Abstract
Neovascular age-related macular degeneration is a leading cause of blindness in the developed world. Currently, the treatment of choice is intravitreal injections of anti-VEGF medications. These require frequent dosing, up to monthly, and impose a substantial burden on patients and the health economy. Ionizing radiation was proposed as a possible treatment for age-related macular degeneration due to its anti-inflammatory and anti-fibrotic properties. Stereotactic radiotherapy is an outpatient-based radiotherapy platform that provides stereotactic application of low energy X-ray to the retina in three highly collimated beams that cross the inferior sclera to overlap at the macula. A randomized, double-masked, sham-controlled trial of 230 patients (INTREPID) showed that a single dose of stereotactic radiotherapy significantly reduces the number of intravitreal anti-VEGF injections needed over 2 years. A larger randomized controlled trial (STAR) is underway.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in individuals over 50 years old in developed nations.Citation1,Citation2 It can have a profound impact on quality of life, and affects more than 500,000 people in the UK alone.Citation3 Approximately 10% of those affected have the neovascular “wet” form of the disease, which is clinically more aggressive and destructive, but also more amenable to treatment. The mainstay of treatment for wet AMD is chronic treatment with anti-VEGF drugs, with regular, ongoing clinical review from the point of diagnosis. Given the rapidly aging population and constrained health care budgets, there is an unmet need for a more cost effective treatment, and one that does not involve such an intensive treatment regimen.
Wet AMD is characterized by the growth of abnormal new blood vessels in a process called choroidal neovascularization. These vessels originate from the choriocapillaris, and traverse Bruch’s membrane into the sub-retinal pigment epithelial and sub-retinal spaces. The new vessels have poor structural integrity and tend to leak, resulting in hemorrhage and edema, which results in degradation of visual function. This process is often irreversible, leading to scarring and ultimately permanent loss of central vision.
At present, the main strategy for the treatment of wet AMD is via modulators of VEGF.Citation4 There are several anti-VEGF drugs available, including pegaptanib, ranibizumab, bevacizumab, and aflibercept. Pivotal studies demonstrated significant visual improvements using ranibizumab, given on a monthly basis.Citation5,Citation6 The Comparison of AMD Treatments Trials (CATT) study, analyzing visual outcomes with bevacizumab and ranibizumab therapy, found no significant difference between the two agents at 1 and 2 years, when given at the same dosing regimen. Additionally, both agents showed better visual outcomes when administered monthly, compared with “as required” (pro re nata [PRN]) dosing.Citation7,Citation8 Monthly dosing imposes a significant economic and personal burden on the health care sectors and patients respectively, and therefore a less intensive treatment modality would be desirable. Furthermore, each injection carries small risks, such as endophthalmitis and retinal detachment, and therefore a reduction in their frequency, without compromising efficacy, would be a substantial advance.Citation9
Radiation and neovascular AMD
Radiation is defined as the outward emission of energy from a central source and is commonly used in medical practice. Most commonly, it is used as a diagnostic tool, for example with plain radiographs and computed tomography. Secondly, radioactive substances can be introduced inside the body and their release of radiation used for therapeutic purposes such as radioisotope treatment of hyperthyroidism. Additionally, high powered X-ray machines or radioactive sources can be used to deliver radiotherapy when they are aimed into the body from an external source.
Radiation is used to selectively and irreversibly damage the DNA of target cells which prevents further replication. Cells that are rapidly dividing, or of abnormal morphology, undergo apoptosis following radiation therapy, whereas non-dividing cells are better able to repair the damage and remain structurally intact.Citation10
Treatment with radiation can be divided into two categories. Brachytherapy, known as internal radiotherapy, refers to a radiation source being directly inserted or placed next to the target site surgically, for example in the treatment of prostate tumors.Citation11,Citation12 Teletherapy uses an external source to channel radiation into a fine beam that is projected to the target tissue. Teletherapy is commonly utilized in oncology, particularly with breast malignancy.Citation13 One form of tele-therapy is stereotactic radiotherapy (SRT), where multiple narrow beams of radiation can be used to target small well defined areas with precision.
Some radioactive sources, for example potassium-40 and strontium-90, emit high-speed electrons or positrons in a process called beta decay. The potential benefit of beta decay in ophthalmology was predicted in animal models in 1987, when cultured monkey ocular fibroblasts were shown to undergo rapidly reduced proliferation following exposure in vitro.Citation14 Since then, there have been reports of radiation used as an adjunctive treatment for pterygium and glaucoma filtration surgery.Citation10,Citation15
Radiation can also be used to treat wet AMD, since this disease is caused by abnormal cell proliferation and radiation preferentially damages these cells.Citation16–Citation19 However, the introduction of anti-VEGF therapy produced better visual outcomes than radiation and therefore the use of radiation diminished.Citation5,Citation20 Studies are now investigating whether radiation might have a role as an adjuvant to anti-VEGF therapy. This adjuvant approach is supported by results in other branches of medicine, where combined treatment produces better results than either treatment modality individually, for example alongside chemotherapy in breast cancer.Citation21 Anti-VEGF agents have a rapid onset of action, but they typically require long-term, repeated dosing to maintain their therapeutic effect. As radiotherapy has a delayed effect, but a long duration of action, it may work synergistically with anti-VEGF therapy.
Epimacular brachytherapy
Epimacular brachytherapy (EMB) was designed as a way of precisely delivering radiation to the macula in patients with wet AMD.Citation22 The EMB device (NeoVista Inc., Fremont, CA, USA) contained a strontium-90/yttrium-90 source that delivered radiation to the macula using an intraocular probe during vitrectomy. The Macular Epiretinal Brachytherapy in Treated Age-related Macular Degeneration (MERITAGE) study was an international, multicenter, prospective, interventional, non-controlled clinical trial of 53 patients with previously treated, active disease. All patients underwent vitrectomy with EMB, and received PRN ranibizumab treatment, governed by pre-defined retreatment criteria. At 1 year follow-up, 81% of participants lost fewer than 15 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters whilst only 3.49 anti-VEGF retreatments were required.Citation23 Secondary outcomes showed a mean best corrected visual acuity change of −4.0 ETDRS letters and a small increase in optical coherence tomography central retinal thickness of 50 μm, that failed to reach significance. Subgroup analysis revealed that predominantly classic lesions did best, with a mean gain of 1.5 ETDRS letters and mean center point thickness decreased by 43 μm.Citation24 The 2-year data on the same cohort showed more frequent ranibizumab retreatment than in year 1, increasing from 0.29 injections per month in months 0–12, to 0.44 injections per month in months 13–24, however both were less than occurred pre-radiation (0.50 injections per month).Citation25 Two participants developed possible non-proliferative radiation retinopathy over 24-month follow-up, but this did not negatively affect their vision. There were no other adverse events attributed to radiation therapy.Citation25
The Choroidal Neovascularization Secondary to AMD Treated with Beta Radiation Epiretinal Therapy (CABERNET) trial was a large, pivotal, multicenter, randomized controlled trial investigating the use of EMB in 495 treatment naïve patients. At 24 months, 77% and 90% of the EMB group and control group lost fewer than 15 ETDRS letters respectively. As such the trial failed to meet its non-inferiority endpoint. Furthermore, 16% of the EMB group versus (vs) 26% of the control group gained more than 15 letters. There was a reduction in the number of injections in the EMB group, at 6.2, compared to 10.4 in the control group, although this comparison did not necessarily indicate a reduced demand for anti-VEGF therapy in the radiation arm, as this arm had fewer mandated injections. Although 3% of the EMB group had mild non-proliferative radiation retinopathy, all these cases lost fewer than 15 ETDRS letters with a mean gain of 4.4 letters at 24 months. Further, the radiation changes were often relatively subtle, and substantially different to the clinical picture observed with high dose radiation for ocular tumors. The participants with retinopathy had better outcomes than those who did not, and overall safety was deemed acceptable.Citation26,Citation27
In summary, the encouraging early clinical trials of EMB for treatment naïve disease were not replicated in a Phase III pivotal trial (CABERNET), and therefore its use in this setting is unproven.Citation28 A pivotal study in previously treated disease (MERLOT; ClinicalTrials.gov identifier NCT01006538) is expected to report soon.
SRT
Introduction
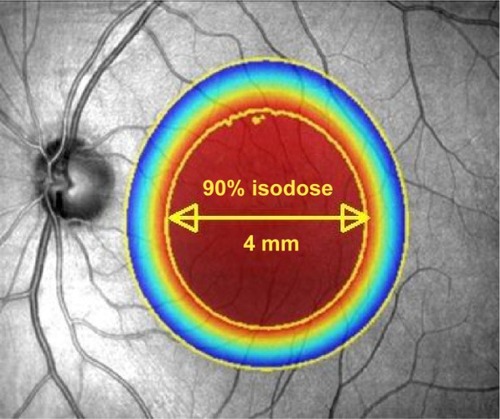
In order to improve the delivery of radiation to the neovascular lesion, a US biotechnology company (Oraya Therapeutics, Newark, CA, USA) developed an SRT device (IRay) designed specifically to treat wet AMD in a single treatment session. The device uses low-voltage X-rays to generate three separate beams of highly collimated radiation that pass through the inferior sclera at different angles to overlap at the macula in a 4 mm diameter treatment zone ( and ). This is designed to minimize the radiation exposure to surrounding healthy tissue, whilst giving a high dose to the desired target. Unlike EMB, SRT does not require vitrectomy and therefore gives a more practical method for radiation delivery. Additionally, this may be advantageous as vitrectomy reduces the half-life of intravitreal drugs, so that any remaining disease activity may be hard to control with anti-VEGF agents.Citation29 Furthermore, with IRay, the entire 4 mm treatment zone receives 90% of the intended dose, whereas with EMB the dose declines exponentially with distance from the source, meaning larger lesions received lower doses peripherally.Citation30
Figure 1 Diagram showing the location of three collimated beams of radiation passing through the inferior sclera (green circles) to avoid the crystalline lens, during stereotactic radiotherapy.
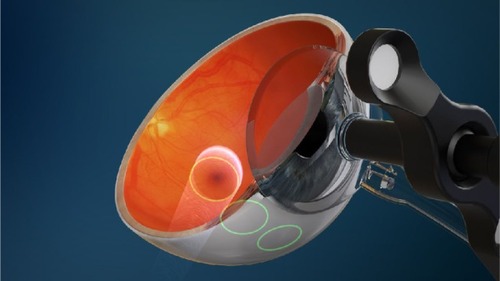
Figure 2 Illustration to show the attenuation effect of the three collimated beams to deliver 90% of the desired radiation dose to a 4 mm diameter at the macula.
Details of the procedure
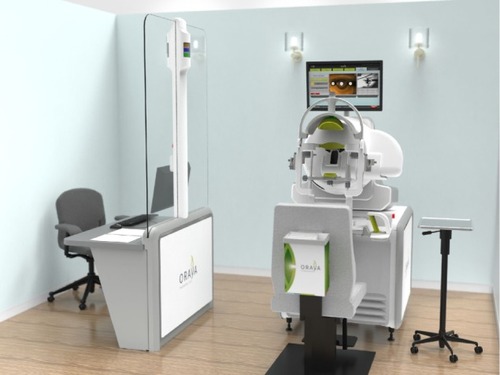
The SRT machine is an outpatient radiotherapy platform that measures approximately 1.5 meters ×2 meters and is designed for use in a standard medical office, without the need for added room radiation shielding ().Citation31,Citation32 The clinician is separated from the patient and protected from radiation by a lead screen. Due to the focused nature of the low-energy beams, no intrinsic radiation protection such as lead aprons are required for the patient or operator.Citation33 The machine has a number of key components in order to accurately and safely deliver treatment. A low energy X-ray tube produces three narrow collimated beams, which are designed to pass through the inferior sclera, avoiding the crystalline lens, and focus on the macula. Built-in computer software is used to robotically position the treatment based on the axial length of the eye that is entered into the machine by the clinician.
Figure 3 The room set-up of the IRay system.
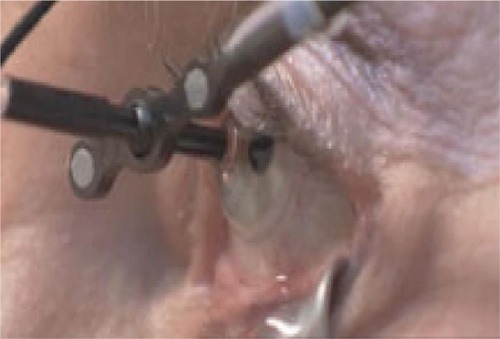
The patient sits normally with their chin placed on a chin rest in order to receive the treatment. A sterile, disposable contact lens is placed centrally on the cornea and sclera, and kept in place by a light suction mechanism (). A small retractor is used to retract the lower eyelid and expose the inferior sclera to the radiation beams. The radiation delivery then commences and treatment usually takes approximately 10 minutes. The machine uses an eye tracking device to continuously monitor the eye position, and radiation treatment is automatically shut down if excessive eye movement is detected.
Figure 4 A contact lens, with light suction mechanism, is used to keep the eye in position during treatment.
Efficacy
Two Phase I studies were carried out to assess the safety and preliminary efficacy of different doses of SRT.Citation34,Citation35 Radiation doses of 16 Gy and 24 Gy in conjunction with ranibizumab produced improvement in visual acuity at 6 months. There were no safety concerns, and in particular no cases of radiation retinopathy, although follow-up was only reported at 6 months.
Following on from the promising Phase I study results, the multicenter, randomized, double-masked, sham-controlled IRay plus Anti-VEGF Treatment For Patients with Wet AMD (INTREPID) trial investigated the use of SRT for previously treated wet AMD (ClinicalTrials.gov identifier NCT01016873).Citation36 A total of 230 patients were randomized 2:1:2:1 to 16 Gy, sham 16 Gy, 24 Gy, or sham 24 Gy – all arms received PRN ranibizumab based on pre-defined retreatment criteria. At 1 year follow-up, both the SRT groups (16 Gy and 24 Gy) received significantly fewer ranibizumab injections than the sham arms (2.64, 2.43 and 3.74 respectively), thus meeting the study’s primary end point.Citation36 The effect on reduction of number of injections was sustained at 2 years (4.5, 5.4 and 6.6 respectively) with statistical significance for the 16 Gy group (P=0.008). Also, 15% of those receiving SRT required no further ranibizumab injections over 2 years.Citation37
The 16 Gy, 24 Gy, and sham arms gained 0 or more letters in 32%, 43%, and 38% of eyes respectively, with 68%, 75%, and 79% losing fewer than 15 letters, respectively at 24 months. A pre-defined year 1 subgroup analysis showed SRT was more effective in lesions ≤4 mm in greatest linear dimension, which correlates with the 4 mm diameter treatment zone. Those with macular volumes greater than the median value of 7.4 mm3 also had better outcomes with radiotherapy. The presence of both a greatest linear dimension ≤4 mm and a macular volume ≥7.4 mm3 was associated with a 55% reduction in the number of ranibizumab injections compared to sham (2.08 vs 4.60; P=0.0002), better visual outcome (2.18 vs –3.15 letters; P=0.0284) and superior structural outcome (−122.6 μm vs −51.5 μm reduction in mean central subfield thickness on optical coherence tomography; P=0.027).Citation38
Based on these subgroup analyses it seems reasonable to use this device only when the patient has significant active leakage, and to avoid any patient whose lesion extends beyond the 4 mm treatment zone, which is centered at the fovea.
Strengths of the INTREPID study are its randomized, double-masked, sham-controlled design. Weaknesses include the fact that it was not designed to establish efficacy beyond year 1; the visits at year 2 and 3 were designed to assess safety. After year 1 the anti-VEGF treatment was dictated by the prevailing standard of care across a range of sites and countries. The study was designed to determine if radiotherapy reduces the number of injections, and not to detect a difference in visual acuity outcomes.
Safety
In terms of safety, over 24 months, there were similar numbers of adverse events and serious adverse events in both arms. Eighteen participants in the radiotherapy arms were identified as having microvascular abnormalities (MVAs) by a panel of experts, of which two were considered likely to have affected vision. These two cases lost 46 letters and 41 letters respectively, with 13 and eight PRN ranibizumab injections needed respectively, but it was concluded that the underlying AMD process was likely to have been the main contributor to visual loss in each case. All other 16 cases had MVAs which were extra-foveal, and ten of these were located outside the 4 mm treatment area. Overall, the mean visual change in the 18 eyes with radiation attributed MVAs was similar to the mean change in the total cohort receiving SRT.Citation37
Conclusion
The clinical, financial, and social burden of AMD is substantial, and continues to rise with an aging population. It is therefore important to explore novel treatment options for the expanding cohort of patients undergoing regular intravitreal injections. Radiation has many theoretical advantages as a means of treating wet AMD, and following the encouraging results of INTREPID, a large randomized, double-masked, sham-controlled clinical trial is underway (STAR). Like INTREPID, the STAR study is designed to establish if SRT reduces the number of anti-VEGF injections that patients require, but it targets the best responder group identified in the INTREPID subgroup analysis. Unlike INTREPID, STAR is powered to determine if visual acuity is non-inferior to anti-VEGF monotherapy, and with extended safety follow-up over 4 years (ClinicalTrials.gov identifier NCT02243878).
Disclosure
Timothy L Jackson’s employer has received research funding from NeoVista Inc., Oraya Therapeutics, and Novartis. The authors have no other conflicts of interest to disclose.
References
- CongdonNO’ColmainBKlaverCCCauses and prevalence of visual impairment among adults in the United StatesArch Ophthalmol2004122447748515078664
- PascoliniDMariottiSPPokharelGP2002 global update of available data on visual impairment: a compilation of population-based prevalence studiesOphthalmic Epidemiol20041126711515255026
- Macularsociety.org [homepage on the Internet]The Macular SocietyUnited Kingdom2013 Available from: www.macularsociety.orgAccessed July 15, 2013
- WongTYLiewGMitchellPClinical update: new treatments for age-related macular degenerationLancet2007370958320420617658379
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- BrownDMKaiserPKMichelsMRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- CATT Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- FalavarjaniKGNguyenQDAdverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literatureEye (Lond)201327778779423722722
- KirwanJFConstablePHMurdochIEKhawPTBeta irradiation: new uses for an old treatment: a reviewEye (Lond)200317220721512640408
- BarnhardHJSupervoltage therapy comes of ageN Engl J Med1958258627527713504458
- PaulsonDFCarcinoma of the prostate: the therapeutic dilemmaAnnu Rev Med1984353413726372662
- LenzMFreidJRMetastases to the skeleton, brain and spinal cord from cancer of the breast and the effect of radiotherapyAnn Surg193193127829317866472
- NevarezJAParrishRD2ndHeuerDKHajekASHoudekPVMallickKSThe effect of beta irradiation on monkey Tenon’s capsule fibroblasts in tissue cultureCurr Eye Res1987657197233595181
- MeadKWBeta irradiation for recurrent pterygiaTrans Ophthalmol Soc Aust19561610110913467984
- ChakravarthyUHoustonRFArcherDBTreatment of age-related subfoveal neovascular membranes by teletherapy: a pilot studyBr J Ophthalmol19937752652738318462
- ReinholdHSBuismanGHRadiosensitivity of capillary endotheliumBr J Radiol19734654154574683331
- ChakravarthyUMackenzieGExternal beam radiotherapy in exudative age-related macular degeneration: a pooled analysis of phase I dataBr J Radiol20007386730531310817048
- JaakkolaAHeikkonenJTommilaPLaatikainenLImmonenIStrontium plaque brachytherapy for exudative age-related macular degeneration: three-year results of a randomised studyOphthalmology2005112456757315808245
- EvansJRSivagnanavelVChongVRadiotherapy for neovascular age-related macular degenerationCochrane Database Syst Rev20105CD00400420464726
- RagazJJacksonSMLeNAdjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancerN Engl J Med1997337149569629309100
- PetrarcaRJacksonTLRadiation therapy for neovascular age-related macular degenerationClin Ophthalmol20115576321311657
- DugelPUPetrarcaRBennettMMacular epiretinal brachytheraoy in treated age-related macular degeneration: MERITAGE study: twelve-month safety and efficacy resultsOphthalmology201211971425143122465819
- PetrarcaRDugelPUNauJSlakterJSJaffeGJJacksonTLMacular epiretinal brachytherapy in treated age-related macular degeneration (MERITAGE): month 12 optical coherence tomography and fluorescein angiographyOphthalmology2013120232833323178157
- PetrarcaRDugelPUBennettMMacular Epiretinal Brachy- therapy in treated Age-Related Macular Degeneration (MERITAGE): Month 24 Safety and Efficacy ResultsRetina201434587487924169101
- DugelPUBebchukJDNauJEpimacular brachytherapy for neovascular age-related macular degeneration: a randomized, controlled trial (CABERNET)Ophthalmology2013120231732723174399
- JacksonTLDugelPUBebchukJDEpimacular Brachytherapy for Neovascular Age-Related Macular Degeneration (CABERNET): Fluoroscein Angiography and Optical Coherence TomographyOphthalmology201312081597160323490325
- Casaroli-MaranoRPAlforjaSGiraltJFarahMEEpimacular brachytherapy for wet AMD: current perspectivesClin Ophthalmol201481661167025210436
- BeerPMBakriRJSinghRKLiuWPetersGB3rdMillerMIntraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injectionOphthalmology2003110468168612689886
- PetrarcaRRichardsonMDouiriASafety testing of epimacular brachytherapy with microperimetry and indocyanine green angiography: 12-Month ResultsRetina20133361232124023508075
- HanlonJLeeCChellEKilovoltage stereotactic radiosurgery for age-related macular degeneration: assessment of optic nerve dose and patient effective doseMed Phys20093683671368119746800
- MoshfeghiDMKaiserPKGertnerMStereotactic low-voltage x-ray irradiation for age-related macular degenerationBr J Ophthalmol201195218518820852318
- Orayainc.com [homepage on the Internet]USAOraya Therapeutics, Inc2014 Available from: http://www.orayainc.comAccessed December 6, 2014
- CantonVMQuiroz-MercadoHVelez-MontoyaR16 Gy low-voltage x-ray irradiation with ranibizumab therapy for AMD: 6-month safety and functional outcomesOphthalmic Surg Lasers Imaging201142646847321830747
- CantonVMQuiroz-MercadoHVelez-MontoyaR24-Gy low-voltage x-ray irradiation with ranibizumab therapy for neovascular AMD: 6-month safety and functional outcomesOphthalmic Surg Lasers Imaging2012431202422251841
- JacksonTLChakravarthyUKaiserPKStereotactic radiotherapy for neovascular age-related macular degeneration: 52-week safety and efficacy results of the INTREPID studyOphthalmology201312091893190023490327
- JacksonTLChakravarthyUSlakterJSStereotactic Radiotherapy for Neovascular Age-Related Macular Degeneration: Year 2 Results of the INTREPID StudyOphthalmology2015122113814525208859
- JacksonTLShustermanEMArnoldussenMChellEWangKMoshfeghiDMStereotactic Radiotherapy for wet age-related macular degeneration (INTREPID): Influence of baseline characteristics on clinical responseRetina (Philadelphia, Pa.)2015352194204
