Topical bevacizumab
Topical bevacizumab drops have also been advocatedCitation51 in the control of corneal and conjunctival neovascularization. A simple 1% solution can readily be prepared by a qualified compounding pharmacy and applied BID to QID over a period of several weeks to several months. This often enables a significant therapeutic antineovascular effect in the cornea and conjunctiva – superficial vessels remain more susceptible to all therapeutic efforts, while deeper interstitial vessels prove more recalcitrant.
Preferred surgical technique for mild pterygia
All pterygia, regardless of size or location (nasal, temporal, superior, or inferior) should be treated if an appropriate surgical technique, with the lowest recurrence rate and the maximum cosmetic effect, is available.
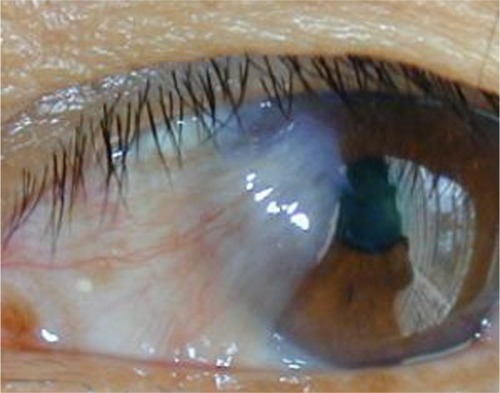
Smaller lesions without lid, caruncular, muscle, forniceal, or diffuse involvement can be remedied with a modified conjunctival rotational allograft technique (). Once the conjunctival lesion has been delineated with Wescott scissors, the pterygium is grasped and carried forward to reveal the limbal region, which must be excised with a 64 Beaver blade (Beaver Visitec International). This dissection is continued anteriorly until all abnormal corneal tissue is removed, hopefully en bloc. After excision, aggressive cleaning of the corneal bed, with the 64 blade, ensures optimal cosmesis and recurrence avoidance. The cleanup can be augmented by gentle superficial removal of scarred anterior corneal stroma and basement membrane, with 0.12 forceps or Maumanee forceps and a Vannas scissors.
Figure 4 Typical fibrovascular nasal pterygium amenable to a superior and inferior rotational conjunctival graft procedure.
Care should always be taken not to create a new deeper plane, which risks unwarranted ectasia and even the threat of iatrogenic perforation in some challenging preexisting ectatic cases. Some surgeons advocate the use of a rotating burr or AlgerBrush (The Alger Company, Lago Vista, TX, USA) to clean the irregular nasal corneal stroma, with variable success, while other surgeons find burrs to be an unnecessary substitute for precise dissection and clean up. Once mitomycin C 0.04% has been applied for the appropriate amount of time, it can be vigorously irrigated away with six separate consecutive washings. It is imperative to remove all residual mitomycin C. After each balanced salt solution (BSS) irrigation, residual saline can be quickly removed using a standard sterile 4 × 4 sponge applied directly to the surgical field.
Mitomycin C can be applied with small pieces of presoaked Weck-Cel sponge cut by the surgical technician. The best location is under the free edges of normal conjunctiva as well as directly upon the scarred corneal stroma and limbus. After excision and aggressive mitomycin C irrigation, there should be no stem cells or fibroblasts on the now bared limbus, so the rotational conjunctival tissue should be spared direct mitomycin C exposure. The astute surgeon should also consider pharmaceutical-grade Mitosol, which provides precut pledgets, precise concentration, and the convenience of a 2-year shelf life.
The rotational allograft is created by gently undermining the superior and the inferior limbus and bulbar conjunctiva immediately adjacent to the conjunctival excision site, then performing a snug peritomy limbal incision at the one- or two o’clock position. Rotation of the anterior limbal portion of each flap preserves limbal stem cell polarity while providing adequate closure tissue. Next, a double mattress suture is fashioned, starting with the scleral side of the superior flap, reentering approximately 5 mm away, then recreating the same mattress pattern inferiorly, with the last stroke passing the needle towards the sclera such that the knot will be buried. Prior to tying the double mattress suture, it must also be adequately anchored into the limbus, usually exactly at the three o’clock position in the right eye and the nine o’clock position in the left eye. This anchor suture must be deep enough into the limbus to avoid cheese-wiring, thereby relinquishing the limbal positioning and causing undesirable posterior migration of the conjunctival closure. The triple throw tie is carefully shimmied together, without placing excessive tension on the nylon suture until the wound is fully approximated. For larger dissections, excess tension with this suture can be relieved by loosening the lid speculum. Then the suture is cinched and the knot tied. The knot is closely trimmed and of course, covered by conjunctiva as well as the THBL at the conclusion of the case. Additional double mattress sutures are usually necessary posteriorly in order to fully close the wound, but anchor passes are not necessary and may be undesirable, particularly over the medial rectus muscle insertion.
Larger defects preclude this rotational technique. As it is imperative to leave no bare sclera, a covering must be provided, either from the superior ipsilateral host conjunctiva or from amniotic membrane. The former adds significant time to the case, while the latter adds significant expense. There is also a risk of pyogenic granuloma formation at the free autograft site, which should itself be closed with nylon suture. Tissue glue, either Tisseel or Evicel, provides excellent cosmesis and speedy surgery. Glue, unfortunately, adds another significant expense to the case, which may render this marvelous technology fiscally unacceptable for a nonhospital-based surgery center. Despite their inherent efficiencies, freestanding, physician-owned ambulatory surgery centers (ASCs) receive 60% of the facility fee given to hospital-based or -owned facilities. Furthermore, nonhospital ASCs are not allowed pass-through spending allowances for indicated supplies, such as amniotic membrane, tissue glue, some glaucoma shunts, donor cornea, donor sclera, pericardium, and many other implanted materials, including postevisceration and enucleation implants. Thus, these supplies can overconsume the facility fee and subject these procedures to a substantial fiscal loss. 10-0 nylon sutures are far more economical, albeit far less “high tech” than amnion or fibrin glue products.
Generally, the knots are very well tolerated and do not require removal in the clinic, even the nylon will resorb and dissolve over the course of 6–12 months. However, many patients manage to extrude the sutures while fully denying any rubbing behavior or excess physical activity. At bedtime, the use of a hard, clear eye shield, secured with paper tape, is routinely recommended for the first 3 weeks postoperatively, longer for patients who are agitated or have insomnia. When the sutures loosen, they can readily be removed at the slit lamp, with a 15 degree blue blade and jewelers forceps. Topical bromfenac and tetracaine (Tetravisc; Ocusoft, Rosenberg, TX, USA) provide more than sufficient analgesia for the vast majority of patients.
Case presentation
The operative approach to a typical case, as managed by one of the authors (AM) is presented ().
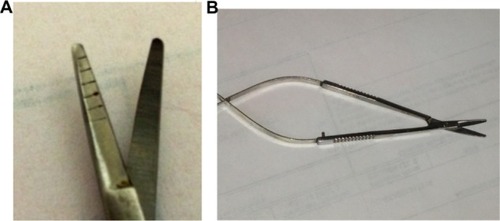
For the procedure, intravenous sedation was used. The eye was prepped and draped in the usual sterile fashion, and a lid speculum was inserted. Brimonidine tartrate (Alphagan P) drops were then applied topically to constrict the blood vessels for the subsequent control of hemostasis, and 2% lidocaine gel was applied for topical anesthesia. The pterygium and the semilunar fold were identified. Using a 0.12 forceps to pick up the semilunar fold, a peritomy was made in front of the semilunar fold and carried superiorly and inferiorly toward the fornix. Then, all of the underlying fibrovascular tissue was picked up, by the 0.12 forceps, as high as possible (to remove most from the medial and inferior fornix) until all visible medial rectus was free from the dense fibrovascular tissue. This was accomplished using the Mansur–Aran Graft Harvesting Pterygium Debunking Scissors (Rhein Medical Inc., St Petersburg, FL, USA) while the scissors were used to truncate it from the base (allowing the remaining tissue to retract into the deep fornix after truncation) (). This dissection left the muscle sheath, as well as the underlying Tenon’s capsule, intact – when the underlying Tenon’s capsule looks glistening, mobile, and thin, it is left intact. The above dissection was carried out superiorly and inferiorly toward the fornix. Hence, the entire pterygium head and body, including the underlying fibrovascular tissue, were dragged by the forceps all the way toward the nasal limbus, where the head was detached with blunt dissection. The remnant tissue over the cornea was cleared by grasping with a 0.12 forceps and #64 blade scraping or dental burr polishing. Sponges soaked in 0.04% mitomycin C were inserted under the surrounding conjunctiva, in the fornix space between the Tenon’s capsule and the conjunctiva from the limbus. A total of two or three sponges was used and incubated for 30 seconds. The edge of the mitomycin C sponge was bent to contact the cut conjunctival edges. After incubation, the sponges were removed, and the area was irrigated with a copious amount of BBS. Using two 0.12 forceps, one grasping the conjunctival edge and the other the edge of Tenon’s capsule, the gaps between the two were identified and sealed by applying one drop each of the two components of the fibrin glue. Thereafter, a free graft of superior conjunctiva containing limbal stem cells was placed on the recipient site and stretched with a surgical spatula. Next, using two 0.12 forceps, one grasping the conjunctival edge and the other the edge of Tenon’s capsule, the gaps in between the two were identified and sealed by sequentially applying one drop each of the two components of the fibrin glue.
Figure 6 Mansur–Aran Graft Harvesting Pterygium Debunking Scissors (Rhein Medical Inc., St Petersburg, FL, USA): (A) tip and (B) full view.
The harvesting site was then covered with conjunctiva glue in a sequential manner. The excess autograft tissue was trimmed, as was as the fibrin glue. Then, 0.220 cc of triamcinolone acetonide 40 mg/cc (Kenalog) was injected into the caruncle region, and topical tobramycin–dexamethasone (Tobradex, Alcon Laboratories, Inc.) ointment was applied. The eye was patched and shielded.
After surgery, topical besifloxacin was administered, one drop TID for 7 days, and then an LE suspension, one drop TID for 5 weeks, then finished, or titrated downward over a longer period of time according to the individual patient’s inflammatory response. documents the pterygium preoperatively and 2-weeks postoperatively.
In our center, the patient is generally seen 1 day later to look for conjunctival gaps only when the technique was aggressive. We have found that only about 1% of cases have a gap, which if greater than 2 mm can be re-glued in any chosen manner. The next follow-up visit is usually scheduled at 1 week, and the final follow-up visit is at 4–6 weeks; generally, 98% of all signs and symptoms of regrowth occur within this period of time. With regrowth, about 1% of cases show aggressive vessels, usually from the inferior cul de sac; these patients are treated with 0.1 cc of bevacizumab and 0.2 cc of triamcinolone acetonide, injected under the sites of aggressive vessels, with attention to the graft itself. Further follow up is then scheduled 2 weeks later, by which time about 99% of cases resolve; the remainder must undergo another surgical procedure.
Overall, this case illustrates the fact that pterygium surgery is very complex and requires vast surgical experience to accomplish the best end result – no recurrence and excellent cosmesis. Debunking of all the fibrovascular tissue, thorough cleaning of the medial rectus, and a limbo–conjunctival autograft with no Tenon’s capsule that covers all of the excised tissue area are vital. Tisseel allows secure placement of the graft, and the application of mitomycin C for 30 seconds in the cul de sac is also extremely important. The impregnation of the graft with triamcinolone acetonide prevents edema. The use of LE suspension, gel, or ointment for 5 weeks gives this surgical approach the final step to accomplish a successful result.
Conclusion
Despite two millennia of experience with this disease, the ultimate objective continues to be achieving a more thorough understanding of its pathogenesis, with the aim of developing interventions to reduce both pterygium incidence and postsurgical recurrence. Until that time, the immediate goal is to optimize surgical practices, to ensure the best possible outcomes. Based on the authors’ experience, LE, including the preservative-free ointment formulation, is a safe and effective component of the perioperative regimen for this complex ocular condition. Prospective clinical studies are needed to further clarify the role of LE in the management of pterygium.
Acknowledgments
The authors wish to thank Anthony Shardt, MD, of Churchill Communications (Maplewood, NJ) for writing assistance. This assistance was funded by Bausch & Lomb. The authors retained full control of the manuscript content.
Supplementary material
Discussion of conjunctival forceps design
The Sheppard conjunctival forceps (Bausch & Lomb, Rochester, NY, USA) has been specifically designed for the intricate needs of pterygium surgery (Figure S1). The long-handle Maumenee design body enables comfortable anatomical hand and wrist positioning parallel to the surgical field, with minimal view obstruction, even while working deep in the nasal canthus. The leveraged grasp reduces surgeon fatigue compared with the classic 0.12 tissue forceps. The angular 45-degree tip configuration allows full visualization of the grasped conjunctival tissues. The rounded rather than toothed Pierse tips prevent damaging button holes or tears in the conjunctiva during tissue manipulation, particularly when the tissue must be stretched during a rotational autograft or lengthy dissection.
Minimization of the use of cautery to the bare scleral surface avoids scarring, inflammation, and unsightly charcoal burns, as well as potential rare but disfiguring scleral ectasia and necrosis. Hemostasis can often be achieved simply with topical 1:10,000 epinephrine solution.
For suturing, a 10-0 nylon suture should be used. It is familiar, the needle is atraumatic, and most importantly, there is no tissue reaction whatsoever. The sutures often remain intact, subconjunctival, unnoticed, and inert until full hydrolysis occurs 6–18 months postoperatively. A double mattress technique should be used, to avoid cheese-wiring through the opposing conjunctival edges. In order to bury the double mattress suture knot subconjunctivally, the first pass should be made from the posterior conjunctival surface, and the last pass should be towards the posterior conjunctival surface. The most anterior conjunctival suture should be anchored in the limbal episcleral tissue with a third bite, to avoid posterior migration.
Fibrin tissue glue and amniotic membrane are outstanding adjuncts to successful surgery. However, for smaller lesions, the potential benefits could be outweighed by the relative costs: hundreds of dollars each for the glue and the membrane, compared with about $30 for the 10-0 nylon suture.
The duration of topical mitomycin C 0.04% treatment should be titrated to the patient’s risk of recurrence. Astute clinical judgment, based upon clinical experience, is required to match application duration to the individual patient’s needs: higher-risk patients should require longer exposure.
Disclosure
JDS, AM, and JAH are consultants for Bausch & Lomb. TLC is an employee of Bausch & Lomb. JDS is also a consultant for Abbvie, Alcon, Allergan, Merck, NiCox, Omeros, and Science Based Health. The authors report no other conflicts of interest in this work.
References
- AndersonDMDorland’s Illustrated Medical Dictionary31st edPhiladelphia, PASaunders2007
- JohnsonRDPaiVCHoftRHHistorical approaches to pterygium surgery, including bare sclera and adjunctive beta radiation techniquesHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated201220122736
- ChuiJJYCoroneoMTPterygium pathogenesis, actinic damage, and recurrenceHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated2012126
- JohnstonSCWilliamsPBSheppardJDJrA Comprehensive System for Pterygium Classification [abstract]Investig Ophthalmol Vis Sci2004452940
- ChuiJDi GirolamoNWakefieldDCoroneoMTThe pathogenesis of pterygium: current concepts and their therapeutic implicationsOcul Surf200861244318264653
- CoroneoMTDi GirolamoNWakefiledDThe pathogenesis of pterygiaCurr Opin Ophthalmol199910428228810621537
- Di GirolamoNChuiJCoroneoMTWakefieldDPathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinasesProg Retin Eye Res200423219522815094131
- van SettenGAspiotisMBlalockTDGrotendorstGSchultzGConnective tissue growth factor in pterygium: simultaneous presence with vascular endothelial growth factor – possible contributing factor to conjunctival scarringGraefes Arch Clin Exp Ophthalmol2003241213513912605268
- KheirkhahACasasVShehaHRajuVKTsengSCRole of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygiumCornea2008271566318245968
- KheirkhahANazariRNikdelMGhassemiHHashemiHBehrouzMJPostoperative conjunctival inflammation after pterygium surgery with amniotic membrane transplantation versus conjunctival autograftAm J Ophthalmol2011152573373821742306
- ComstockTLDecoryHHAdvances in corticosteroid therapy for ocular inflammation: loteprednol etabonateInt J Inflam2012201278962322536546
- AlberthMWuWMWinwoodDBodorNLipophilicity, solubility and permeability of loteprednol etabonate: a novel, soft anti-inflammatory steroidJ Biopharm Sci199122115125
- BodorNBuchwaldPSoft drug design: general principles and recent applicationsMed Res Rev20002015810110608921
- SamudreSSLattanzioFAWilliamsPBSheppardJDComparison of topical steroids for acute anterior uveitisJ Ocul Pharmacol Ther200420653354715684812
- BodorNLoftssonTWuWMMetabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonatePharm Res1992910127512781448425
- WuWMHuangFLeeYBuchwaldPBodorNPharmacokinetics of the sequential metabolites of loteprednol etabonate in ratsJ Pharm Pharmacol200860329129718284808
- AmonMBusinMLoteprednol etabonate ophthalmic suspension 0.5%: efficacy and safety for postoperative anti-inflammatory useInt Ophthalmol201232550751722707339
- BartlettJDHorwitzBLaibovitzRHowesJFIntraocular pressure response to loteprednol etabonate in known steroid respondersJ Ocul Pharmacol1993921571658345288
- HollandEJBartlettJDPaternoMRUsnerDWComstockTLEffects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteersCornea2008271505518245967
- HollandEJDjalilianARSandersonJPAttenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantationCornea200928101139114319770719
- NovackGDHowesJCrockettRSSherwoodMBChange in intraocular pressure during long-term use of loteprednol etabonateJ Glaucoma1998742662699713785
- StewartRHorwitzBHowesJNovackGDHartKDouble-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1J Cataract Refract Surg19982411148014899818338
- The Loteprednol Etabonate Postoperative Inflammation Study Group 2A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammationOphthalmology19981059178017869754192
- ComstockTLPaternoMRSinghAErbTDavisESafety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgeryClin Ophthalmol2011517718621383946
- FongRLeitritzMSiou-MermetRErbTLoteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trialClin Ophthalmol201261113112422888209
- RajpalRKRoelLSiou-MermetRErbTEfficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgeryJ Cataract Refract Surg201339215816723218817
- NguyenHTSamudreSSLattanzioFAJrWilliamsPBSheppardJDJrLoteprednol etabonate (Lotemax, 0.5%) reduces inflammatory response associated with pterygium progressionPoster presented at: the 2008 Meeting of the Association for Research in Vision and Ophthalmology (ARVO)April 27–May 1, 2008Fort Lauderdale, Florida
- NguyenHTSamudreSSLattanzioFAWilliamsPBSheppardJDLoteprednol etablonate reduces Inflammatory Response associated with Pterygium ProgressionInvestig Ophthalmol Vis Sci2008496027
- AngLPChuaJLTanDTCurrent concepts and techniques in pterygium treatmentCurr Opin Ophthalmol200718430831317568207
- KaufmanSCJacobsDSLeeWBDengSXRosenblattMIShteinRMOptions and adjuvants in surgery for pterygium: a report by the American Academy of OphthalmologyOphthalmology2013120120120823062647
- ZhengKCaiJJhanjiVChenHComparison of pterygium recurrence rates after limbal conjunctival autograft transplantation and other techniques: meta-analysisCornea201231121422142722643650
- ChoHChuckRSPterygium excision and placement of amniotic membrane graftsHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated201291100
- TananuvatNMartinTThe results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograftCornea200423545846315220729
- LuanratanakornPRatanapakornTSuwan-ApichonOChuckRSRandomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excisionBr J Ophthalmol200690121476148016837545
- HovanesianJABehesnilianASPterygium excision with a conjunctival autograft and prophylactic placement of subconjunctival amniotic membrane surrounding the excision siteHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated2012101110
- HardtenDRFibrin tissue adhesiveHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated20124954
- SinghPKSinghSVyasCSinghMConjunctival autografting without fibrin glue or sutures for pterygium surgeryCornea201332110410722735311
- AlmondMCDastrupBTKaufmanSC5-fluorouracil and mitomycin-C: adjuncts to pterygium surgeryHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated20125564
- RaiskupFSolomonALandauDIlsarMFrucht-PeryJMitomycin C for pterygium: long term evaluationBr J Ophthalmol200488111425142815489487
- MastropasquaLCarpinetoPCiancagliniMEnrico GallengaPLong term results of intraoperative mitomycin C in the treatment of recurrent pterygiumBr J Ophthalmol19968042882918703875
- RubinfeldRSPfisterRRSteinRMSerious complications of topical mitomycin-C after pterygium surgeryOphthalmology19929911164716541454338
- KheirkhahAHashemiHAdelpourMNikdelMRajabiMBBehrouzMJRandomized trial of pterygium surgery with mitomycin C application using conjunctival autograft versus conjunctival-limbal autograftOphthalmology2012119222723222153864
- SharmaAGuptaARamJGuptaALow-dose intraoperative mitomycin-C versus conjunctival autograft in primary pterygium surgery: long term follow-upOphthalmic Surg Lasers200031430130710928667
- YamamotoTVaraniJSoongHKLichterPREffects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblastsOphthalmology1990979120412102122349
- BekibeleCOAshayeAOlusanyaB5-Fluorouracil versus mitomycin C as adjuncts to conjunctival autograft in preventing pterygium recurrenceInt Ophthalmol20123213822246200
- KumarDAAgarwalAPterygium: postoperative management and complicationsHovanesianJAPterygium: Techniques and Technologies for Surgical SuccessThorofare, NJSlack Incorporated2012111120
- Torres-GimenoAMartínez-CostaLAyalaGPreoperative factors influencing success in pterygium surgeryBMC Ophthalmol2012123822873737
- HirstLWRecurrence and complications after 1,000 surgeries using pterygium extended removal followed by extended conjunctival transplantOphthalmology2012119112205221022892149
- SheppardJDScoperSVSamudreSTopical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye diseaseJ Ocul Pharmacol Ther2011271232721133792
- CoffeyMJDecoryHHLaneSSDevelopment of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic dropClin Ophthalmol2013729931223430378
- KimSWHaBJKimEKTchahHKimTIThe effect of topical bevacizumab on corneal neovascularizationOphthalmology Epub4242008