Abstract
A potential association between Alzheimer’s disease (AD) and chronic glaucoma has been suggested but results of epidemiological studies have been inconsistent. Therefore, we performed a systematic review and critical appraisal of this literature. We searched systematically in PubMed from December 1964 to September 2013 and identified 239 articles potentially relevant for abstract and full-text review. Statistical heterogeneity (variability) across studies was evaluated using the Cochran Q test and the I2 statistic, and the Newcastle-Ottawa score was used to assess study quality. Ten studies were finally selected. Compared to non-demented participants, patients with AD had a statistically significant decreased risk of glaucoma but the results were very heterogeneous, and thus summary estimates were not reported (I2, 89%; Pheterogeneity, <0.001). The study results ranged from large positive relative risks identified in small and poorly-conducted studies to weak inverse associations or null estimates observed in some cohort and record-linkage studies, but the summary estimates were essentially driven by a large retrospective cohort using medical claims that may be afflicted by underdiagnosis bias. There was also evidence for substantial publication bias (Egger’s P≤0.01). The association of AD and glaucoma is heterogeneous and most studies are small and inadequately designed. Large prospective studies with long follow-ups are warranted to clarify this association.
Introduction
Recent evidence has suggested a potential association between Alzheimer’s disease (AD) and chronic glaucoma. Glaucoma is a group of optic neuropathies that are characterized by progressive neurodegeneration of retinal ganglion cells and their axons, resulting in structural changes of the optic nerve and visual field defects.Citation1 Elevated intraocular pressure is a major risk factor of glaucoma,Citation2 and open-angle glaucoma is its most prevalent form worldwide.Citation3 Dementia is also a group of neurodegenerative disorders that occurs in the elderly and leads to impaired cognition. AD is the most common type of dementiaCitation4 and is characterized by the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles consisted of hyperphosphorylated tau protein.Citation5
Therefore, it has been suggested that AD and glaucoma are indirectly associated via common pathophysiological mechanisms or risk factors.Citation6–Citation12 Neuro-inflammation may be an important mechanism for the development of both AD and glaucoma.Citation6 The complement component 1q is upregulated in AD, as well as in mouse and monkey glaucoma models.Citation7,Citation8 Elevated tumor necrosis factor-α concentrations contribute to the neurodegeneration process in AD and glaucoma.Citation9,Citation10 In addition, some studies have shown similar levels of both amyloid β1–42 and tau protein in the cerebrospinal fluid of AD patients and in the vitreous fluid of glaucoma patients.Citation11,Citation12
However, it is unclear if the AD and glaucoma association is causal. Some epidemiological studies have reported a higher risk of glaucoma in patients with AD or a higher risk of AD in patients with glaucoma,Citation13–Citation18 whereas other studies reported null or even inverse associations.Citation19–Citation22 The reasons underlying these heterogeneous findings need to be investigated, but no formal evaluation of this literature has been published. Therefore, we performed a systematic review to describe and critically appraise the findings of the AD and chronic glaucoma literature.
Materials and methods
Study identification
We searched systematically in PubMed from December 1964 through September 2013 to identify observational studies that investigated the association between AD or dementia with chronic glaucoma using the following algorithm: “(glaucoma or primary open-angle glaucoma or pseudoexfoliation glaucoma) and (dementia or Alzheimer’s disease or vascular dementia)”. No language restrictions were imposed. We excluded articles that did not have glaucoma or dementia as the outcome, that had no human or original data, and that did not have a control group. We also excluded screening and case-report studies. Our search identified 239 studies potentially relevant for abstract review (). There were 100 studies claimed for full-text review based on information in the abstracts. Of these, ten articles were established as pertinent.Citation13–Citation22 Abstract and full-text review was conducted independently by two investigators (AGT and KKT) and discrepancies were resolved by consensus. The methodological quality of the included studies was assessed independently by AGT and KKT using the Newcastle-Ottawa scale, which accords a maximum of nine points to each study, with five or less points indicating a high risk of bias.Citation23 Data on each study, including location, population, design, number of cases and controls, method of assessment of glaucoma and AD, mean age, percentage of males, type of statistical analysis, relative risk (RR) and 95% confidence interval (CI), matching and adjustment factors were independently abstracted into a standardized form. We further systematically searched for relevant articles in EMBASE and the COCHRANE databases, but no further articles were deemed eligible.
Statistical analysis
We abstracted the maximally adjusted RR estimates and 95% CIs for the association of AD or dementia and glaucoma. The eligible studies reported odds ratios, hazard ratios (HRs) or standardized incidence ratios, which were considered equivalent given that AD and glaucoma are relatively rare except in populations over 80 years.Citation24,Citation25 Some studies did not provide RR estimates in the publication, and we calculated matched or unmatched odds ratios from 2×2 tables.Citation13–Citation16,Citation18 Bayer et al published two small case-control studies in 2002 with insufficient information as to whether they used independent samples.Citation14,Citation15 Further information was requested from the authors but no details were supplied, and the samples were assumed independent for this meta-analysis.
The statistical synthesis was performed using the fixed effects method with each RR estimate weighted by the inverse of its variance. We did not conduct a random effects synthesis, because it tends to be overtly inflated in the presence of small study effects, as the small studies receive increased relative weight in random effects calculations.Citation26 We performed a meta-analysis for the association between AD and glaucoma (eight studies) and separately for dementia and glaucoma (nine studies), but the results were very heterogeneous and were therefore not reported in detail in the text or in tables and figures.
Subgroup analyses were also performed to investigate potential sources of heterogeneity. We repeated the fixed effects synthesis after omitting one study at a time. The association of dementia and glaucoma was assessed by study location (Europe, USA, Asia), design (cohort, record linkage, case-control), number of cases (≥100, <100), use of matching or adjustment (for at least one factor, crude) and mean age of the study population at recruitment (≥75, <75 years). To test whether the summary estimates differed between strata of the latter characteristics, we conducted meta-regression analyses.
Statistical heterogeneity (variability) across studies was evaluated using the Cochran Q test and the I2 statisticCitation27,Citation28 with its corresponding 95% CIs.Citation29 An I2 value of 0% implies lack of heterogeneity, whereas values of 25%, 50% and 75% imply low, medium, and high heterogeneity respectively. Publication bias (bias against the publication of negative results or publication of those results after considerable delay) was quantified from the visual inspection of a funnel plot,Citation30 from the Begg rank correlation methodCitation31 and the Egger’s regression asymmetry test (publication bias considered present if P≤0.10).Citation30 A nonparametric “trim and fill” method that accounts for publication bias was also applied.Citation32 All statistical analyses were performed with STATA software version 12 (StataCorp LP, College station, TX, USA), and all tests were two-sided.
Results
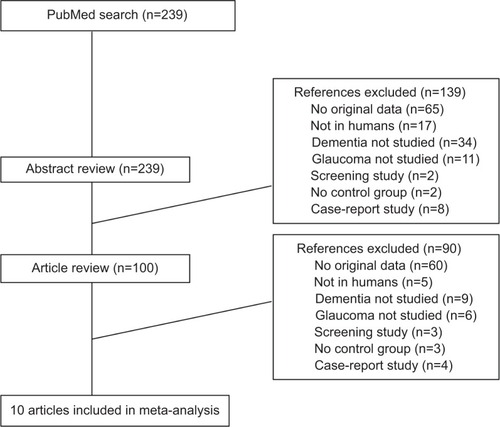
Ten studies were selected according to our inclusion criteria (, and ). Six studies were conducted in Europe,Citation14,Citation15,Citation17,Citation19,Citation20,Citation22 two in the United StatesCitation13,Citation21 and two in Asia.Citation16,Citation18 Five reports were case-control studies,Citation13–Citation16,Citation18 of which two used prevalent cases,Citation14,Citation15 two were record-linkage studiesCitation20,Citation22 and another three were cohort studies,Citation17,Citation19,Citation21 of which one was a retrospective cohort.Citation21 All studies used patients with open angle glaucoma, a form of glaucoma that has been hypothesized to have neurodegenerative elements. The number of cases with glaucoma in these studies ranged between 21 and 63,325. Participants had a mean age at recruitment that ranged from 64 to 83 years. Most case-control studies had less than 350 total participantsCitation14–Citation16,Citation18 except for one study using death certificates that had more than 20,000 participants but very few exposed cases,Citation13 and all observed large positive and strongly significant RRs for the association between AD or dementia and glaucoma. In contrast, the large retrospective cohort study with 63,325 cases of glaucoma observed a statistically significant inverse association,Citation21 whereas other prospective cohort and record-linkage studies reported mixed results.Citation17,Citation19,Citation20,Citation22 One study was further excluded from the meta-analysis, because it did not identify any case with AD, and thus calculated a zero RR.Citation22
Table 1 Study characteristics of the ten eligible studies on dementia and glaucoma risk
Table 2 Population characteristics of the ten eligible studies on dementia and glaucoma risk
The median Newcastle-Ottawa quality score was 4 with an interquartile range (IQR) from 4 to 7 (Tables S1 and S2). The five case-control studiesCitation13–Citation16,Citation18 scored poorly in the quality scale (median, 4; IQR, 2–4) because most studies did not independently validate the case definition; they did not use population-based controls, and did not control for important potential confounders. The four cohort and record-linkage studiesCitation17,Citation19–Citation21 had a median quality score of 7 (IQR, 5.5–7), and all but oneCitation20 had scores of 7.
Compared to non-demented participants, patients with AD (eight studies; RR, 0.92; 95% CI, 0.89–0.94; I2, 89%; Pheterogeneity, <0.001) or with dementia (nine studies; RR, 0.94; 95% CI, 0.92–0.96; I2, 89.4%; Pheterogeneity, <0.001) had a statistically significant decreased risk of glaucoma, respectively ( and ). The study results were very heterogeneous and tended to follow the finding of the largest study in our sample.Citation21 After excluding the Ou et alCitation21 study, the findings changed to a statistically significant positive association, but they were still very heterogeneous.
Figure 2 Study-specific relative risks and 95% confidence intervals (CI) for the association of Alzheimer’s disease (AD) and chronic glaucoma.
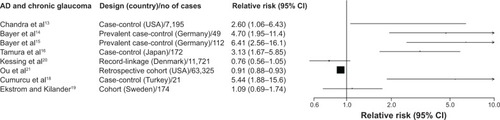
Figure 3 Study-specific relative risks and 95% confidence intervals (CI) for the association of dementia in general and chronic glaucoma.
Funnel plots and statistical tests (AD and glaucoma: Egger’s Pheterogeneity, 0.01; Dementia and glaucoma: Egger’s Pheterogeneity, <0.001) provided evidence for substantial publication bias, as there were several very small studies with large and positive RRs.Citation14–Citation18 When we attempted to correct for publication bias using the non-parametric trim and fill method, the results of the meta-analysis remained identical, as adding several small studies with strong inverse associations to the funnel plot did not leverage the results of the large Ou et al study. In meta-analyses performed by study location, design, number of cases, use of adjustments for confounders and mean age, dementia was associated with a lower risk of glaucoma in the subgroups where the Ou et al study belonged (studies in USA, cohorts, number of cases ≥100, adjusted results, and mean age ≥75 years), whereas the summary results were significantly positive in all other subgroups (Table S3).
Discussion
In this systematic review, we critically appraised ten studies for the association between AD or dementia and chronic glaucoma. The findings of the ten studies were extremely heterogeneous and ranged from large positive RRs identified in small and poorly-conducted case-control studies to weak inverse associations or null estimates observed in some cohort and record-linkage studies. The summary estimate was identical to the result of the large Ou et al retrospective cohort study, which reported an inverse association between AD and glaucoma but this study used medical claims to infer about diagnosis of dementia and glaucoma and it may be afflicted by underdiagnosis bias.
It is unclear pathophysiologically why there should be an inverse association between AD and glaucoma. Although the literature has not provided potential mechanistic clues for an inverse association, many researchers have suggested mechanisms explaining a positive association between the two diseases. It has been shown that patients with AD exhibit a cerebrospinal fluid stasis due to decreased secretion and increased resistance to cerebrospinal fluid outflow,Citation33–Citation36 resulting in reduced clearance of the toxic molecules in the subarachnoid space of the optic nerve and a low cerebrospinal fluid pressure, which may lead to an abnormal high trans-lamina cribrosa pressure difference and to a larger cup-to-disc ratio followed by glaucomatous damage.Citation37–Citation40 However, other studies have suggested that even though an abnormal high trans-lamina cribrosa pressure difference is created, there is no observable alteration in the lamina cribrosa resulting in no optic disc cupping,Citation41 which does not support an association between AD and glaucoma. Several factors should be considered for the interpretation of our findings. AD and chronic glaucoma are both age-related neurodegenerative diseases that may very well co-exist in the elderly due to common risk factors or pathophysiological mechanisms. However, to infer a direct and causal association between them, prospective designs are warranted where chronic glaucoma is evaluated many years after the diagnosis of AD (or vice versa) in participants who are free of glaucoma (or any early signs of it) at the start of the study. Sensitivity analyses where the first 2–5 years of follow-up are discarded may assist in identifying a potential causal effect.
However, half of the studies that were included in this systematic review had a case-control design that precludes a valid assessment of the association between AD and glaucoma, as both diseases were evaluated cross-sectionally at recruitment.Citation13–Citation16,Citation18 The study sample also included one record-linkageCitation20 and three cohort studies.Citation17,Citation19,Citation21 The retrospective cohort by Ou et al used Medicare data to identify 63,325 participants with glaucoma and 63,325 participants without glaucoma who had no AD or other dementia at recruitment, and were followed up for 14 years.Citation21 This was by far the largest study in our sample, and found a statistically significant inverse association between glaucoma and AD (HR, 0.91; 95% CI, 0.88–0.93). The use of administrative data in this study may lead to misclassification of the diagnoses of glaucoma and/or dementia and to potential underdiagnosis bias. Patients with cognitive decline are perhaps less likely to undergo formal ophthalmologic testing and this could cause the non-glaucoma group to contain a larger proportion of individuals who are subsequently diagnosed with dementia. The French cohort by Helmer et al studied 812 volunteers and identified 41 cases of dementia in 3 years of total follow-up.Citation17 They observed a strong statistically significant positive association between dementia and glaucoma (HR, 3.90; 95% CI, 1.50–10.4). Although this study was prospective and excluded demented participants at recruitment, its short follow-up led to a small number of incident dementia cases who most likely had brain pathology at least at a mild level at baseline. The third cohort study was conducted in Sweden among 1,123 city residents; it identified 174 new cases of AD in a maximum follow-up of 25 years and reported a non-significant HR of 1.09 (95% CI, 0.69–1.74) between AD and chronic glaucoma.Citation19
High heterogeneity was ubiquitous in all of our analyses. Heterogeneity may be introduced because of the methodologic or demographic differences among studies. However, we were unable to identify the exact sources of heterogeneity in our systematic review, because the summary results were always driven by the large Ou et al study, even though we performed subgroup and meta-regression analyses on several factors. However, even when we reran the meta-analysis after excluding the Ou et al study, the results remained heterogeneous. Therefore, we decided not to report in detail the summary RRs.
Meta-analyses of observational studies are also vulnerable to residual confounding inherent in the original studies. The Ou et al and Helmer et al studies both adjusted for a range of confounders that included age, sex, race, education, family history of glaucoma, and comorbidities.Citation17,Citation21 The case-control studies adjusted only for age and/or sex, whereas some studies did report frequency matching for a wider set of confounders;Citation14–Citation16,Citation18 however, RRs were not reported in these studies and had to be calculated crudely from 2×2 tables, which may explain at least partially why the authors observed strong positive associations, as some comorbidities and the black race are positively associated with both AD and glaucoma and could cause the overestimation of the unadjusted results.
Conclusion
In summary, the association of AD and chronic glaucoma is heterogeneous in the literature and most studies are small and inadequately designed. Large and high-quality prospective studies with long follow-ups are needed to clarify the existence, magnitude, and natural history of this potential association.
Author contributions
All authors (AGT, KKT, S-HP, GK) contributed to the conception and design of the study. AGT and KKT acquired the data for the systematic review, performed the statistical analysis, and wrote the paper. S-HP and GK contributed critically to the revisions of the paper and had valuable input to the clinical interpretation of the discussion. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Supplementary materials
Table S1 Newcastle-Ottawa scale for the quality assessment of five case-control studies on dementia and glaucoma risk
Table S2 Newcastle-Ottawa scale for the quality assessment of four cohort and record-linkage studies on dementia and glaucoma risk
Table S3 Summary fixed-effects relative risks (RR) and 95% confidence intervals (CI) for the association of dementia in general and glaucoma in subgroups
Disclosure
None of the authors had financial support related to this study. The authors declare that there is no conflict of interests regarding the publication of this paper. This submission has not been published elsewhere previously and it is not currently under consideration by another journal.
References
- BolandMVErvinAMFriedmanDTreatment for Glaucoma: Comparative EffectivenessRockville (MD)Agency for Healthcare Research and Quality (US)2012
- NickellsRWHowellGRSotoIJohnSWUnder pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathyAnnu Rev Neurosci20123515317922524788
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- ReitzCBrayneCMayeuxREpidemiology of Alzheimer diseaseNat Rev Neurol20117313715221304480
- Bossy-WetzelESchwarzenbacherRLiptonSAMolecular pathways to neurodegenerationNat Med200410SupplS2S915272266
- McKinnonSJThe cell and molecular biology of glaucoma: common neurodegenerative pathways and relevance to glaucomaInvest Ophthalmol Vis Sci20125352485248722562847
- FonsecaMIChuSHBerciAMContribution of complement activation pathways to neuropathology differs among mouse models of Alzheimer’s diseaseJ Neuroinflammation201181421235806
- StasiKNagelDYangXComplement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyesInvest Ophthalmol Vis Sci20064731024102916505037
- TezelGTNF-alpha signaling in glaucomatous neurodegenerationProg Brain Res200817340942118929124
- TobinickETumour necrosis factor modulation for treatment of Alzheimer’s disease: rationale and current evidenceCNS Drugs200923971372519689163
- EngelborghsSDe VreeseKVan de CasteeleTDiagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementiaNeurobiol Aging20082981143115917428581
- YonedaSHaraHHirataAFukushimaMInomataYTaniharaHVitreous fluid levels of beta-amyloid(1–42) and tau in patients with retinal diseasesJpn J Ophthalmol200549210610815838725
- ChandraVBharuchaNESchoenbergBSConditions associated with Alzheimer’s disease at death: case-control studyNeurology19863622092113945392
- BayerAUKellerONFerrariFMaagKPAssociation of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s diseaseAm J Ophthalmol2002133113513711755850
- BayerAUFerrariFErbCHigh occurrence rate of glaucoma among patients with Alzheimer’s diseaseEur Neurol200247316516811914555
- TamuraHKawakamiHKanamotoTHigh frequency of open-angle glaucoma in Japanese patients with Alzheimer’s diseaseJ Neurol Sci20062461–2798316564058
- HelmerCMaletFRougierMBIs there a link between open-angle glaucoma and dementia? The Three-City-Alienor CohortAnn Neurol201374217117923686609
- CumurcuTDorakFCumurcuBEErbayLGOzsoyEIs there any relation between pseudoexfoliation syndrome and Alzheimer’s type dementia?Semin Ophthalmol201328422422923662834
- EkstromCKilanderLPseudoexfoliation and Alzheimer’s disease: a population-based 30-year follow-up studyActa Ophthalmol201492435535823879292
- KessingLVLopezAGAndersenPKKessingSVNo increased risk of developing Alzheimer disease in patients with glaucomaJ Glaucoma2007161475117224749
- OuYGrossmanDSLeePPSloanFAGlaucoma, Alzheimer disease and other dementia: a longitudinal analysisOphthalmic Epidemiol201219528529222978529
- Bach-HolmDKessingSVMogensenUFormanJLAndersenPKKessingLVNormal tension glaucoma and Alzheimer disease: comorbidity?Acta Ophthalmol201290768368521332678
- Ottawa Hospital Research Institute [homepage on the Internet]WellsGASheaBO’ ConnellDPetersonJWelchVLososMTugwellPThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxfordAccessed October 2, 2013
- FerriCPPrinceMBrayneCGlobal prevalence of dementia: a Delphi consensus studyLancet200536695032112211716360788
- FriedmanDSWolfsRCO’ ColmainBJPrevalence of open-angle glaucoma among adults in the United StatesArch Ophthalmol2004122453253815078671
- HigginsJGreenSCochrane Handbook for Systematic Reviews of InterventionsEngland: ChichesterThe Cochrane Collaboration and John Wiley & Sons Ltd2008
- CochranWGThe combination of estimates from different experimentsBiometrics1954101101129
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- IoannidisJPPatsopoulosNAEvangelouEUncertainty in heterogeneity estimates in meta-analysesBMJ2007335762691491617974687
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- DuvalSTweedieRA nonparametric “trim and fill” method of accounting for publication bias in meta-analysisJournal of the American Statistical Association2000958998
- SerotJMBeneMCFaureGCChoroid plexus, aging of the brain, and Alzheimer’s diseaseFront Biosci20038s515s52112700093
- SilverbergGDHeitGHuhnSThe cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s typeNeurology200157101763176611723260
- SerotJMZmudkaJJouannyPA possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer’s diseaseJ Alzheimers Dis2012301172622349684
- SilverbergGDMayoMSaulTRubensteinEMcGuireDAlzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesisLancet Neurol20032850651112878439
- WostynPAudenaertKDe DeynPPAn abnormal high trans-lamina cribrosa pressure difference: a missing link between Alzheimer’s disease and glaucoma?Clin Neurol Neurosurg2008110775375418603354
- WostynPAudenaertKDe DeynPPAlzheimer’s disease and glaucoma: is there a causal relationship?Br J Ophthalmol200993121557155919286688
- WostynPAudenaertKDe DeynPPMore advanced Alzheimer’s disease may be associated with a decrease in cerebrospinal fluid pressureCerebrospinal Fluid Res200961419917128
- WostynPDe GrootVVan DamDAudenaertKDe DeynPPSenescent changes in cerebrospinal fluid circulatory physiology and their role in the pathogenesis of normal-tension glaucomaAm J Ophthalmol2013156151423608683
- HayrehSSCerebrospinal fluid pressure and glaucomatous optic disc cuppingGraefes Arch Clin Exp Ophthalmol2009247672172418987870