Abstract
Nystagmus refers to involuntary, typically conjugate, often rhythmic oscillations of the eyes. The most common cause of nystagmus in children is infantile nystagmus syndrome (INS). INS presents within the first few months of life and is sometimes accompanied by an ocular condition associated with sensory impairment. Because this condition affects a person throughout life, it is important to understand the options available to manage it. This review focuses on the underlying nystagmus etiology, psychosocial and functional effects of nystagmus, as well as current principles of management, including optical, pharmacological, surgical, and rehabilitative options. Currently, the neural mechanisms underlying INS are not fully understood. Treatment options are designed to increase foveation duration or correct anomalous head postures; however, evidence is limited to mainly pre- and post-study designs with few objective comparisons of treatment strategies. Management of INS should be individualized. The decision on which treatment is best suited for a particular patient lies with the patient and his/her physician.
Introduction
Nystagmus refers to involuntary, typically conjugate, often rhythmic oscillations of the eyes. There are three types of nystagmus that are most likely to be encountered in children: infantile nystagmus syndrome (INS), fusion maldevelopment syndrome nystagmus (previously known as latent/manifest latent nystagmus), and spasmus nutans. This review focuses on INS. Some believe that fusion maldevelopment syndrome nystagmus represents a monocular form in the same spectrum as INS.Citation1 The term congenital nystagmus is often used synonymously with INS; however, it is technically incorrect as nystagmus does not typically develop at birth but more likely at 2–3 months of age. INS may be associated with retinal or optic nerve maldevelopment (previously known as sensory nystagmus) or may occur in isolation (previously known as congenital motor nystagmus). Conditions commonly associated with nystagmus include albinism, aniridia, achromatopsia, cone dystrophy, optic nerve hypoplasia, foveal hypoplasia, congenital cataracts, corneal opacities, retinopathy of prematurity, Leber congenital amaurosis, and syndromic causes associated with early-onset retinal degenerations. The nystagmus associated with visual sensory deficit is identical to that which presents in isolation; however, acuity is typically worse in those with sensory deficit.Citation2
Assessment of nystagmus
When assessing an infant or child with nystagmus, it is important to determine the age of onset as well as the child’s birth, developmental, medical, and family history. It is imperative to identify an underlying etiology if present, as the associated ocular or associated systemic condition may require intervention. Newer high-resolution imaging modalities such as optical coherence tomography (OCT) are increasingly being used for determining the cause of INS in children. The ability to use imaging to augment the clinical evaluation is important as patients are often misdiagnosed as having idiopathic INS, when in fact there is an underlying ophthalmic diagnosis and foveal maldevelopment can be found on OCT.Citation3 Neuro-imaging should be considered when nystagmus onset is after 3 months and not associated with an underlying sensory deficit or when associated with optic nerve hypoplasia.Citation4
Clinically, the evaluation of nystagmus includes measurement of best-corrected visual acuity and a description of characteristics of the waveform (eg, direction, type, amplitude, frequency) as well as documentation about any head turns or tilts and location of the null point, if present. To further characterize nystagmus, eye movement recordings are utilized. These recordings may be used to determine change after medical or surgical intervention. There are at least three methods used to quantify foveation characteristics in patients with nystagmus. The first, developed in Dell’Osso’s lab, is the Expanded Nystagmus Acuity Function (NAFX) ().Citation5 Two additional measures (the nystagmus optimal fixation functionCitation6,Citation7) have been developed that are fully automated and correlate well with the NAFX.
Figure 1 Fixation recording in a patient with oculocutaneous albinism collected using a 30 Hz eye tracker.
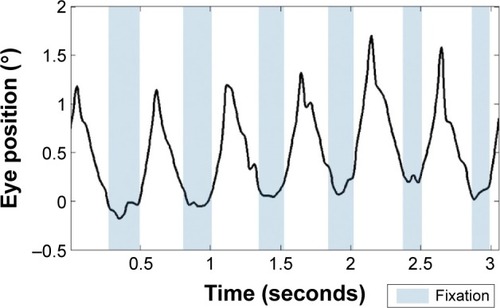
Dell’Osso and DaroffCitation2 identified at least 12 waveforms that range from pure pendular to pure jerk. While not clinically evident, the intermediate waveforms are often punctuated by embedded saccades that increase the time the eye position is on the fovea. Nystagmus with acceleration of movement during the slow phase is considered characteristic of infantile nystagmus.Citation8 The earliest form of infantile nystagmus seen tends to be pendular and develops into a jerk form in the first 2 years of life.Citation8 The presence of a null point or zone is also characteristic of infantile nystagmus. The null point is typically within 10° of fixation with lateral head turns the most common adaptation.Citation9 How the null point develops is poorly understand and has received surprisingly little research attention. The absence of illusory movement of the visual world (oscillopsia) is a characteristic of infantile nystagmus, which separates it from acquired forms. Visual perception is normally suppressed during saccades to prevent smear of the visual image.Citation10 It has been postulated that the eye movement generation systems produce a copy of the nystagmus eye movement to be executed and send it to higher cortical areas. This “efference copy” serves as the template for the expected movement at the cortical level.Citation11 Since there is a match between the true eye movement and the expected eye movement, no perception of visual motion is generated. In acquired nystagmus, a mismatch between the expected and actual location occurs, or possibly, multiple signals are misinterpreted, resulting in the illusion of motion of the visual world.
Genetic analysis may also further define what appear to be idiopathic cases. INS may be inherited in a dominant, recessive, or X-linked fashion. Five different chromosomal abnormalities (NYS1–5) have been linked to INS. Mutations in the X chromosome at locus q26 have been associated with typical clinical INS as well as periodic alternating nystagmus. The FRMD7 gene associated with this defect is found in areas of the brain involved in eye movement control and may play a role in neurite development. The defect has a relatively high penetrance in females. Mutations in FRMD7 have been found in less than 10% of sporadic cases.Citation12
Mechanisms for development of nystagmus
The underlying mechanism of infantile nystagmus is not fully understood, although theories abound that attempt to explain its development.Citation13,Citation14 Most implicated defects are found in fixation, saccades, the optokinetic reflex, the neural integrator, or the pursuit eye movement systems. No single theory has as yet been able to explain the development of the condition in all its complexity in an oculomotor movement system that largely has intact saccades, pursuits, and vestibulo-ocular reflex (VOR) movements outside the nystagmus.Citation15,Citation16 Three systems play a role in maintaining a stable image on the retina: fixation, the VOR, and gaze control. During the first few weeks of life, fixation is poor as the fovea is maturing, and orientation to targets is largely mediated by subcortical pathways through the superior colliculus.Citation17 This rapidly changes as top-down pathways from the cerebral cortex establish greater control of eye movements at approximately 2–3 months of age.Citation18 Larger waveform saccadic oscillations and intrusions have been reported to precede the onset of the infantile nystagmus waveform.Citation2
The vestibulo-ocular system holds the eyes stable during head movement. The VOR is one of the earliest developing of the image stabilization control systems. In patients with INS, the VOR system appears to function normally with a superimposed nystagmus.Citation16 Invoking the VOR, head movement, or head shaking does not suppress movement or improve visual acuity in nystagmus.Citation19
The gaze control system holds the eyes stable when they are in an eccentric position and is coordinated by the neural integrator.Citation20 The neural integrator is proposed to convert the velocity of the head’s movement generated by the VOR to a neural signal that is to be sent to the eye muscles for target tracking. In gaze-evoked nystagmus, it is presumed that the neural integrator becomes “leaky” allowing the eyes to drift away from the object of regard with a correcting saccade back to the target. During early infant development, it is likely that the neural integrator is “calibrating” itself. Inappropriate input or feedback during this calibration period could lead to a lifelong error signal. A number of theories of the development of nystagmus have postulated that errors in the inputs, outputs, or feedback loops within the neural integrator are at fault in the development of infantile nystagmus.Citation21–Citation23
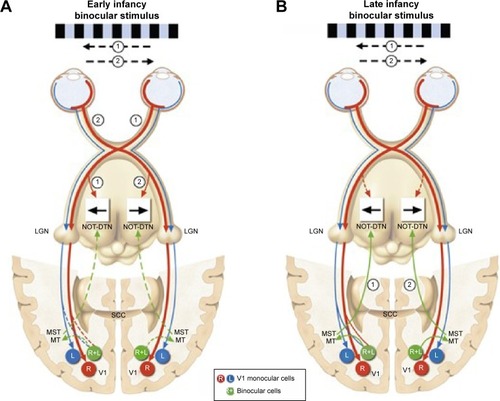
The optokinetic system is fundamental for maintaining a steady image as image flow moves across the retina. It is functionally present shortly after birth for large slow-moving targets. Optokinetic nystagmus (OKN) is nystagmus in response to stimuli drifting across the subject’s visual field. OKN’s similarity to nystagmus waveforms has led to theories that propose it as a possible driver of INS. The OKN response is mediated through the dorsal terminal nucleus, lateral terminal nucleus, and medial terminal nucleus in the pretectum which collectively comprise the accessory optic system (AOS) as well as the nucleus of the optic tract (NOT).Citation24 Symmetry in the monocular OKN develops by months 3–5 as binocular-driven motion pathways from the middle temporal and medial superior temporal cortex provide greater control and effectively override any direct retinal input ().Citation24 Brodsky and Dell’Osso have recently proposed that delays in cortical pathway maturation could allow the retinal–AOS–NOT system to act unimpeded with the resulting development of infantile nystagmus.Citation1 They have posited the unimpeded AOS–NOT system activity as the unifying process of infantile nystagmus. Tychsen et al has suggested that a failure of binocular cortical connections could also lead to nystagmus in and of itself.Citation25 These two opposing theories are referred to as bottom-up (Brodsky) or top-down mechanisms (Tychsen). While connections may not be fully developed for several months, the cortex does appear to have some very early connectivity with brainstem structures. Infants born without cortical structures do not develop OKN, and OKN is severely impaired in early cortical damage.Citation26,Citation27
Figure 2 Depiction of the brain, as viewed from above, showing normal cortical and subcortical projections during early human development.
Abbreviations: L, left eye monocular cells; LGN, lateral geniculate nucleus; R, right eye monocular cells; R+L, cortical binocular cells; SCC, splenium of the corpus callosum; V1, primary visual cortex.
The role that the eye muscles might play in nystagmus has received little attention. Biopsy of ocular muscle from nystagmus surgery patients has shown that there are more centrally nucleated muscle fibers with greater variation between them than is seen in normal extraocular muscle tissue.Citation28 Unique proprioceptors are found within the extraocular muscles and appear to be altered in INS compared to normal. It is possible that proprioceptor function changes after muscle surgery for nystagmus.Citation29
Brain imaging studies are also relatively rare. Hüfner et al using a voxel-based morphology analysis, found that the areas associated with motion processing in middle temporal lobe and areas near the cerebellar vermis had greater volumes in those with higher amplitude infantile nystagmus than those with lower amplitudes.Citation30 Schlindwein et al reported positron emission tomography scan results in a patient with infantile pendular nystagmus that showed increased activity in the cerebellar nodulus, an area thought to be associated with the torsional optokinetic response.Citation31 Leguire et al report that activity in the cerebellar declive, a subregion of the vermis, shows increased activity when the gaze is focused away from the null zone compared to when the gaze is directed into the null zone.Citation32 Harris has pointed out that care needs to be taken while interpreting these results and that changes in function in the cerebellum may be a result of, rather than a cause of, nystagmus.
Functional implications and quality of life
Relatively little is known about vision-specific, health-related quality of life in children with vision impairment. The few studies that do exist evaluated groups of children with varied etiologies for vision impairment, only some of which are associated with nystagmus. Most studies of the effects of nystagmus on quality of life and visual function have been conducted using adult participants. There is clearly a need for more research in this area.
DeCarlo et al investigated the effects of pediatric vision impairment on quality of life using parallel focus groups of children with vision impairment and their parents. Nystagmus was present in 76% of participating children.Citation33 The focus groups utilized a semi-structured script that covered topics related to general vision, mobility, school functioning, social relations and activities, self-care, medical treatment, and family impact. In focus groups of children, the largest number of negative comments pertained to the psychosocial aspects of their vision impairment. One child was noted to say “It’s weird when people look at my eyes. They’re like what’s wrong with your eyes, stop doin’ that and I can’t….”Citation33 Parents also reported difficulties with teasing because of nystagmus but expressed the most negative comments about school functioning. The negative effects of nystagmus are twofold: it is an easily observable manifestation of their ocular condition that makes them different from their peers, and it is associated with decreased vision which can also have negative effects if appropriate accommodations are not made. However, there were also a substantial number of positive comments, specifically with regard to things they could do, and the beneficial effects of adaptive technology and accommodations as well as positive peer support from friends.
In a study of adults with nystagmusCitation34 using a structured interview, 18 out of 21 participants specifically mentioned difficulties with the cosmetic appearance of nystagmus as well as other’s avoidance responses. Participants also reported poor self-esteem and increased dependency. In contrast to the previously discussed study of childrenCitation33 that reported both positive and negative experiences, this studyCitation34 revealed universally negative experiences about living with nystagmus.
Another study using the VF-14 and a social function questionnaire mailed to members of the Nystagmus Network UK found that the impact of nystagmus on visual function was low compared to other chronic eye conditions that result in low vision.Citation35 They also found that children with nystagmus had poorer social function than adults with nystagmus and that poor visual function was associated with poor social function. However, the authors caution that membership in the Nystagmus Network UK may be biased toward those with poorer visual function.
Children with vision impairment on average read slower than their normally sighted peers. The majority of children with vision impairment have nystagmus; however, no studies have focused solely on those children with nystagmus. Barot et al’s study of reading in adults with nystagmusCitation36 found that maximum reading speeds were 18.8% slower in participants with albinism and 14.7% slower in those with idiopathic infantile nystagmus when compared to normally sighted controls. Woo and BedellCitation37 also studied adults with nystagmus and found that they read more slowly than controls when using either continuous text or rapid serial visual presentation (RSVP) text. Continuous text was read more quickly, presumably due to a lack of contextual clues in RSVP text. The most significant finding of this work was that reading speed exceeded the nystagmus frequency, suggesting that reading is still occurring during non-foveating periods. Readers with INS sometimes read much better than expected; this may be due to the strategies they use to manipulate their nystagmus to best obtain information from text.Citation38 Nystagmus in and of itself cannot be the root cause of reading difficulties in children with nystagmus.
Nystagmus waveforms have been reported to be favorably,Citation39 negatively,Citation40 and unaffectedCitation41 by increased visual demand. The differences in these findings have been suggested to be related to the importance of the visual task.Citation41 This has implications for how the relationship between nystagmus characteristics and visual acuity should be measured post-intervention. Typically, the two are not measured simultaneously, where the changes of interest might be noted.Citation39
There are many anecdotal reports of increased nystagmus intensity under conditions of stress, but only few studies have quantified it. Cham et alCitation42 induced stress by having participants perform mental arithmetic orally, while they were responding to a Landolt C visual acuity task via a keyboard. A reward/penalty system was utilized for correct/incorrect responses. Heart rate as well as amplitude, frequency, and intensity of nystagmus increased during the task, although the two did not correlate. To further investigate this relationship, Jones et alCitation43 used a two-alternative forced choice staircase procedure for Landolt C visual acuity under conditions of acclimation and relaxation as well as when subjects received a small electrical shock for incorrect answers (task demand condition) or at random intervals (anticipatory anxiety). They found that although nystagmus amplitude and intensity significantly increased while foveation periods decreased, visual acuity was not reduced. Shock increased reaction times, and INS participants were slower to respond than controls, consistent with reports that nystagmus contributes to a “slow-to-see” phenomenon.Citation44
Interventions for nystagmus
Refractive management
Interventions for INS all strive to increase fixation stability (by lengthening foveation time and increasing accuracy during foveation).Citation45 Improving cosmesis by decreasing the nystagmus intensity (amplitude × frequency) is a secondary goal but one that is often very important to patients. The first intervention to be considered is optimal refractive correction, as children with nystagmus commonly have refractive error, especially with-the-rule astigmatism.Citation46,Citation47 This is important even at young ages, as the prevalence and magnitude of with-the-rule astigmatism increase with age, and there is little evidence of emmetropization through age 8.Citation48 Adding yoked prism to the spectacles of patients with anomalous head posture (apex pointed in the direction of the patient’s gaze) can be helpful in alleviating the anomalous head posture when the angle is small. We have found that prism greater than 10 prism diopters becomes heavy and cosmetically unacceptable to most patients even when using high-index lenses. Using Fresnel prism will degrade image quality, so this technique is typically reserved for patients with small head turns. Prism may also be useful for patients with fusion whose nystagmus dampens in convergence. In this case, base-out prism may be trialed binocularly to stimulate convergence (additional minus lens power must be incorporated into the spectacles to compensate for convergence-induced accommodation). Positive response to prism treatment is a good predictor of response to surgical management.Citation49
Contact lenses can also be considered. Many patients needlessly worry that due to their nystagmus, contact lenses will not be an option. Contact lenses have the advantage that they maintain the optical center of the correction on the patients’ visual axis, which may make them preferable to spectacle lenses, especially for those with high refractive errors. Contact lenses broaden the high foveation-quality field, presumably through afferent feedback from the ophthalmic division of the trigeminal nerve.Citation50 Most reports are case studies and have conflicting results.Citation49,Citation51,Citation52 However, the only randomized controlled trial published to dateCitation53 found no differences in nystagmus intensity comparing soft contact lenses, rigid gas-permeable lenses, and spectacles using an unmasked cross-over study design. They did, however, find a statistically but not clinically significant decrease in acuity with soft contact lenses compared to spectacles or gas-permeable contact lenses. Pharmacological and/or surgical treatment of INS is typically initiated after optimal refractive correction has been in place, but visual acuity or function is still not satisfactory or there is an anomalous head posture present.Citation54
Pharmacological treatment
Several case reports have described the benefit of various drugs in treating INS. Diethylpropionate was reported to improve visual acuity, stereopsis, exotropia, and ocular motility recordings in a female adult with INS.Citation55 Ocular motility recordings showed increased foveation time and broadened null zone. Another report showed improvement in visual acuity and nystagmus intensity after smoking 10 mg of cannabis.Citation56 Botulinum toxin injections have also been shown in a case series to be beneficial.Citation57
While several studies have been done on pharmacological treatment of acquired nystagmus, little research has been conducted on the treatment of INS.Citation58 One such study looked at the effect of baclofen on infantile periodic alternating nystagmus.Citation59 The proposed mechanism of action is to restore inhibitory control of the gamma-aminobutyric acid (GABA)-mediated vestibular nuclei with baclofen, a GABA agonist. Baclofen was started at 15 mg and increased until the patient felt a significant improvement clinically. Four out of eight patients had improved binocular Snellen visual acuity, and four reported improvement in head posture; however, four patients had to withdraw due to side effects. The authors concluded that a trial of baclofen in periodic alternating nystagmus might be valuable before considering surgical intervention. A cross-over, double-masked trial of topical brinzolamide (Azopt) versus placebo in treating INSCitation60 showed improvement in nystagmus waveform characteristics and NAFX in all five patients. Four out of the five patients also had improved visual acuity. The mechanism of action of carbonic anhydrase inhibitors on INS is not well understood.
Gabapentin and memantine have also been shown to be beneficial in acquired nystagmus. Gabapentin binds a subunit of voltage-dependent calcium channels. Memantine selectively blocks excess glutamatergic activity. However, the mechanism by which these drugs reduce nystagmus is not known.Citation58 One small study reported improvements in visual acuity and nystagmus amplitude in both acquired nystagmus and INS.Citation61 These early reports of improvement led to a randomized, placebo-controlled trial of gabapentin and memantine in 48 adults with INS.Citation58 The starting dose of memantine was 5 mg and could be increased up to 40 mg, while gabapentin was started at 300 mg and increased up to 2,400 mg. There was a statistically significant improvement in visual acuity for both treatment groups over the placebo group. Interestingly, the idiopathic infantile nystagmus group had a significant visual acuity improvement, while those with secondary nystagmus (infantile nystagmus due to ocular pathology) did not. Eye movement recordings also showed a statistically significant improvement in foveation and decrease in nystagmus intensity in all positions of gaze for both the drugs. However, self-reported subjective improvements in vision were equal among the three groups. There were few adverse effects in both treatment groups, and 13 patients from each group opted to continue treatment after the study ended. The authors concluded that pharmacologic treatment of INS is possible and reported that the study revealed no difference between the effects of memantine and gabapentin.
Surgical treatment
Surgery for nystagmus has been performed since the 1950s when Kestenbaum and Anderson first proposed surgical alleviation of abnormal head posture (AHP).Citation62 The aim of surgery for nystagmus is to correct AHP or to improve visual acuity by decreasing nystagmus amplitude or frequency. Patients with INS are candidates for surgery when they have AHP because of an eccentric gaze null point and strabismus, or when nystagmus and visual acuity improve with convergence.Citation63 The most common surgeries performed involve extraocular muscle recession, resection, tenotomy, or a combination of these.
Another less commonly performed procedure called artificial divergence surgery is based on the observation of dampened nystagmus with convergence.Citation62 The surgery aims to create a divergent resting position (exophoria) that must be overcome by fusional convergence, thus decreasing the nystagmus intensity. Preoperative fusional amplitudes must be measured to assure adequate potential.
Recession and resection procedures
KestenbaumCitation64 and Anderson’sCitation65 recession and resection procedures were initially intended to correct AHP; however, visual acuity also improved in some cases. Anderson recommended recession of the recti muscles whose action was in the direction of the face turn. Kestenbaum performed resections and recessions on all four horizontal recti. The goal was to move the eyes in the direction of the face turn, thus placing the eccentric null zone in primary position to decrease the AHP.Citation68 Their procedures were modified by ParksCitation66 and Calhoun and HarleyCitation67 to reduce the rate of recurrence due to undercorrection.Citation68 These alterations have led to the most commonly performed procedure, known as the augmented modified Kestenbaum procedure. The appropriate goal of this procedure is to improve cosmesis and reduce AHP with a small potential for improvement in visual acuity.Citation68
Several studies have looked at the effects of multiple variations of the Kestenbaum procedure on AHP, visual acuity, and eye movements in infantile nystagmus. Kumar et al used an Anderson–Kestenbaum procedure or a modified Anderson (recession of yoke muscles) procedure with or without tenotomy in 28 patients (mean age 10.9).Citation69 After excluding five patients with 20/20 vision, 12 patients had improved acuity, and the rest were unchanged. There was no difference in visual acuity or residual AHP between the surgical techniques. In another prospective study, 58 patients with infantile nystagmus were assigned to one of three groups based on preoperative measures of AHP, visual acuity, and heterotropia.Citation70 Each group underwent a different variation of recession and resection procedure to correct the AHP. Overall, they saw significant improvement in distance monocular visual acuity in all groups. Interestingly, mean distance binocular visual acuity only improved when two horizontal rectus muscle recessions were performed and in INS subjects with sensory impairment. Eye movement recordings showed changes in nystagmus intensity in all groups; however, the change was only significant for the four recti muscle recession surgery.
Other variations of recession and resection procedures have also been reported for INS, including symmetric recession of all four horizontal recti muscles with new insertion posterior to the equator causing less rotational effect on the globe.Citation71 Initial reports showed significant improvement in visual acuity, amplitude, and AHP without significantly limiting ocular motility.Citation72,Citation73 Twelve patients underwent this procedure, and 63.6% had increased visual acuity, and 81.8% had decreased nystagmus amplitude and frequency with no limitations on ocular motility.Citation71 The effects were more beneficial to the subjects with idiopathic INS than sensory INS. Recession procedures also have the advantage of being reversible. A retrospective study of this procedure showed an improvement in AHP in addition to visual acuity.Citation74 Out of 20 patients, AHP was present in seven patients preoperatively, and only two surgeries were planned to address the AHP. Still, AHP improved in all patients. Because AHP was not treated in five of the subjects, these findings may be due to a decrease in primary gaze nystagmus.
Most studies look at the effect of horizontal rectus muscle recession and resection on horizontal AHP. There are fewer reports on the effects of vertical rectus or oblique muscle surgery on AHPs with INS. Hertle et al looked at the effect of surgery in 24 patients with chin up or down AHP of greater than 10°.Citation75 Each patient had horizontal nystagmus with decreased intensity in vertical eccentric null gaze. Thirteen patients with chin-down position (up gaze null) received bilateral superior rectus recession, bilateral inferior oblique recession, resection, or tenotomy of one horizontal rectus in each eye. Eleven patients with chin-up position (down gaze null) had bilateral inferior rectus recession, bilateral superior oblique tenectomy, and the same treatment on horizontal recti. Postoperatively, there was a 12%–15% symmetric, bilateral limitation in vertical gaze in the direction of the recessed vertical rectus. There was a significant improvement in vertical head posture after surgery; however, none showed complete resolution. In addition, there was a statistically significant improvement in both visual acuity and foveation time.
There is a risk of anterior segment ischemia with recession and resection of all four horizontal recti.Citation76 When resection is utilized, blood vessels supplying the anterior segment can be severed which can complicate further procedures for AHP. One retrospective study utilized a tucking procedure instead of resection as a variation of Kestenbaum procedure.Citation77 On long-term follow-up (36/42 subjects), median head turn was 10°. Out of the 36 patients, 72% had residual head turn less than 10°, and 83% had head turn of 15° or less. Importantly, there were no reported long-term anterior segment alterations.Citation77
Tenotomy
Improved visual acuity and foveation with Kestenbaum procedures led to the hypothesis that tenotomy with reattachment at the original insertion would have a similar effect.Citation78 The mechanism of action is believed to be an interruption of the afferent proprioceptive loop, possibly tied to recently discovered nerve endings at the tendino-scleral interface in the extraocular muscles.Citation79 This interruption will produce a dampened peripheral oculomotor response to the nystagmus signal. Hertle et al looked at the effect of horizontal rectus muscle tenotomy in five children with INS.Citation79 Postoperatively, there was improvement in NAFX immediately and up to 1 year after surgery in two patients. Four out of five patients had improvement in visual acuity. Another study by Wang et al examined the effects of tenotomy on foveation and gaze angle in patients with INS.Citation80 For practical purposes, testing NAFX improvement across all gaze angles is necessary because the eyes do not remain in primary position. They used the NAFX in nine subjects at different gaze angles to determine if tenotomy increased potential acuity across these gazes. Eight out of nine subjects had increased NAFX at primary position. Seven out of nine patients showed improved NAFX at the various gaze angles. Six patients had improved visual acuity; acuity was unchanged in three. They concluded that tenotomy can broaden the area of heightened visual function and those patients with more impaired visual function have greater postsurgical improvement.
Timing of surgical intervention
Because there are currently no cures for the visual sensory deficits leading to INS, surgical treatment should be aimed at reducing nystagmus intensity, increasing foveation at null position and various gaze angles, or decreasing velocity of slow phase during foveation.Citation2 It is believed that these alterations to the nystagmus waveform will allow for improved visual acuity and visual function. INS usually manifests by 3 months, but surgery is typically deferred until 7 years or 8 years of age to be sure that the AHP has stabilized.Citation62 Still, there is a chance of recurrence of an AHP. Felius and MuhannaCitation81 found that nystagmus-induced partial binocular visual deprivation during the sensitive period of visual development contributes to decreased vision in idiopathic INS and suggest that timing of interventions should consider this.
Low-vision rehabilitation
Visual acuity ranges widely in patients with nystagmus, with some patients having 20/20 vision, while others are legally blind. For those with decreased acuity that impairs their ability to perform everyday tasks, vision rehabilitation, or vision habilitation in the case of people born with vision impairment, is an important treatment modality to maximize visual function. Vision habilitation may include optical or electronic magnification devices as well as training in their use and other adaptive strategies. One common strategy used by children with nystagmus is large print. The use of electronic devices such as tablet computers for reading is increasing. These devices can provide variable amounts of magnification, which make them ideal for children with nystagmus and vision impairment. There are no large-scale studies of the outcomes of vision rehabilitation in children. A recent systematic review of low-vision rehabilitation in children identified only 22 studies, mostly case series.Citation82 All studies reviewed had favorable outcomes supporting the use of low-vision rehabilitation in children.
Intervention summary
Since nystagmus has negative psychosocial and functional consequences as discussed earlier, there is a great deal of interest in its treatment. Intervention should be individualized based on patient age, function, needs, and concerns. The first step in intervention is provision of optimal refractive correction, possibly with the inclusion of prism to stimulate convergence or correct an AHP. If this does not meet the patient’s needs, then pharmacological or surgical intervention should be considered. Unfortunately, there are no randomized controlled trial data comparing pharmacological treatment to surgical treatment or comparing surgical techniques. In fact, most reports contain pre–posttreatment data for small sample sizes. The literature does not provide sufficient evidence to establish a preferred practice pattern for the treatment of nystagmus, although it does offer treatment choices. The decision on which treatment is best suited for a particular patient lies with the patient and his or her physician. While treatments are focused on decreasing nystagmus intensity and improving acuity, vision habilitation should also be recommended for those with vision impairment that affects the ability to perform everyday tasks.
Disclosure
The authors have no conflicts of interest.
References
- BrodskyMCDell’OssoLFA unifying neurologic mechanism for infantile nystagmusJAMA Ophthalmol2014132676176824525626
- Dell’OssoLFDaroffRBCongenital nystagmus waveforms and foveation strategyDoc Ophthalmol19753911551821201697
- HolmstromGBondesonMLErikssonUAkerblomHLarssonE‘Congenital’ nystagmus may hide various ophthalmic diagnosesActa Ophthalmol201492541241623889849
- ProudlockFAGottlobINystagmus in childhoodHoytCSTaylorDPediatric Ophthalmology and StrabismusAmsterdamElsevier Limited2013909923
- Dell’OssoLFJacobsJBAn expanded nystagmus acuity function: intra- and intersubject prediction of best-corrected visual acuityDoc Ophthalmol2002104324927612076015
- FeliusJFuVLBirchEEHertleRWJostRMSubramanianVQuantifying nystagmus in infants and young children: relation between foveation and visual acuity deficitInvest Ophthalmol Vis Sci201152128724873122003105
- TaiZHertleRWBilonickRAYangDA new algorithm for automated nystagmus acuity function analysisBr J Ophthalmol201195683283621059596
- ThurtellMJLeighRJNystagmus and saccadic intrusionsHandb Clin Neurol201110233337821601073
- HarrisCBerryDA developmental model of infantile nystagmusSemin Ophthalmol2006212636916702071
- Martinez-CondeSMacknikSLHubelDHThe role of fixational eye movements in visual perceptionNat Rev Neurosci20045322924014976522
- Dell’OssoLFThe mechanism of oscillopsia and its suppressionAnn N Y Acad Sci2011123329830621951008
- ThomasMGCrosierMLindsaySThe clinical and molecular genetic features of idiopathic infantile periodic alternating nystagmusBrain2011134pt 389290221303855
- GottlobIProudlockFAAetiology of infantile nystagmusCurr Opin Neurol2014271839124346039
- AbadiRVMechanisms underlying nystagmusJ R Soc Med200295523123411983762
- Dell’OssoLFvan der SteenJSteinmanRMCollewijnHFoveation dynamics in congenital nystagmus. II: smooth pursuitDoc Ophthalmol199279125491568421
- Dell’OssoLFvan der SteenJSteinmanRMCollewijnHFoveation dynamics in congenital nystagmus. III: vestibulo-ocular reflexDocumenta Ophthalmologica Adv Ophthalmol19927915170
- StjernaSSairanenVGröhnRVisual fixation in human newborns correlates with extensive white matter networks and predicts long-term neurocognitive developmentJ Neurosci201535124824482925810513
- BraddickOAtkinsonJDevelopment of human visual functionVision Res201151131588160921356229
- CarlJROpticanLMChuFCZeeDSHead shaking and vestibulo-ocular reflex in congenital nystagmusInvest Ophthalmol Vis Sci1985268104310503874850
- ArnoldDBRobinsonDAThe oculomotor integrator: testing of a neural network modelExp Brain Res1997113157749028775
- RahmanWProudlockFGottlobIOral gabapentin treatment for symptomatic Heimann-Bielschowsky phenomenonAm J Ophthalmol2006141122122216387013
- OpticanLMZeeDSA hypothetical explanation of congenital nystagmusBiol Cybern1984501191346722208
- TusaRJZeeDSHainTCSimonszHJVoluntary control of congenital nystagmusClin Vis Sci19927195210
- KnappCMProudlockFAGottlobIOKN asymmetry in human subjects: a literature reviewStrabismus2013211374923477776
- TychsenLRichardsMWongAFoellerPBradleyDBurkhalterAThe neural mechanism for Latent (fusion maldevelopment) nystagmusJ Neuroophthalmol201030327628320818206
- TychsenLAbsence of subcortical pathway optokinetic eye movements in an infant with cortical blindnessStrabismus199641111421314414
- MorroneMCAtkinsonJCioniGBraddickOJFiorentiniADevelopmental changes in optokinetic mechanisms in the absence of unilateral cortical controlNeuroreport199910132723272910511430
- BergKTHunterDGBothunEDAntunes-FoschiniRMcLoonLKExtraocular muscles in patients with infantile nystagmus: adaptations at the effector levelArch Ophthalmol2012130334334922411664
- ChengHYangJCXiaHUltrastructural study of proprioceptors in extraocular muscles of congenital nystagmus subjectsZhonghua Yan Ke Za Zhi20033912427 Chinese12760809
- HüfnerKStephanTFlanaginVLCerebellar and visual gray matter brain volume increases in congenital nystagmusFront Neurol201126021994501
- SchlindweinPSchreckenbergerMDieterichMVisual-motion suppression in congenital pendular nystagmusAnn N Y Acad Sci2009116445846019645948
- LeguireLEKashouNHFogtNNeural circuit involved in idiopathic infantile nystagmus syndrome based on FMRIJ Pediatr Ophthalmol Strabismus201148634735621261242
- DecarloDKMcGwinGJrBixlerMLWallanderJOwsleyCImpact of pediatric vision impairment on daily life: results of focus groupsOptom Vis Sci20128991409141622863790
- McLeanRJWindridgeKCGottlobILiving with nystagmus: a qualitative studyBr J Ophthalmol201296798198622517800
- PillingRFThompsonJRGottlobISocial and visual function in nystagmusBr J Ophthalmol200589101278128116170116
- BarotNMcLeanRJGottlobIProudlockFAReading performance in infantile nystagmusOphthalmology201312061232123823462273
- WooSBedellHEBeating the beat: reading can be faster than the frequency of eye movements in persons with congenital nystagmusOptom Vis Sci200683855957116909075
- ThomasMGGottlobIMcLeanRJMaconachieGKumarAProudlockFAReading strategies in infantile nystagmus syndromeInvest Ophthalmol Vis Sci201152118156816521862655
- WigginsDWoodhouseJMMargrainTHHarrisCMErichsenJTInfantile nystagmus adapts to visual demandInvest Ophthalmol Vis Sci20074852089209417460265
- AbadiRVBjerreAMotor and sensory characteristics of infantile nystagmusBr J Ophthalmol200286101152116012234898
- TkalcevicLAAbelLAThe effects of increased visual task demand on foveation in congenital nystagmusVision Res20054591139114615707922
- ChamKMAndersonAJAbelLATask-induced stress and motivation decrease foveation-period durations in infantile nystagmus syndromeInvest Ophthalmol Vis Sci20084972977298418390641
- JonesPHHarrisCMWoodhouseJMMargrainTHEnnisFAErichsenJTStress and visual function in infantile nystagmus syndromeInvest Ophthalmol Vis Sci201354137943795124222308
- WangZIDell’OssoLFBeing “slow to see” is a dynamic visual function consequence of infantile nystagmus syndrome: model predictions and patient data identify stimulus timing as its causeVision Res200747111550156017328937
- WangZIDell’OssoLFEye-movement-based assessment of visual function in patients with infantile nystagmus syndromeOptom Vis Sci200986898899519609232
- DuJWSchmidKLBevanJDFraterKMOllettRHeinBRetrospective analysis of refractive errors in children with vision impairmentOptom Vis Sci200582980781616189490
- FresinaMBenedettiCMarinelliFVersuraPCamposECAstigmatism in patients with idiopathic congenital nystagmusGraefes Arch Clin Exp Ophthalmol201325161635163923456100
- WangJWyattLMFeliusJOnset and progression of with-the-rule astigmatism in children with infantile nystagmus syndromeInvest Ophthalmol Vis Sci201051159460120019374
- HertleRWExamination and refractive management of patients with nystagmusSurv Ophthalmol200045321522211094245
- TaibbiGWangZIDell’OssoLFInfantile nystagmus syndrome: broadening the high-foveation-quality field with contact lensesClin Ophthalmol20082358558919668758
- BiousseVTusaRJRussellBThe use of contact lenses to treat visually symptomatic congenital nystagmusJ Neurol Neurosurg Psychiatry200475231431614742616
- GolubovicSMarjanovicSCvetkovicDManicSThe application of hard contact lenses in patients with congenital nystagmusFortschr Ophthalmol19898655355392583648
- JayaramachandranPProudlockFAOdedraNGottlobIMcLeanRJA randomized controlled trial comparing soft contact lens and rigid gas-permeable lens wearing in infantile nystagmusOphthalmology201412191827183624811959
- HertleRWNystagmus in infancy and childhood: characteristics and evidence for treatmentAm Orthopt J201060485821061884
- HertleRWMaybodiMBauerRMWalkerKClinical and oculographic response to Dexedrine in a patient with rod-cone dystrophy, exotropia, and congenital aperiodic alternating nystagmusBinocul Vis Strabismus Q200116425926411720591
- PradeepAThomasSRobertsEOProudlockFAGottlobIReduction of congenital nystagmus in a patient after smoking cannabisStrabismus2008161293218306120
- CarruthersJThe treatment of congenital nystagmus with BotoxJ Pediatr Ophthalmol Strabismus19953253063088531035
- McLeanRProudlockFThomasSDeggCGottlobICongenital nystagmus: randomized, controlled, double-masked trial of memantine/gabapentinAnn Neurol200761213013817279539
- ComerRMDawsonELLeeJPBaclofen for patients with congenital periodic alternating nystagmusStrabismus200614420520917162442
- HertleRWYangDAdkinsonTReedMTopical brinzolamide (Azopt) versus placebo in the treatment of infantile nystagmus syndrome (INS)Br J Ophthalmol201599447147625336575
- SheryTProudlockFASarvananthanNMcLeanRJGottlobIThe effects of gabapentin and memantine in acquired and congenital nystagmus: a retrospective studyBr J Ophthalmol200690783984316556621
- LeeJSurgical management of nystagmusJ R Soc Med200295523824111983764
- HertleRWAnningerWYangDShatnawiRHillVMEffects of extraocular muscle surgery on 15 patients with oculo-cutaneous albinism (OCA) and infantile nystagmus syndrome (INS)Am J Ophthalmol2004138697898715629289
- KestenbaumAA Nouvelle operation de nystagmus [A new operation for nystagmus]Bull Soc Ophtalmol Fr1954210711078 French
- AndersonJRCauses and treatment of congenital eccentric nystagmusBr J Ophthalmol195337526728113042022
- ParksMMSymposium: nystagmus. Congenital nystagmus surgeryAm Orthopt J19732335394710214
- CalhounJHHarleyRDSurgery for abnormal head position in congenital nystagmusTrans Am Ophthalmol Soc1973717083 discussion 84–7710949591
- NelsonLBErvin-MulveyLDCalhounJHHarleyRDKeislerMSSurgical management for abnormal head position in nystagmus: the augmented modified Kestenbaum procedureBr J Ophthalmol198468117968006498134
- KumarAShettySVijayalakshmiPHertleRWImprovement in visual acuity following surgery for correction of head posture in infantile nystagmus syndromeJ Pediatr Ophthalmol Strabismus201148634134621261243
- BagheriAAletahaMAbrishamiMThe effect of horizontal rectus muscle surgery on clinical and eye movement recording indices in infantile nystagmus syndromeStrabismus2010182586420521880
- AtillaHErkamNIsikcelikYSurgical treatment in nystagmusEye199913pt 1111510396377
- von NoordenGKSprungerDTLarge rectus muscle recessions for the treatment of congenital nystagmusArch Ophthalmol199110922212241993031
- HelvestonEMEllisFDPlagerDALarge recession of the horizontal recti for treatment of nystagmusOphthalmology1991988130213051923370
- BagheriAFarahiAYazdaniSThe effect of bilateral horizontal rectus recession on visual acuity, ocular deviation or head posture in patients with nystagmusJ AAPOS20059543343716213392
- HertleRWYangDAdamsKCaterinoRSurgery for the treatment of vertical head posturing associated with infantile nystagmus syndrome: results in 24 patientsClin Experiment Ophthalmol2011391374620662845
- ParkCMinBMWrightKWEffect of a modified rectus tuck on anterior ciliary artery perfusionKorean J Ophthalmol19915115251942598
- SchildAMThoenesJFrickeJNeugebauerAKestenbaum procedure with combined muscle resection and tucking for nystagmus-related head turnGraefes Arch Clin Exp Ophthalmol2013251122803280923835757
- Dell’OssoLFHertleRWWilliamsRWJacobsJBA new surgery for congenital nystagmus: effects of tenotomy on an achiasmatic canine and the role of extraocular proprioceptionJ AAPOS19993316618210428591
- HertleRWDell’OssoLFFitzGibbonEJYangDMellowSDHorizontal rectus muscle tenotomy in children with infantile nystagmus syndrome: a pilot studyJ AAPOS20048653954815616501
- WangZDell’OssoLFJacobsJBBurnstineRATomsakRLEffects of tenotomy on patients with infantile nystagmus syndrome: foveation improvement over a broadened visual fieldJ AAPOS200610655256017189150
- FeliusJMuhannaZAVisual deprivation and foveation characteristics both underlie visual acuity deficits in idiopathic infantile nystagmusInvest Ophthalmol Vis Sci20135453520352523687170
- ChavdaSHodgeWSiFDiabKLow-vision rehabilitation methods in children: a systematic reviewCan J Ophthalmol2014493e71e7324862788
