Abstract
Numerous brands and types of artificial tears are available on the market for the treatment of dysfunctional tear syndrome. Past literature has focused on comparing the components of these products on patient’s clinical improvement. The wide array of products on the market presents challenges to both clinicians and patients when trying to choose between available tear replacement therapies. Different formulations affect patients based on etiology and severity of disease. In order to provide an unbiased comparison between available tear replacement therapies, we conducted a literature review of existing studies and National Institutes of Health clinical trials on commercially available, brand name artificial tears. Outcomes evaluated in each study, as well as the percent of patients showing clinical and symptomatic improvement, were analyzed. Fifty-one studies evaluating different brands of artificial tears, and their efficacy were identified. Out of the 51 studies, 18 were comparison studies testing brand name artificial tears directly against each other. Nearly all formulations of artificial tears provided significant benefit to patients with dysfunctional tear syndrome, but some proved superior to others. From the study data, a recommended treatment flowchart was derived.
Introduction
Dysfunctional tear syndrome (DTS), commonly known as dry eye syndrome, describes the multifactorial condition where the ocular system fails to produce good quality tears or a sufficient amount of tears to keep the eye moisturized.Citation1 Human tears, composed of electrolytes, water, proteins (eg, antibodies, lysozymes), and lipids, function to moisturize the ocular surface and minimize damage to the corneal epithelium. These components come together to form three distinct layers: 1) the outermost lipid layer, 2) a middle aqueous layer, and 3) the epithelium-covering mucoid layer. Dysfunction in any of these layers can yield tear film instability and hyperosmolarity.Citation2,Citation3 External causes of such dysfunction are widespread including environmental factors, systemic diseases, and medications.Citation4–Citation12
DTS is among the most commonly encountered ocular morbidities, affecting as many as 15%–25% of individuals over the age of 65 and up to 6% of adults over the age of 40.Citation12–Citation15 Inadequate lubrication results in ocular surface damage and discomfort. In addition to increasing the risk of ocular infection, DTS can cause irreversible scarring and fibrosis due to unprotected corneal epithelial exposure.Citation16–Citation19 Many clinicians have begun to treat the condition with increased vigilance.Citation14 Furthermore, prompt intervention can offer substantial benefits with regards to quality of life and comfort.Citation20
Artificial tears are currently the mainstay of therapy of DTS. They account for at least $540 million in annual sales globally and are the preferred first-line therapy due to their noninvasive nature and low side effect profile.Citation14,Citation21,Citation22 However, a dizzying array of brands and marketing strategies have made it a challenge for patients and clinicians alike to identify the product that best suits individual patients.
Previous review articles have studied and compared the active ingredients within the artificial tears, but none have compared the full formulations available on the market.Citation23 With a focus on artificial tear brands, this study aims to provide a useful literature-based comparison between available tear replacement therapies for clinicians and patients considering starting therapy to manage DTS. The following is a detailed overview of the commercial agents available (summarized in Tables S1–S5) for treating dry eye, with a particular emphasis placed upon those agents and practices that are most effective in mitigating symptoms of DTS.
Materials and methods
Literature search
This study involved a review of the literature analyzing artificial tear treatments of DTS. The resources utilized were the electronic databases Medline (PubMed; http://www.ncbi.nlm.nih.gov), National Institutes of Health (NIH) clinical trials (http://clinicaltrials.gov), Google Scholar, and the Cochrane Library. Keywords used in the search included dry eye, dysfunctional tear syndrome, Actimist, Advance Eye Relief, Akorn, Akwa, Blink, Clarymist, Clear Eyes, Freshkote, GenTeal, Hylogel, Isopto, Just Tears, Lacril, Lacrisert, Liposic, Lubrifresh, Murine Tears, Natural Balance, Nature’s Tears, Nutratear, Oasis Tears, Paralube, Refresh, Rohto Hydra, Systane, Soothe, Tearisol, Tears Again, Tears Natural, Thera Tears, Ultra Tears, Visco Tears, and Vizulize Dry Eye Mist. Both published articles and data from clinical trials (including multicenter, blinded studies as well as open label, industry-funded studies) were included in this study. Studies were excluded if the brand and specific type of artificial tear could not be identified. Data from unpublished clinical trials and articles from artificial tears that are no longer being produced were excluded. No other exclusion criteria were applied.
The etiology of dry eye syndrome in the studies reviewed included the following conditions: environmental (humidity, pollution, etc), situational (reading/prolonged focus), contact lens wear, LASIK surgery, autoimmune syndromes, nutritional deficiencies, vitamin A, Stevens–Johnson syndrome, and irritant exposure. The patient subgroups were not excluded but were also not specifically identified in many of the studies during analysis of the treatment of DTS, and thus we cannot readily distinguish between these patient populations. In future studies, this is an issue which will need to be clearly addressed.
Data compilation
Data from all studies were compiled into one group. Each study had its own means of data collection and definition for successful treatment with artificial tears. Methods for collecting the subjective data were mainly through questionnaires including McMonnies Dry Eye Symptom survey,Citation24 Ocular Surface Disease Index (ODSI) questionnaire,Citation18 impact of dry eye on everyday life (IDEEL) questionnaire,Citation25 Visual Analogue Scale, Salisbury Eye Evaluation (SEE) questionnaire,Citation26 Ocular Discomfort Severity questionnaire, direct brand comparison with drop preference selection, quality of life through the Measure Yourself Medical Outcome Profile-2 (MYMOP-2),Citation27 and Dry Eye Disease Comfort Assessment Score. Several studies also utilized custom questionnaires.Citation28–Citation31 Objective data took the form of ocular surface staining using various dyes, usually fluorescein and rose bengal. Additionally, comparative data was collected through analysis of a variety of other parameters including Schirmer’s test, tear break-up time, post-therapy corneal topography, tear meniscus volume, mucinous layer analysis with rose bengal staining, tear osmolarity, lipid layer thickness (LLT), improvement of visual acuity, conjunctival hyperemia, Ocular Protection Index (OPI) (examining the tear break-up time divided by the interblink interval), number of eyelid parallel conjunctival folds, blinking time analysis through the OPI 2.0 system, Global Staining Score, the TearLab Osmolarity System, and tear film normalization test (measurement of lines of improvement in visual acuity after administration of artificial tears).Citation28,Citation29,Citation32–Citation36
Commercially available tear film substitutes
To provide maximum utility for the patient and clinician, commercially available artificial tears were identified and categorized based on active ingredient (Tables S1–S5). Other over-the-counter treatments for DTS including gels, ointments, and sprays/mists were included in the compilation (Table S4). Active ingredients and preservatives were verified via package inserts for each product. Artificial tears were divided into groups based on active ingredients including hydroxypropyl methylcellulose (HPMC), carboxy methylcellulose (CMC), polyvinyl alcohol, homeopathic remedies, and the liquid polyols. A final group was made for other delivery methods including gel/ointments, spray/mist over-the-counter treatments, and the prescribed ophthalmic insert, Lacrisert® (Valeant, Bridgewater, NJ, USA). Lacrisert was included in the study because its composition and active ingredient mimic those of some artificial tears.
Results
A total of 18 articles comparing subjective and objective outcomes of artificial tear brands were identified in our literature search. All articles were written in English or translated to English from other languages, including German, Spanish, and Chinese. The outcomes from these studies are summarized in . Many of these studies utilized different parameters to determine treatment efficacy.
Table 1 Summary of published papers and clinical trials comparing artificial tears
Refresh versus Systane
The first study compared Systane® (Alcon Laboratories, Inc., Fort Worth, TX, USA) to Refresh Tears® (Allergan, Irvine, CA, USA) in 87 patients with dry eyes over a 6-week period.Citation37 Both investigators and patients were blinded to the artificial tear being used. The study evaluated the conjunctival/corneal staining and a custom symptom questionnaire at days 7, 14, 28, and 42. At days 14 and 28, patients using Systane® showed significantly improved conjunctival staining compared to patients using Refresh Tears®. At days 14 and 42, patients using Systane also had significantly decreased temporal corneal staining compared to patients using Refresh. Furthermore, subjective symptomatic improvement was significantly increased in patients using Systane when compared to those using Refresh.
Another study comparing Systane versus Refresh products utilized a three-way cross-over study design comparing Systane®, Refresh Tears®, and Refresh Endura® (now called Refresh Optive®; Allergan).Citation38 Including only patients with a history of dry eye signs or symptoms, 50 patients were evaluated using tear film breakup time (TBUT) and the OPI over three separate clinical visits. TBUT measurements were taken at 5, 10, 15, 20, 30, 45, and 60 minutes after tear application. Systane® significantly increased TBUT compared to both Refresh Tears® and Refresh Endura® at 5, 10, 15, 20, and 60 minutes after artificial tear application.
A third study analyzing Refresh and Systane products compared the effects of Refresh Liquigel® and Systane® on corneal staining and symptomatic improvement in 60 patients.Citation30 A reduced sum score of corneal staining and a reduction of corneal staining from baseline were only observed in the Refresh Liquigel® group (P=0.008 and P=0.019, respectively). Patient’s tear preference and comfort were also analyzed in this study; however, patients were not blinded to the artificial tear assigned. Using one eye for each artificial tear, patient preference was recorded 5 minutes after application with 36% of patients preferring Refresh and 24% preferring Systane. Limited value may be drawn from the subjective component of this study since unilateral symptoms may have been present in the study participants.
The largest of the studies comparing artificial tears was completed in Germany.Citation39 The study was a multicenter, observational study involving patients from 835 ophthalmologists. Data from 5,277 patients who required a change in their artificial tear formulation or were naive to artificial tear treatment were analyzed after 2–4 weeks of treatment with Refresh Optive®. Patients had previously been using either Systane®, Hylo-Comod®, or Lacophtal® (Ursapharm Arzneimittel GmbH, Saarbrücken, Germany). Nearly 85% of patients reported improvement in ocular comfort with Refresh Optive®, and nearly 75% experienced an improvement in their symptoms after changing artificial tear treatment regimens. TBUT also significantly increased in patients using Refresh Optive® from a mean of 7.7 seconds to 10.0 seconds (P<0.001).
In an industry-sponsored, Alcon, head-to-head clinical trial, visual acuity was measured after application of Systane® Ultra and Refresh Optive®.36 The study population included 48 patients with a history of dry eye. Each patient underwent visual acuity evaluation while completing a computer task at 15, 45, and 90 minutes postapplication. The amount of time the patients maintained their best-corrected visual acuity was the measured end point. At 90 minutes, the mean time best-corrected visual acuity was maintained for 9.17 seconds with Systane versus 6.84 seconds with Refresh. These data were not statistically analyzed.
Alcon sponsored another clinical trial comparing Systane® Ultra and Refresh Optive® with an emphasis on TBUT, corneal staining, and conjunctival staining after 0, 7, 14, 28, and 42 days of artificial tear use.Citation40 A total of 109 patients with a diagnosis of dry eye were enrolled in the clinical trial with 53 in the Systane® Ultra group and 56 in the Refresh Optive® group. Mean TBUT after 42 days was 4.5 seconds with Systane® Ultra and 4.2 seconds with Refresh Optive®. The study also compared corneal and conjunctival staining using a 0–10 scale, with 0 equaling no staining present. At day 14 and 42, Systane® Ultra-treated eyes had a mean corneal staining score of 2.9 on both visits while Refresh Optive®-treated eyes had a mean score of 4.5 and 4.2 for the respective visits.
Refresh versus Refresh
Allergan funded a project studying the efficacy of Refresh Tears®, Refresh Ultra®, and Refresh Optive® in relieving the signs and symptoms of dry eye.Citation43 A total of 37 participants completed the study; 18 with a history of dry eye and 19 controls without a history of dry eye. In this three-way cross-over study, each patient used each artificial tear formulation for 2 weeks while TBUT, tear evaporation, osmolarity, tear structure, and patient symptoms were evaluated. All patients with a history of dry eye experienced improvement in both their signs and symptoms of dry eye with all formulations (P<0.05). In the control group, Refresh Optive® and Refresh Ultra® treatment resulted in a decreased rate of evaporation, significantly greater than Refresh Tears® on cross-group comparison. Refresh Tears® was the only formulation observed to decrease tear osmolarity in the control group (P<0.05). No other statistical difference appeared on cross-group analysis.
A combination of three separate, independent studies aimed to find the differences in corneal staining and OPI in patients treated with either Refresh Tears® or Refresh Liquigel®, an artificial tear product with a thicker consistency than most drops.Citation29 A total of 607 patients were evaluated after 1 month of using either Refresh Tears® or Refresh Liquigel®. With regard to change in OPI, Refresh Liquigel®-treated patients showed greater improvements than those treated with Refresh Tears® (P<0.05). Additionally, after 1 week and 1 month of use, patients treated with Refresh Liquigel® showed significantly reduced corneal staining compared to those treated with Refresh Tears® (P<0.001 and P=0.011, respectively). Although study subjects did report increased blurring after application with Refresh Liquigel®, both artificial tears had statistically equivalent acceptability. Over a 1-month period, patients also reported using the Refresh Liquigel® less frequently compared to Refresh Tears® (P=0.05).
To assess the effects on blurring, distortion, and contrast sensitivity, Allergan funded a study to quantify changes in visual acuity after administration of Refresh Liquigel® and Refresh Celluvisc®, two products of thicker consistency than most artificial tears.Citation42 In 20 normal subjects without a history of dry eye disease, artificial tears were applied at different time points followed by testing of contrast sensitivity via a computer controlled stimulus. Both artificial tears significantly reduced contrast sensitivity immediately after application (P<0.001); however, Refresh Celluvisc® did so to a greater degree (P<0.001).
Further research compared the effect of Refresh Liquigel® and Refresh Tears® on TBUT in 39 patients.Citation42 For each patient, either Refresh Liquigel® or Refresh Tears® was initially applied, and TBUT was measured at 5, 10, 15, 20, 30, 45, and 60 minutes. This was repeated 1 week later with the other artificial tear formulation. Order of administration was randomly assigned. At 5 minutes, both drops increased TBUT; however, only Refresh Liquigel® significantly increased TBUT beyond 5 minutes. This effect was notable for up to 20 minutes postapplication.
Blink versus Refresh versus Systane
In a study of 60 participants, Blink® Intensive (Abbott Laboratories, Abbott Park, IL, USA), Systane®, and Refresh Celluvisc® were compared at baseline and after 30 days of product use.Citation44 Tear osmolarity measurement, Schirmer tear test, TBUT, fluorescein staining, corneal wavefront aberrometry, and visual acuity were all measured. All three treatment groups demonstrated improvement in all measured endpoints. However, cross–group comparison found Blink to better reduce the tear osmolarity compared to both Systane® and Refresh Celluvisc® (P<0.001). No other statistical differences were found between the groups.
One unpublished, Abbot-funded NIH clinical trial with 80 patients compared Blink® Tears to Systane® Ultra with regard to TBUT and visual acuity 1 month after treatment.Citation45 Blink® Tears proved superior to Systane® Ultra in both TBUT (P=0.003) and improvement in visual acuity (P<0.001).Citation46
Blink® Tears was also compared to Refresh Optive® in a trial of 51 patients with a history of dry eye.Citation31 The primary goal of this Allergan-sponsored study was to improve subjective symptoms on the Dry Eye Disease Comfort Assessment questionnaire score over a 16-day period. No statistical analysis was performed on these data, but changes between the two groups appeared similar with a mean decrease on the questionnaire score of 1.41 with Refresh Optive® and 1.47 with Blink® Tears.
In an Alcon-sponsored clinical trial, Systane® Ultra, Refresh Optive®, GenTeal Moderate® (Novartis Pharmaceuticals, East Hanover, NJ, USA), and Blink® Tears were compared with regard to postapplication comfort.Citation46 Drop comfort grading was measured using a 0–9 scale, with 0 representing the highest level of comfort. The design was a randomized, double-masked, cross-over study with 20 patient participants. After drop administration, the comfort scores were 0.7±1.26 for Systane® Ultra, 1.05±1.10 for Refresh Optive®, 1.84±2.19 for Blink® Tears, and 1.1±1.21 for GenTeal®. No statistical analysis was performed.
Soothe versus Refresh or Systane
Soothe® (Bausch & Lomb Incorporated, Bridgewater, NJ, USA) is a lipid-based artificial tear. A pair of separate studies aimed to elucidate its effects on the LLT within the tear film. The first study evaluated the effectiveness of Soothe® versus Refresh Optive®. Enrollment included 41 patients with a LLT under 70 nm in both eyes and baseline visual acuity greater than 20/70. Each patient received a drop of Soothe® in one eye and Refresh Optive® in the contralateral eye. A custom-designed lipid layer interferometer quantified the LLT at 1, 5, and 15 minutes after application. Both artificial tears increased the LLT from a baseline of 61.5±1.6 nm (P<0.001), with a mean LLT of 83.2±3.6 nm for Refresh Optive® and 121.5±3.8 nm for Soothe® (P<0.001).Citation47
A second study utilized the same inclusion criteria and study design but compared Soothe® to Systane®.48 A total of 40 patients were included in this study. Results showed a mean LLT of 124.4±4.9 nm for Soothe® and a mean LLT of 71.3±2.6 nm for Systane®. This represents an increase from baseline of 107% for Soothe and only 16% for Systane. Both studies were industry sponsored by Ocular Research (Boston, MA, USA) and Alimera Sciences (Alpharetta, GA, USA), which have partial ownership of Soothe.
Other comparative studies
Another randomized controlled study analyzing lipid-based tear substitutes looked at their effect on TBUT, Schirmer’s test, tear meniscus, and subjective symptoms.Citation49 This crossover study compared Tears Again® (OcuSoft, Rosenberg, TX, USA) to Liposic® (Bausch & Lomb) in 74 patients with a history of dry eye over two separate 6-week periods. In both the initial treatment and after cross-over, Tears Again® improved all the aforementioned subjective and objective endpoints (P<0.05); 62.5% of patients preferred Tears Again®, 25% preferred Liposic®, and 12.5% found the preparations to be equal.
GenTeal Tears® with preservative, GenAqua®, and preservative-free Tears Naturale® (Alcon Laboratories, Inc.) were compared in an open-label, two-treatment, two-period study.Citation50 After 4 weeks of treatment, patients were evaluated with TBUT, Schirmer’s test, and corneal staining as well as via a symptom questionnaire. A total of 37 patients completed the study. Both TBUT and Schirmer testing improved in the GenTeal group but not in the Tears Naturale® group (P=0.27). Both artificial tears were rated as excellent for tolerability and convenience. Subjective symptoms were not different between the two treatments.
Clinical improvement
summarizes the clinical improvement of dry eye subjects based on objective and subjective criteria used on each study included in this review. Due to the heterogeneity of criteria used on these studies, a percent of improvement was calculated for each of the artificial tears evaluated. The percent of improvement was calculated based on the amount of subjects with symptomatic and clinical improvement with respect to the total subjects treated.
Table 2 Clinical data for performance of artificial tears and their corresponding percent improvement based upon respective subjective and objective criteria
Discussion
In all 18 head-to-head studies, patients with dry eyes had clinical improvement both immediately after application and over the long term when using tear replacements. This occurred in both preserved and preservative-free artificial tear formulations. With head-to-head comparisons, the results varied greatly and often depended on the funding source. To no surprise, each study that was industry sponsored found the respective company’s artificial tear to be most effective. Further, the newer artificial tears, Refresh Optive® and Systane® Ultra, definitively outperformed the older Refresh Tears® and Systane® in both subjective and objective tests. Interestingly, Soothe® dramatically increased the lipid layer of the tear film compared to its Systane and Refresh counterparts. A lipid layer under 60 nm indicates a higher likelihood of having dry eye symptoms. Conversely, having a LLT greater than 75 nm decreases symptoms, and generally, a thicker lipid layer directly correlates with decreased symptoms.Citation50 This is an important finding since studies have shown that a deficient lipid layer is the most common cause of DTS.Citation52 After reviewing all the studies, we elaborated a set of recommendations that may help both the physician and the patient in decision making when a tear replacement therapy is needed. Due to the lack of standardization and bias from industry-funded studies, these recommendations are not intended to be conclusive and final, but a good resource based on the comparative data we gathered on the discussed head-to-head studies.
Recommendations
Our recommendations for suggested drop brands in each of the respective categories is based upon both the number of studies completed on these brands as well as the trials that compared their efficacy against other brand name artificial tears (see and ). Due to the heterogeneous data available and lack of standardization between studies, the recommendations made are not intended to be definite and should be individualized based on disease severity and patient’s expectations. This is a step approach, initiating therapy with the most studied artificial tears. Treatment recommendations (summarized in ) are as follows:
Figure 1 Treatment flowchart.
Abbreviations: CMC, carboxy methylcellulose; DTS, dysfunctional tear syndrome; HPMC, hydroxypropyl methylcellulose; PEG, polyethylene glycol; PG, propylene glycol.
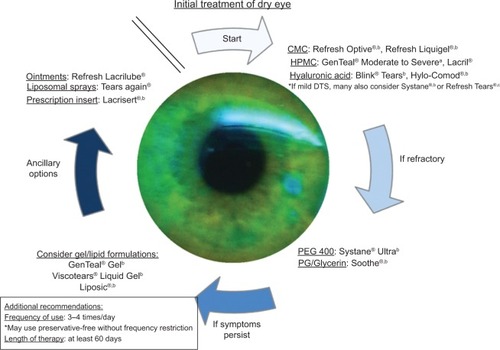
Step 1
The treatment algorithm allows for initial therapies to be divided into three categories of drops based on the active ingredient: CMC-based, HPMC-based, and hyaluronic acid-based. In a recent comprehensive review of the active ingredients contained in artificial tears, the above listed ingredients have been shown to be the most beneficial in improving patient comfort levels.Citation53 For each of these categories, the most studied brands of artificial tears were recommended. Additionally, Systane® and Refresh Tears® have been well studied and have been beneficial in the treatment of mild dry eye syndrome (see and ).
Step 2
Systane® Ultra and Soothe® have both been shown in clinical research to out-perform the CMC/HPMC/hyaluronic acid-based formulations listed in Step 1 (see and S1). In the instance that initial therapy fails to adequately control the symptoms of DTS, either of these two drops should be considered as the next therapy.
Steps 3 and 4
In the event that standard artificial tears fail to adequately abate the patient’s symptoms and/or in the case of severe DTS, lid malposition, or exposure keratopathy, the implementation of additional therapies such as gels, ointments, liposomal sprays, is indicated.Citation37,Citation54–Citation61 These therapies may need to be implemented earlier based on severity of disease.
Frequency/duration
Based on the duration and frequency of artificial tear use reported in all the studies referenced in and , a mean of 3.47 doses/day over a period 60.1 days was established. Thus, our recommendation would be to use the artificial tears three to four times per day over a period of 2 months before transitioning to the next step. If artificial tear use extends beyond four to six times per day, then a preservative-free formulation should be used.Citation62
Preserved versus preservative-free
As seen in Tables S1–S5, many different tear replacement formulations include preservatives, but not all preservatives have the same effects. The most commonly used preservative was benzalkonium chloride (BAK). BAK has more antimicrobial activity than any other preservative in both animal and human subjects. However, BAK has been shown to damage the corneal epithelium and disrupt the tear film immediately after administration, which directly contradicts the goal of artificial tears.Citation63,Citation64 Some of the newer preservative compounds appear to have a better safety profile than BAK but do not entirely prevent corneal epithelium damage.Citation65 These preservatives include Purite® (Bio-Cide International Inc., Norman, OK, USA), Polyquad® (Alcon Laboratories, Inc.), GenAqua® (Novartis Ophthalmics, East Hanover, NJ, USA), OcuPure® (Abbott Laboratories Inc.), Dissipate® (OCuSOFT, Rosenberg, TX, USA).Citation50,Citation63,Citation66 Since most of the corneal changes occur when the preservatives reach high concentrations, if one chooses to use a tear replacement with preservatives, daily use should be limited to four to six times.Citation62 Due to the risk of contamination, if a preservative-free artificial teardrop is chosen, single dose vials are recommended over multidose administration bottles.Citation67 Increased cost represents another downside to preservative-free artificial tears compared to those with preservatives. All things considered, a choice between preservative-free and preserved artificial tears should be discussed on an individual basis between physician and patient.
Limitations
The US is currently the epicenter of clinical research on DTS as it has been previously documented to conduct 70.8% of the registered clinical trials around the world which focused on dry eye.Citation23 The data presented in this paper may not be applicable in a global setting, as the American diet, and cultural and daily activities may play a role in the development of DTS and its corresponding treatment. With growing research in other countries, such as Japan, Australia, UK, and the Netherlands, treatment of DTS with artificial tears will progressively be refined on a global scale.Citation68 Furthermore, the research that has been presented in this article is based on a nonregulated health product, and the US permits distribution of artificial tears without any data revealing positive efficacy.Citation68,Citation37 Any research investments made into this field may be affected by financial interests of the investigators and call into question the integrity of the outcomes, which may have influenced our recommendations. A recent meta-analysis of the comprehensive research on dry eye disease revealed that the pharmaceutical industry sponsored 78% of 185 clinical trials on this topic in the US.Citation68 For this reason, care should be taken when analyzing and applying data from industry-funded studies. Independent, unbiased research must increase on a worldwide level in order to more objectively and accurately elucidate the management of DTS.
In addition, this review article addressed a myriad of subjective and objective data with a multitude of data collection methods and varying result parameters, including dosing and length of treatment phase. For these reasons, further statistical analyses of improvements were not completed in this paper, but our study does suffer from lack of statistical backing. Furthermore, many of the individual papers which contributed to our information pool validated their data prior to their release. In future clinical trials, standardization of measurement instruments as well as dosage and time of treatment could enrich the generalizability of the outcomes and lead to a more consistent review of resultant data.
Finally, our study provides recommendations based on comparative studies previously published in the literature. Most of the artificial tears available in the market, as seen in and S1–S5, were not included in the recommendations since they have not been included in comparative studies. We recognize that the recommendations made are still a good guide for clinicians and patients when initiating DTS therapy based on the data available.
Conclusion
With the expansive amount of commercially available artificial tear options, specific recommendations are needed to help guide both the clinician and patient. Although limited by the lack of congruent methodology throughout the included studies and heterogeneous population, this paper aimed to provide unbiased recommendations based on the available data and should be a step towards the standardization of future studies regarding artificial tears. Utilizing both direct comparison and patient improvement following artificial tear use, a treatment flowchart was created (). Ultimately, artificial tear selection should be individualized to the patient’s specific needs. In the future, a standardized method to evaluate dry eye, as well as efficacy of artificial tear treatment, will allow for improved recommendations to be formed.
Supplementary materials
Table S1 Methylcellulose-based artificial tears commercially available
Table S2 Polyvinyl alcohol-based artificial tears commercially available
Table S3 Liquid polyol-based artificial tears available on the market
Table S4 Gels, ointments, sprays, and an ophthalmic insert for dysfunctional tear syndrome
Table S5 Artificial tears with combinations of active ingredients
Disclosure
The authors report no conflicts of interest in this work.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf200752759217508116
- LempMAContact lenses and allergyCurr Opin Allergy Clin Immunol20088545746018769201
- TiffanyJThe normal tear filmDev Ophthalmol20084112018453758
- LuPChenXLiuXDry eye syndrome in elderly Tibetans at high altitude: a population-based study in ChinaCornea200827554555118520503
- HimebaughNLBegleyCGBradleyAWilkinsonJABlinking and tear break-up during four visual tasksOptom Vis Sci2009862E106E11419156014
- WilsonSEStultingRDAgreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye diseaseCornea200726328428917413954
- KabalakGDobbersteinSBMatthiasTAssociation of immunoglobulin-like transcript 6 deficiency with Sjögren’s syndromeArthritis Rheum200960102923292519790059
- LermanSOcular side effects of acutane therapyLens Eye Toxic Res199293–44294381301795
- ShellansSRichLFLouiselleIConjunctival goblet cell response to vasoconstrictor useJ Ocul Pharmacol1989532172202625617
- GöbbelsMJAchtenCSpitznasMEffect of topically applied oxymetazoline on tear volume and tear flow in humansGraefes Arch Clin Exp Ophthalmol199122921471492044975
- HazinRAbuzetunJYDaoudYJAbu-KhalafMMOcular complications of cancer therapy: a primer for the ophthalmologist treating cancer patientsCurr Opin Ophthalmol200920430831719491683
- BjerrumKBKeratoconjunctivitis sicca and primary Sjögren’s syndrome in a Danish population aged 30–60 yearsActa Ophthalmol Scand19977532812869253975
- McCartyCABansalAKLivingstonPMStanislavskyYLTaylorHRThe epidemiology of dry eye in Melbourne, AustraliaOphthalmology19981056111411199627665
- LatkanyRDry eyes: etiology and managementCurr Opin Ophthal2008194287291
- ScheinODMuñozBTielschJMBandeen-RocheKWestSPrevalence of dry eye among the elderlyAm J Ophthalmol199712467237289402817
- HuangTWangYLiuZWangTChenJInvestigation of tear film change after recovery from acute conjunctivitisCornea200726777878117667608
- MertzanisPAbetzLRajagopalanKThe relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sampleInvest Ophthalmol Vis Sci2005461465015623753
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the Ocular Surface Disease IndexArch Ophthalmol2000118561562110815152
- VitaleSGoodmanLAReedGFSmithJAComparison of the NEI-VFQ and OSDI questionnaires in patients with Sjögren’s syndrome-related dry eyeHealth Qual Life Outcomes200424415341657
- ReddyPGradORajagopalanKThe economic burden of dry eye: a conceptual framework and preliminary assessmentCornea200423875176115502474
- KymionisGDBouzoukisDIDiakonisVFSiganosCTreatment of chronic dry eye: focus on cyclosporineClin Ophthalmol20082482983619668437
- Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf20075216317817508120
- AlvesMFonsecaECAlvesMFDry eye disease treatment: a systemic review of published trials and a critical appraisal of therapeutic strategiesOcul Surf201311318119223838019
- NicholsKKNicholsJJMitchellGLThe reliability and validity of McMonnies Dry Eye IndexCornea200423436537115097131
- AbetzLRajagopalanKMertzanisPBegleyCBarnesRChalmersRImpact of dry eye on everyday life (IDEEL) Study GroupDevelopment and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patientsHealth Qual Life Out20119111
- WestSKMunozBRubinGSFunction and visual Impairment in a population-based study of older adultsInvest Ophthalmol Vis Sci199738172829008632
- KimTHKangJWKimKHAcupuncture for dry eye: a multicentre randomised controlled trial with active comparison intervention (artificial tear drop) using a mixed method approach protocolTrials20101110721078194
- GiffordPEvansBJMorrisJA clinical evaluation of SystaneCont Lens Anterior Eye2006291314016473547
- SimmonsPAVehigeJGClinical performance of a mid-viscosity artificial tear for dry eye treatmentCornea200726329430217413956
- NoeckerRJComparison of initial treatment response to two enhanced-viscosity artificial tearsEye Contact Lens200632314815216702870
- AllerganEfficacy and acceptability of two lubricant eye drops. NLM Identifier: NCT00756678 [webpage on the Internet]Bethesda, MDClinicaltrials.gov2008 Available from: http://clinicaltrials.gov/show/NCT00756678Accessed September 24, 2013
- NilforoushanMLatkanyRASpeakerMGEffect of artificial tears on visual acuityAm J Ophthalmol2005140583083516310460
- ChartersLArtificial tear normalizes blink patternOphthalmology Times72011
- RolandoMAutoriSBadinoFBarabinoSProtecting the ocular surface and improving the quality of life of dry eye patients: a study of the efficacy of an HP-guar containing ocular lubricant in a population of dry eye patientsJ Ocul Pharmacol Ther200925327127819366323
- LatkanyRLockBGSpeakerMTear film normalization test: a new diagnostic test for dry eyesCornea200625101153115717172889
- Alcon ResearchThe effect of systane ultra lubricant eye drops (FID112903) on visual performance. NLM Identifier: NCT00673764 [webpage on the Internet]Bethesda, MDClinicaltrials.gov2008 Available from: http://clinicaltrials.gov/show/NCT00673764Accessed September 24, 2013
- ChristensenMTCohenSRinehartJClinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eyeCurr Eye Res2004281556214704914
- OuslerGWMichaelsonCChristensenMTAn evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye dropsCornea200726894995217721294
- KaercherTBuchholzPKimmichFTreatment of patients with keratoconjunctivitis sicca with Optive: results of a multicenter, open-label observational study in GermanyClin Ophthalmol20093333919668542
- Alcon ResearchEvaluation of SYSTANE ultra lubricant eye drops. NLM Identifier: NCT00702377 [webpage on the Internet]Bethesda, MDClinicaltrials.gov2008 Available from: http://clinicaltrials.gov/ct2/show/NCT00702377Accessed September 24, 2013
- SimmonsPVehigeJThe effects of artificial tear composition on tear-film break up timeAm Academy of Optometry2004CN-00525769
- LaMotteJORidderWHKuanTChangJOlejnikOVehigeJThe effect of artificial tears with different CMC formulations on contrast sensitivityInvest Ophthalmol Vis Sci200243 E-Abstract 3151
- TomlinsonAMaddenLCSimmonsPAEffectiveness of dry eye therapy under conditions of environmental stressCurr Eye Res201338222923623294168
- BenelliUNardiMPosarelliCAlbertTGTear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectivenessCont Lens Anterior Eye2010332616720153684
- Innovative MedicalCross-over evaluation of two lubricating eye drops. NLM Identifier: NCT00493662 [webpage on the Internet]Bethesda, MDClinicaltrials.gov2007 Available from: http://clinicaltrials.gov/show/NCT00493662Accessed September 24, 2013
- Alcon ResearchAcute comfort and blurring profile evaluation of marketed lubricant eye drops. NLM Identifier: NCT00756093 [webpage on the Internet]Bethesda, MDClinicaltrials.gov2008 Available from: http://clinicaltrials.gov/ct2/show/results/NCT00756093Accessed September 24, 2013
- ScaffidiRCKorbDRComparison of the efficacy of two lipid emulsion eyedrops in increasing tear film lipid layer thicknessEye Contact Lens2007331384417224677
- KorbDRScaffidiRCGreinerJVThe effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptomsOptom Vis Sci200582759460116044071
- DauschDLeeSDauschSKimJCSchwertGMichelsonWComparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutesKlin Monbl Augenheilkd200622312974983 German17199193
- ClimentAOComparison of GenAqua-preserved GenTeal in multidose bottles vs preservative-free tears naturale in single dose units in patients with moderate to severe dry eyeInvest Ophthalmol Vis Sci200647 E-Abstract 261–B439
- BlackieCASolomonJDScaffidiRCGreinerJVLempMAKorbDRThe relationship between dry eye symptoms and lipid layer thicknessCornea200928778979419574906
- KnopEKnopNMillarTObataHSullivanDAThe international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian glandInvest Ophthalmol Vis Sci20115241938197821450915
- WhiteCJThomasCRByrneMEBringing comfort to the masses: a novel evaluation of comfort agent solution propertiesCont Lens Anterior Eye2014372819123999507
- WaduthantriSYongSSTanCHHtoonHMTongLLubricant with gelling agent in treating dry eye in adult Chinese patientsOptom Vis Sci201289111647165323069726
- TauberJEfficacy, tolerability and comfort of a 0.3% hypromellose gel ophthalmic lubricant in the treatment of patients with moderate to severe dry eye syndromeCurr Med Res Opin200723112629263617868503
- DedhiaNEfficacy and tolerability of GenTeal Gel in post-menopausal women with moderate to severe dry eye: results of a post marketing observational studyIndian Medical Gazette2012186192
- HartsteinIKhwargSPrzydrygaJAn open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult populationCurr Med Res Opin200521225526015801996
- ChiambarettaFPouliquenPMenerathJMPilotazFRebikaHRigalDEfficacy and safety of a fluid carbomer gel versus a conventional carbomer gel in dry eye treatmentJ Fr Ophtalmol2004272130135 French15029039
- WanderAHKofflerBHExtending the duration of tear film protection in dry eye syndrome: review and retrospective case series study of the hydroxypropyl cellulose ophthalmic insertOcul Surf20097315416219635248
- HillJCSlow-release artificial tear inserts in the treatment of dry eyes in patients with rheumatoid arthritisBr J Ophthalmol19897321511542649149
- LeeSYTongLLipid-containing lubricants for dry eye: a systematic reviewOptom Vis Sci201289111654166123096494
- AsbellPAIncreasing importance of dry eye syndrome and the ideal artificial tear: consensus views from a roundtable discussionCurr Med Res Opin200622112149215717076975
- FurrerPMayerJMGurnyROcular tolerance of preservatives and alternativesEur J Pharm Biopharm200253326328011976014
- CharnockCAre multidose over-the-counter artifical tears adequately preserved?Cornea200625443243716670481
- LazarusHMImperiaPSBottiREMackRJLassJHAn in vitro method which assesses corneal epithelial toxicity due to antineoplastic, preservative and antimicrobial agentsLens Eye Toxic Res198961–259852488034
- RolandoMCriderJYKahookMYOphthalmic preservatives: focus on polyquaternium-1Expert Opin Drug Deliv20118111425143821905766
- KimMSChoiCYKimJMChangHRChungHRWooHYMicrobial contamination of multiply used preservative-free artificial tears packed in reclosable containersBr J Ophthalmol200892111518152118782802
- CömezATTufanHAKocabıyıkOGencerBEffects of lubricating agents with different osmolalities on tear osmolarity and other tear function tests in patients with dry eyeCurr Eye Res201338111095110323841565
- LiuZPflugfelderSCCorneal Surface regularity and the effect of artificial tears in aqueous tear deficiencyOphthalmology1999106593994310328393
- AvundukAMAvundukMCVarnellEDKaufmanHEThe comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical studyAm J Ophthalmol2003136459360214516798
- AlbietzJMLentonLMMcLennanSGEarlMLA comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patientsCLAO J20022829610012054380
- PerryHDoshi-CarnevaleSDonnenfeldEDSolomonRBiserSABloomAHEfficacy of commercially available topical cyclospoine A 0.05% in the treatment of meibomian gland dysfunctionCornea200625217117516371776
- BronAJDaubasPSiou-MermetRTrinquandCComparison of the efficacy and safety of two eye gels in the treatment of dry eyes: Lacrinorm and ViscotearsEye (Lond)199812Pt 583984710070521
- DumbletonKWoodsCFonnDAn investigation of the efficacy of a novel ocular lubricantEye Contact Lens200935314915519421022
- Garcia-LázaroSBelda-SalmerónLFerrer-BlascoTCerviñoAMontés-MicóRComparison of two artificial tear formulations for dry eye through high-resolution optical coherence tomographyClin Exp Optom201194654955621929716
- PrabhasawatPTesavibulNKasetsuwanNPerformance profile of sodium hyaluronate in patients with lipid tear deficiency: randomised, double-blind, controlled, exploratory studyBr J Ophthalmol2007911475016973668
- LeeSDauschSMaierhoferGDauschDA new therapy concept for the treatment of dry eye – the usefulness of phospholipid liposomesKlin Monbl Augenheilkd20042211082583615499517
- CraigJPPurslowCMurphyPJWolffsohnJSEffect of a liposomal spray on the pre-ocular tear filmCont Lens Anterior Eye2010332838720096622
- JacobiCKruseFECursiefenCProspective, randomized, controlled comparison of SYSTANE UD eye drops versus VISINE INTENSIV 1% EDO eye drops for the treatment of moderate dry eyeJ Ocul Pharmacol Ther201228659860322813209
- BhojwaniRCellesiFMainoAJalilAHaiderDNobleBTreatment of dry eye: an analysis of the British Sjögren’s syndrome association comparing substitute tear viscosity and subjective efficacyCont Lens Anterior Eye201134626927321689965
- MarnerKMøollerPMDillonMRask-PedersenEViscous carbomer eye drops in patients with dry eyes. Efficacy and safety. A randomized, open, cross-over, multicentre studyActa Ophthalmol Scand19967432492528828721
- VehigeJUse of a new non-preserved artificial tear for dry eye treatment after LASIK surgeryAm Academy of Optometry2009CN-00746208
- SindtCWFoulksGNEfficacy of an artificial tear emulsion in patients with dry eye associated with meibomian gland dysfunctionClin Ophthalmol201371713172224039391
- KraderCDrops decrease tear evaporationOphthalmology Times42011
- ChristensenMTCorneal staining reductions observed after treatment with Systane lubricant eye dropsAdv Ther200825111191119918972076
- PaughJRNguyenALHuangPHwangJSRetention and retention of effect of topical formulations in dry eye subjectsOptom Vis Sci200885987387918772723
- SánchezMAArriola-VillalobosPTorralbo-JiménezPThe effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometric studyEye (Lond)20102481331133720300126
- VersuraPProfazioVCamposECOne month use of Systane improves ocular surface parameters in subjects with moderate symptoms of ocular drynessClin Ophthalmol20082362963519668764
- DurrieDStahlJA randomized clinical evaluation of the safety of Systane Lubricant Eye Drops for the relief of dry eye symptoms following LASIK refractive surgeryClin Ophthalmol20082497397919668456
- Montés-MicóRCerviñoAFerrer-BlascoTGarcía-LázaroSOrtí-NavarroSOptical quality after instillation of eyedrops in dry-eye syndromeJ Cataract Refract Surg201036693594020494764
