Abstract
Aim
To compare the visual outcomes of an urban population with age-related macular degeneration (AMD) undergoing ranibizumab monotherapy to the results from major clinical trials.
Procedures
Prospective data was collected from 164 wet AMD patients receiving intravitreal ranibizumab. Visual acuities were obtained with the Early Treatment Diabetic Retinopathy Study chart. All patients underwent a loading phase of three monthly treatments of ranibizumab. Patients were monitored monthly using a retreatment criterion. Treatment was further individualized by sequentially lengthening follow-up intervals when stable.
Results
At 12 and 24 months, respectively, the percentage of eyes that maintained vision was 91% and 88.6%. We found that 20.3% of eyes had improved vision at 12 months and 20% at 24 months. At 12 months, 8.3% of eyes’ vision worsened and 12% worsened at 24 months.
Conclusion
Individualized ranibizumab monotherapy is effective in preserving vision in wet AMD and follows the same trends as the pivotal trials.
Introduction
Age-related macular degeneration (AMD) is the commonest cause of severe visual impairment in older people in the developed world.Citation1,Citation2 The two late phenotypes of AMD are geographic atrophy and wet AMD; together, these are responsible for two-thirds of registrations of visual impairment or blindness in the United Kingdom.Citation2 Vision loss in wet AMD is caused by formation of a choroidal neovascular membrane (CNVM) responding to vascular endothelial growth factor (VEGF). It is thought that wet AMD accounts for only 10%–15% of all AMD cases but is responsible for over 80% of AMD-related severe visual impairment or visual loss.Citation3
The minimally classic/occult trial of the anti-VEGF antibody ranibizumab in the treatment of neovascular AMD (MARINA) and anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularization in AMD (ANCHOR) trials were large multicenter double-blind studies which established that continuous treatment with intravitreal ranibizumab was an effective treatment for wet AMD.Citation4,Citation5 Following this, comparison of age-related macular degeneration treatment trials (CATT) proved that treatment with ranibizumab on an as-required basis following the initial three monthly loading dose was equally as effective as continuous monthly treatments.Citation6,Citation7
The CATT trial retreatment criteria was based on signs of CNVM activity: fluid on optical coherence tomography (OCT), new or persistent hemorrhage, decreased visual acuity as compared with the previous examination, dye leakage, or increased lesion size on fundus fluorescein angiography.Citation6
Following these trials, ranibizumab intravitreal injections have been employed as first-line treatment for wet AMD. Since this change in the treatment of wet AMD, little has been published in the UK regarding real population visual outcomes. We wanted to analyze our inner-city AMD patient cohort and compare their visual outcomes following ranibizumab monotherapy to the major clinical trials.
Aims
To compare the results from an inner-city population to the results achieved in the major clinical trials described in the introduction.
Methods
From December 2007 to January 2012, prospective data were collected from 164 patients who underwent intravitreal ranibizumab treatment for wet AMD and entered into an in-house, password-protected spreadsheet. Baseline and follow-up visual acuity measurements were obtained with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Patient consent to publish their demographics and visual outcome data was gained when the decision to proceed with treatment was made. The study was approved by the ethics committee at London North West Healthcare NHS Trust.
The patients who were included in the study were aged 50 years or older; had visual acuity between 0.00 and 2.30 logMAR; and had the presence in the study eye of previously untreated active CNVM as demonstrated by hemorrhage or leakage on FFA and OCT.
Patients who had received previous treatment, transferred to another trust, or who subsequently had another diagnosis were excluded from our analysis, as were patients who had no more than the three treatments in the loading phase. After applying these exclusions, 27 patients were removed from the study and the data for 123 eyes of 106 patients were analysed. None of the patients refused treatment. Data were collected for age, sex, baseline visual acuity, and monthly visual acuities over 2 years and for the mean number of treatments per year. This study did not analyze the disease progression of different AMD types; we solely wanted to report the visual outcome data of a real-life population undergoing ranibizumab monotherapy.
All patients underwent a loading phase of three monthly treatments of 0.5 mg in 0.05 mL of intravitreal ranibizumab. Following this, patients were monitored monthly and the following retreatment criteria were used to establish whether further treatment was required:
Loss of letters with fluid on OCT
Any fluid on OCT – intraretinal fluid, subretinal fluid
Increased size of pigment epithelial detachment
New retinal hemorrhage
New CNVM.
In addition, if FFA revealed active CNVM, treatment was repeated each month for three months. Treatment was further individualized by sequentially lengthening follow-up intervals when a period of stability had been established.
Each intravitreal ranibizumab injection was performed in theatre under aseptic conditions and chloramphenicol 0.5% eye drops were administered four times a day for 5 days following the treatment. During and post-procedure, the patients were monitored for topical and general adverse events.
Similar to both the ANCHOR and MARINA trials, efficacy analyses were performed on an intention-to-treat basis with the use of a last-observation-carried-forward method for missing data.Citation4,Citation5
Results
Demographics
Similar to the ANCHOR trial but in contrast to the MARINA and CATT trials, our results had a majority of male patients (59%). Our population with a mean age of 83 years was much older than patients included in the other trials. Our largest age group was between 80 and 89 years compared to 75–84 years in the ANCHOR and MARINA trials and 70–79 years in the CATT trial. All the trials had a Caucasian majority of between 97% and 98%; we also demonstrate a Caucasian majority but of only 62% with 38% of patients from other ethnicities. Demographics are summarized in .
Table 1 Comparison of present study demographics and mean baseline vision to pivotal trials
Visual outcomes
We compared our 1-year and 2-year visual outcomes to those found from the ANCHOR, MARINA, and CATT trials ( and ). We used their same criteria for defining the three visual outcomes. The primary outcome measure was the percentage of eyes that maintained vision, meaning the percentage of eyes that lost less than 15 letters from baseline.Citation4–Citation6 Secondary outcome measures included the percentage of eyes that improved vision (meaning those that gained 15 letters or more from baseline) and the mean change in visual acuity.Citation4–Citation6 All 123 eyes underwent at least 12 months of monitoring and 61 eyes (50% of the initial study population) had visual outcome data for 24 months.
Figure 1 Graph comparing the visual outcomes after 1 year from this study to the CATT, ANCHOR, and MARINA trials.
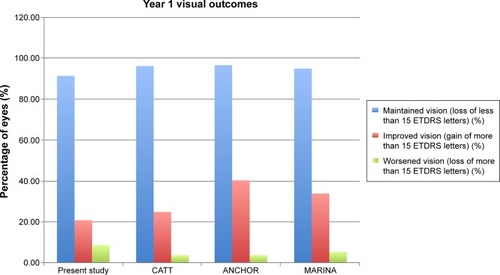
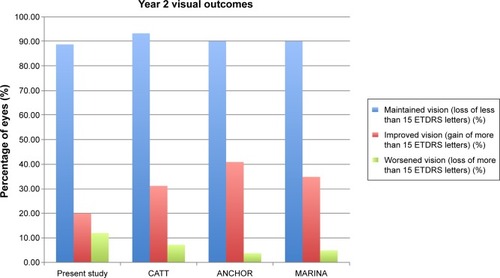
Figure 2 Graph comparing the visual outcomes after 2 years from this study to the CATT, ANCHOR, and MARINA trials.
At 12 months, the percentage of eyes that maintained vision in our study was 91% compared to 96% in the CATT trial, 96.4% in the ANCHOR trial, and 94.6% in the MARINA trial.Citation4–Citation6 We found that 20.3% of eyes had improved vision compared to 25% in the CATT trial, 40.3% in the ANCHOR trial, and 33.8% in the MARINA trial.Citation4–Citation6 Finally, 8.1% of our study eyes’ vision worsened compared to 4% in the CATT trial, 3.6% in the ANCHOR trial, and 5.4% in the MARINA trial ().Citation4–Citation6
Table 2 Comparison of present study visual outcomes and number of ranibizumab treatments to pivotal trials
At 24 months, the percentage of eyes that maintained vision in our study was 88.6% compared to 93% in the CATT trial and 90% in both the ANCHOR and MARINA trials.Citation4–Citation6 We found that 19.7% of eyes had improved vision compared to 33% in the CATT trial, 41% in the ANCHOR trial, and 35% in the MARINA trial.Citation4–Citation6 In our study, 11.5% of eyes worsened compared to 7% in the CATT trial, 4% in the ANCHOR trial, and 6% in the MARINA trial ().Citation4–Citation6
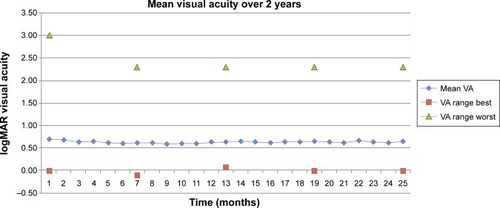
When comparing the mean visual progress over 2 years, our results did not show the initial steep gain of letters that was shown in the trials (). Instead, the mean change in vision over 2 years was only between zero and three ETDRS letters. We also found that the change in mean visual acuity was minimal ranging between 0.58 and 0.70 logMAR over 2 years (). The mean number of injections given per eye was ten over 2 years; six in year 1 (range three to 12) and four in year 2 (range one to 12), compared to 12.6 in the CATT trial ().Citation6
Figure 3 A graph comparing the mean visual progression of this study to the CATT, ANCHOR, and MARINA trials
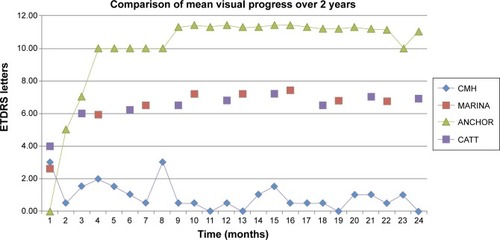
Figure 4 A graph comparing the mean visual acuity of this study to the CATT, ANCHOR, and MARINA trials.
On investigating this further we found that from the group whose vision worsened by 15 letters or more, 73% of these eyes had severe vision loss (30 letters or more; range 16–97). Although this is a relatively small percentage of the total number of eyes (9%), this significant loss of letters may have been enough to skew the mean visual acuity and mean change in vision, thus hiding any initial dramatic increase in vision.
Discussion
The pivotal continuous and individualized clinical trials have changed how wet AMD is treated today. The trials were either continuous, where monthly injections of ranibizumab were given over 2 years, or individualized where, after the three monthly loading doses, the patient was evaluated monthly but ranibizumab therapy was only given according to set criteria relating to the activity of wet AMD in that individual. The ANCHOR and MARINA trials were the two continuous trials that recruited 423 and 716 patients, respectively.Citation4,Citation5 They both observed that 90% of patients maintained vision, and improvement in vision occurred in 41% of patients in the ANCHOR trial and 35% in the MARINA trial.Citation4,Citation5 The mean change in visual acuity from baseline was 10.7 letters in the ANCHOR trial and 6.6 letters in the MARINA trial.Citation4,Citation5
The recently published CATT trial was significantly larger with 1,208 patients.Citation6 They compared visual outcomes of eyes that underwent treatment with ranibizumab to those that had bevacizumab and also the visual outcomes of patients who underwent continuous treatment versus individualized, as-needed treatment.Citation6 The arm of the trial that observed individualized treatment with ranibizumab showed maintenance of vision in 93% of patients, improvement of vision in 33% of patients, and a mean change in visual acuity of 6.7 letters over 2 years.Citation6
The results from our study follow a similar pattern to the pivotal trials, where the initial findings from year 1 were largely maintained throughout year 2. At 2 years, the percentage of eyes that maintained vision was 88.6%, compared to 93% in the CATT trial and 90% in the ANCHOR and MARINA trials.Citation4–Citation6 However, the number of eyes that improved vision was approximately a third less than the pivotal trials; 19.5% compared to 33% in the CATT trial, 41% in the ANCHOR trial, and 35% in the MARINA trial.Citation4–Citation6 The number of eyes whose vision worsened by losing more than 15 letters was greater than in all the trials; 11.5% compared to 7% in the CATT trial, and 4% and 6% in the ANCHOR and MARINA trials, respectively.Citation4–Citation6 The lack of dramatic visual improvement was also observed in the mean visual progress over 2 years, which was quite variable and failed to demonstrate the initial steep rise following the 3-month loading phase. This is further supported by the mean visual acuity over 2 years, which remained fairly static at 0.6 log-MAR (range −0.1 to 2.30). As mentioned previously, this may be due to the fact that 9% of eyes did particularly poorly, losing between 30 and 97 letters, which may have skewed the average visual outcomes.
Our results do follow the same trend as the pivotal trials but not to the same degree of success. This may be due to the smaller sample size of 123 eyes, of which only 61 had follow-up for the entire 24 months. The main reason for this reduction of 50% of the study population was that those patients had only undergone 12 months of treatment at the time of analysis, as despite the study including patients whose treatment began in 2007, the initial recruitment was slow with low patient numbers. Other reasons included: patients who failed to attend further appointments (n=4), patients whose treatment was stopped due to poor visual prognosis (n=3), and patients who died from other illness (n=3).
We also wanted to see how our visual outcome data compared to other single centers. Currently, there is no data published from the United Kingdom; however, ten other papers have been published by other centers (see and ). Our study results compared to these ten studies were very similar. The mean number of eyes that maintained vision from the other studies was 88.8%, compared to 88.6% in our study. The mean number of eyes that improved vision from the other studies was 20.7%, compared to 19.7% in our study. Finally, the mean number of eyes that worsened from the other studies was 10.3%, compared to 11.5% in our study.
Figure 5 A graph comparing the visual outcomes of this study to ten other single-center studies of real-life populations.
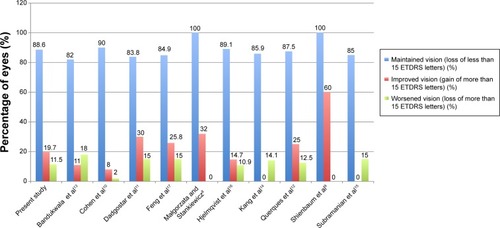
Table 3 Comparison of present study results to ten other studies of real life AMD populations treated with ranibizumab monotherapy around the world
Conclusion
This prospective study of individualized ranibizumab monotherapy over 24 months shows that individualized therapy in an inner-city population is effective in preserving vision in wet AMD and follows the same trends as the pivotal trials. Although our results, and those from other clinical settings, do not quite match the degree of vision preservation and gain as the large clinical trials, they are not dramatically dissimilar. We also hope this study encourages more AMD clinical settings to publish their data for comparison, particularly centers in the United Kingdom.
Disclosure
The authors report no conflicts of interest in this work.
References
- BresslerNMBresslerSBCongdonNGPotential public health impact of Age-Related Eye Disease Study results: AREDS report no 11Arch Ophthalmol20031211621162414609922
- rcophth.ac.uk [homepage on the Internet]LondonThe Royal College of Ophthalmologists2009 [cited 2013]. Available from: http://www.rcophth.ac.ukAccessed April 17, 2014
- BrownDMRegilloCDAnti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patientsAm J Ophthalmol2007144462763717893015
- RosenfeldPJBrownDMHeierJRanibizumab for Neovascular Age-Related Macular DegenerationN Engl J Med20063551419143117021318
- BrownDMMichelsMKaiserPKRanibizumab versus verteporfin photodynamic therapy for neovascular ade-related macular degeneration: Two-year results of the ANCHOR studyOphthalmology20091161576519118696
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- LalwaniGARosenfeldPJFungAEA variable-dosing regimen with intravitreal ranibizumab for neovascular age-related maculuar degeneration: year 2 of the PrONTO StudyAm J Ophthalmol20091481435819376495
- MałgorzataFStankiewiczAEffectiveness of ranibizumab intravitreal injections for exudative age-related macular degeneration treatment: 12-month outcomesMed Sci Monit2011179CR48549021873944
- ShienbaumGGarcia FilhoCAFlynnHWJrNunesRPSmiddyWERosenfeldPJManagement of submacular hemorrage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapyAm J Ophthalmol201315561009101323465269
- CohenSYDuboisLTadayoniRResults of one-year’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical settingAm J Ophthalmol2009148340941319477713
- DadgostarHVenturaAAChungJYSharmaSKaiserPKEvaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degenerationOphthalmology200911691740174719643484
- QuerquesGAzryaSMartinelliDRanibizumab for exudative age-related macular degeneration: 24 month outcomes from a single centre institutional settingBr J Ophthalmol201094329229619951942
- BandukwalaTMuniRHSchwartzCEngKTKertesPJEffectiveness of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration in a Canadian retina practice: a retrospective reviewCan J Ophthalmol201045659059521135894
- KangSRohYJOne-year results of intravitreal ranibizumab for neovascular age-related macular degeneration and clinical responses of various subgroupsJpn J Ophthalmol200953438939519763757
- SubramanianMLAbediGNessSBevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trialEye (Lond)2010241708171520885427
- HjelmqvistLLindbergCKanulfPDahlgrenHJohanssonISiewertAOne-year outcomes using ranibizumab for neovascular age-related macular degeneration: results of a prospective and retrispective observational multicentre studyJ Ophthalmol2011201140572422174994
- FengXFConstableIJMcAllisterILIsaacsTComparison of visual acuity outcomes between ranibizumab and bevacizumab treatment in neovascular age-related macular degenerationInt J Ophthalmol201141858822553617
