Abstract
Background
Nuclear sclerosis (NS) based on the Emery–Little classification and refractive values after lens-sparing vitrectomy was compared between proliferative diabetic retinopathy (DR) patients and nondiabetic patients.
Methods
Progression of NS based on the Emery–Little classification and changes in refractive values were compared between 13 proliferative DR patients (14 eyes, DR group) and 14 nondiabetic patients (14 eyes, non-DR group) who underwent lens-sparing vitrectomy. All patients revealed grade I NS based on the Emery–Little classification. Mean patient age and refractive value just after surgery were 56.07 years and −0.33 diopters (D) in the DR group, and 57.06 years and −0.96 D in the non-DR group.
Results
The Emery–Little classification in the DR group at 6 and 24 months postoperative were grade I (13 eyes)/grade II (one eye) and grade I (eleven eyes)/grade II (three eyes), respectively. Mean refractive values in the DR group at 6, 12, and 24 months postoperative were +0.28 D, +0.27 D, and +0.37 D, respectively. The Emery–Little classification in the non-DR group at 6 and 24 months (or preoperative for patients undergoing cataract surgery) were grade I (five eyes)/grade II (eight eyes) and grade I (zero eyes)/grade II (eight eyes)/grade III (five eyes), respectively. The mean refractive value in the non-DR group at 6 months postoperative was −3.20 D. All eyes exhibited myopic changes and progression of NS.
Conclusion
The findings of this study show that the progression of NS postvitrectomy is mild, even for DR patients 50 years of age or older, thus suggesting the need to reconsider the indications for simultaneous cataract surgery with vitrectomy.
Keywords:
Introduction
Vitreous surgery is used to treat many types of vitreoretinal disease, and it is often performed in phakic eyes as well as aphakic and pseudophakic eyes. It is reported that lens-sparing vitrectomy in patients over 50 years of age is associated with postoperative nuclear sclerosis (NS) and the development of myopia.Citation1–Citation5 The occurrence of progressive NS postvitrectomy was first described in patients undergoing vitrectomy for epiretinal membrane causing macular pucker.Citation1 Numerous reports have shown that as many as 98% of older individuals who undergo vitreous surgery will develop clinically significant NS within 2 years postoperative.Citation1 However, it has been reported that the progression of nuclear cataracts following vitrectomy for proliferative diabetic retinopathy (PDR) patients is slower than that in nondiabetic patients.Citation6 To date, there has been no report which compared postoperative refractive values between these two groups. The purpose of the present retrospective study was to investigate and compare the progression of NS based on both the Emery–Little classification and postoperative refractive values after lens-sparing vitrectomy between patients 50 years of age or older with PDR and similar-age nondiabetic patients.
Materials and methods
This study involved 13 patients (14 eyes) 50 years of age or older who underwent lens-sparing vitrectomy for PDR and were followed-up for ≥2 years postoperatively without complications (DR group), and a control group comprised of 14 nondiabetic patients (14 eyes) 50 years of age or older who underwent lens-sparing vitrectomy for other disorders (non-DR group). In both groups, the criteria for performing lens-sparing vitrectomy was that the patients have either no cataracts or only mild cataracts (grade I of the Emery–Little classification in all cases) which had little influence on visual acuity.
In the DR group, patient age ranged from 50 to 65 years (mean age: 56.07±5.77 years), and the condition leading to surgery was vitreous hemorrhage in eight eyes, macular traction retinal detachment in three eyes, and extramacular traction retinal detachment in three eyes. Fluid-gas (20% sulfur hexafluoride) exchange during surgery was performed in five eyes.
In the non-DR group, patient age ranged from 53 to 68 years (mean age: 57.06±5.05 years), similar to that in the DR group, and the primary disease being treated was branch retinal vein occlusion in four eyes, central retinal vein occlusion in two eyes, macular hole in four eyes (stage III in three eyes and stage IV in one eye), macular epiretinal membrane in three eyes, and age-related macular degeneration in one eye. All cases of branch retinal vein occlusion, central retinal vein occlusion, and age-related macular degeneration were complicated with vitreous hemorrhage. Fluid-gas (20% sulfur hexafluoride) exchange during surgery was performed in four eyes with macular hole.
Postoperative progression of NS was evaluated based on the Emery–Little classification and changes in refractive values. NS based on the Emery–Little grade and baseline refractive values just after surgery (in eyes with fluid-gas exchange: when the intraocular gas disappeared) and those at 6, 12, and 24 months postoperative were compared. The refractive values in all cases were measured by use of an autorefract/keratometer (ARK-1®; NIDEK Co., Ltd., Gamagori, Japan). In the DR group, refractive values in each patient’s fellow eye were also compared during the 24-months follow-up period.
Results
Based on the Emery–Little classification, 13 eyes remained grade I at 6 months and eleven eyes remained grade I, and only three eyes progressed from grade I to grade II at 24 months postoperative in the DR group. On the other hand, eight eyes progressed from grade I to grade II at 6 months, and all eyes progressed from grade I to greater than grade II (five eyes from grade I to grade III) at 24 months postoperative (or preoperative for eyes that underwent cataract surgery) in the non-DR group.
Mean refractive values in the DR group at 6, 12, and 24 months postoperative were +0.28 diopters (D), +0.27 D, and +0.37 D, respectively. Slight hyperopic changes tended to be seen, with almost no NS (). The mean refractive value in the non-DR group at 6 months postoperative was −3.20 D. The mean duration of intraocular gas tamponade was 14±0.9 days.
Table 1 Mean refractive values in the diabetic retinopathy group
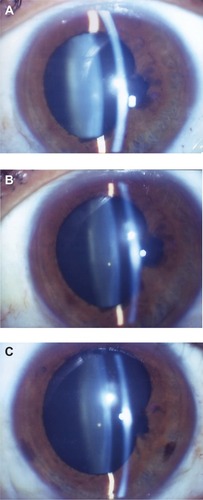
Changes in the Emery–Little grade and refractive values in the DR group showed no progression of NS in most of the operated eyes even after 24 months postoperative, yet the development of slight hyperopia was observed. In addition, no development of NS and myopic change were seen in the fellow eyes (). None of the DR-group eyes required cataract surgery during the follow-up period. In six patients (seven eyes) who were followed-up for ≥6 years postoperative, no significant myopic changes () or progression of NS was observed (–C).
Figure 1 Changes in refractive values.
Abbreviation: D, diopters.
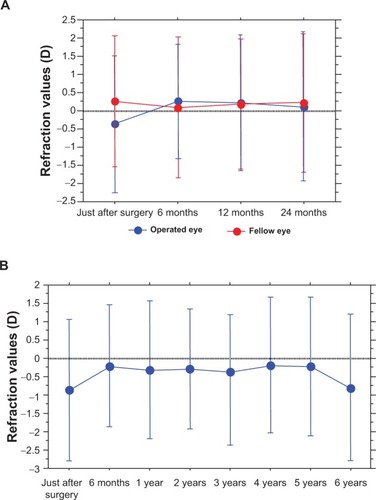
Figure 2 Change of nuclear sclerosis (NS) in a typical case of the diabetic retinopathy group.
Abbreviation: D, diopters.
Changes in refractive values in the non-DR group revealed significant myopic changes at 6 months postoperative, and eight eyes underwent cataract surgery due to the progression of NS from 6 to 12 months postoperative (). In addition, another five eyes underwent cataract surgery from 12 to 24 months postoperative. In all 13 eyes, the mean postoperative period until cataract surgery was 10.1±5.2 months. The corrected visual acuity of the patients who underwent cataract surgery ranged from 20/200 to 20/40. In the DR group, the mean refractive values of the cases with and without fluid-gas exchange at 24-months postoperative were +0.39 D and +0.36 D, respectively. In the non-DR group, the mean refractive values of the cases with and without fluid-gas exchange at 6 months postoperative were −3.24 D and −3.18 D, respectively. There were no differences in refractive values between the cases with and without fluid-gas exchange during surgery.
Table 2 Mean refractive values in the nondiabetic retinopathy group
Discussion
It has also been reported that with the progression of NS of the lens associated with aging, refractive values become more myopic.Citation7 In the present study, the evaluation of the progression of NS was based on the Emery–Little classification and the changes in refractive values. Our findings revealed that in the DR group, there was no progression of NS during the 24-months follow-up period following vitrectomy. However, significant progression of NS was observed in the non-DR group.
A higher rate of cortical and posterior subcapsular opacification and probability of cataract surgery has been reported in diabetic patients compared to nondiabetic patients.Citation8,Citation9 However, in those studies, diabetes mellitus was reportedly not associated with an increased risk of nuclear sclerotic cataract. Fluid-gas exchange reportedly increases the rate of progression of NS postvitrectomy,Citation10 yet no progression of NS was observed in the five eyes in the DR group in the present study that underwent fluid-gas exchange. However, the operated DR-group eyes did develop slight postoperative hyperopia. Although the cause is unclear, changes in anterior chamber depth following vitrectomy might be one reason in phakic eyes.Citation11
A previous study investigating the crystalline lens following vitrectomy for DR reported that moderate or greater progression of NS occurred in 7% of the cases.Citation12 Furthermore, it has occasionally been reported that less postvitrectomy NS occurs in DR patients compared to nondiabetic patients.Citation13,Citation14 It has also been reported that at the 2-year follow-up point following lens-sparing vitrectomy in a DR group compared to a non-DR group, a significantly lower rate of cataract extraction occurred in the DR group (P<0.006).Citation3 Moreover, the findings of the Age-Related Eye Disease Study Report No 5 showed a lower incidence of nuclear cataracts in diabetic patients (odds ratio, 0.68; 95% confidence interval, 0.47–0.98).Citation15 NS was originally thought to be less likely to progress in diabetic patients. Likewise, the findings of the present study showed no progression of NS in the DR-group eyes, even in the nonoperated eyes.
It should be noted that the underlying mechanism for less progression of NS in the DR-group eyes in the present study remains unclear. Previous studies have examined the distribution of oxygen in the intraocular fluids, the vitreous body, and the aqueous humor.Citation16,Citation17 Studies of retinal oxygenation revealed that much of the oxygen in the vitreous body is derived by diffusion from the retina.Citation18–Citation20 DR is reportedly associated with a decreased oxygen supply to the inner retina.Citation21,Citation22 Since the retina is the source of most of the oxygen in the vitreous body, it seems likely that diabetic patients would have decreased levels of oxygen in the vitreous body. Increased intraocular oxygen concentration is reportedly a risk factor for the progression of NS,Citation23,Citation24 and a previous study reported the significantly lower intravitreal oxygen tension in diabetic patients than in nondiabetic patients (P<0.001) as a reason for less progression of NS in diabetic patients.Citation25 Lower oxygen tension results in reduced production of activated oxygen in nuclear proteins, which may inhibit the progression of lens NS in diabetic patients.Citation26
Another possibility is that hyperglycemia may play a protective role against the oxidative damage. A previous study reported that high glucose induces an increase in antioxidant enzyme levels in human endothelial cells;Citation27 however, it was speculated in that study that hyperglycemia may cause increased production of free radicals, and evidence supports a prominent role for these reactive molecules as mediators of endothelial cell dysfunction in diabetes. Those findings appear to be contradictory, so further investigation is necessary to fully elucidate the mechanism for less progression of NS in diabetic patients.
The vision loss attributable to NS may easily be overestimated in patients with diabetes after undergoing vitrectomy. With vitrectomy of a phakic eye in patients 50 years of age or older in Japan, lensectomy also tends to be performed. Reportedly, lensectomy, particularly in diabetic patients, may also increase the incidence of postoperative neovascular glaucoma.Citation28,Citation29 The findings of this present study suggest the need to reconsider the indications for simultaneous cataract surgery with vitrectomy.
Acknowledgments
The authors wish to thank John Bush for reviewing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- CherfanGMMichelsRGde BustrosSEngerCGlaserBMNuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular puckerAm J Ophthalmol199111144344382012145
- OguraYTakanashiTIshigookaHOginoNQuantitative analysis of lens changes after vitrectomy by fluorophotometryAm J Ophthalmol199111121791831992738
- MichelsRGVitrectomy for macular puckerOphthalmology19849111138413886514308
- LeonardRE2ndSmiddyWEFlynnHWJrFeuerWLong-term visual outcomes in patients with successful macular hole surgeryOphthalmology199710410164816529331206
- ThompsonJTGlaserBMSjaardaRNMurphyRPProgression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holesAm J Ophthalmol1995119148547825689
- SmiddyWEFeuerWIncidence of cataract extraction after diabetic vitrectomyRetina200424457458115300079
- SamarawickramaCWangJJBurlutskyGTanAGMitchellPNuclear cataract and myopic shift in refractionAm J Ophthalmol2007144345745917765431
- YataKFujiwaraTYamamotoAItoKTsuyamaYDiabetes as risk factor of cataract: differentiation by retroillumination photography and image analysisOphthalmic Res199022Suppl 178802388757
- WestSKValmadridCTEpidemiology of risk factor for age-related cataractSurv Ophthalmol19953943233347725232
- ThompsonJTThe role of patient age and intraocular gases in cataract progression following vitrectomy for macular holes and epiretinal membranesTrans Am Ophthalmol Soc200310148549814971590
- LiYYangCXQingGPWeiWBChanges in anterior chamber depth following vitrectomyChin Med J (Engl)2013126193701370424112167
- NovakMARiceTAMichelsRGAuerCThe crystalline lens after vitrectomy for diabetic retinopathyOphthalmology19849112148014846521988
- de BustrosSThompsonJTMichelsRGRiceTAVitrectomy for progressive proliferative diabetic retinopathyArch Ophthalmol198710521961993813949
- SchachatAPOyakawaRTMichelsRGRiceTAComplications of vitreous surgery for diabetic retinopathy. II. Postoperative complicationsOphthalmology19839055225306192378
- Age-Related Eye Disease Study Research GroupRisk factors associated with age-related nuclear and cortical cataract: a case-control study in the Age-Related Eye Disease Study, AREDS Report No 5Ophthalmology200110881400140811470690
- MaedaNTanoYIntraocular oxygen tension in eyes with proliferative diabetic retinopathy with and without vitreousGraefes Arch Clin Exp Ophthalmol1996234Suppl 1S66S698871152
- KwanMNiinikoskiJHuntTKIn vivo measurements of oxygen tension in the cornea, aqueous humor, and anterior lens of the open eyeInvest Ophthalmol19721121081145009103
- AlderVAYuDYCringleSJVitreal oxygen tension measurements in the rat eyeExp Eye Res19915232932992015858
- ItoYBerkowitzBAMR studies of retinal oxygenationVision Res20014110–111307131111322975
- YuDYCringleSJAlderVAThe response of rat vitreal oxygen tension to stepwise increases in inspired percentage oxygenInvest Ophthalmol Vis Sci19903112249324992265989
- RivaCELogeanEFalsiniBVisually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retinaProg Retin Eye Res200524218321515610973
- ShonatRDOxygen delivery to the retina and related visual pathology. Brief reviewAdv Exp Med Biol200351024925412580436
- BarbazettoIALiangJChangSZhengLSpectorADillonJPOxygen tension in the rabbit lens and vitreous before and after vitrectomyExp Eye Res200478591792415051473
- HolekampNMShuiYBBeebeDCVitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formationAm J Ophthalmol2005139230231015733992
- HolekampNMShuiYBBeebeDLower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataractAm J Ophthalmol200614161027103216765670
- HolekampNMBaiFShuiYBAlmonyABeebeDCIschemic diabetic retinopathy may protect against nuclear sclerotic cataractAm J Ophthalmol20101504543550e120688316
- CerielloAdello RussoPAmstadPCeruttiPHigh glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stressDiabetes19964544714778603769
- BlankenshipGWThe lens influence on diabetic vitrectomy results. Report of a prospective randomized studyArch Ophthalmol19809812219621987447772
- RiceTAMichelsRGMaguireMGRiceEFThe effect of lensectomy on the incidence of iris neovascularization and neovascular glaucoma after vitrectomy for diabetic retinopathyAm J Ophthalmol19839511116184998
