Abstract
Purpose
To evaluate and compare the efficacy and safety of two investigational artificial tear formulations (CHO-1 and CHO-2) containing carmellose sodium, hyaluronic acid at different concentrations, and osmoprotectants, with a standard carmellose sodium-containing formulation (Refresh Tears [RT]) in the treatment of dry eye disease.
Subjects and methods
In this 3-month, double-masked, multicenter study, subjects (n=305) were randomized 1:1:1 to receive CHO-1, CHO-2, or RT, used as needed but at least twice daily. The primary endpoint was change in ocular surface disease index (OSDI) score from baseline to day 90. Other key outcomes included symptoms evaluated on a visual analog scale, corneal and conjunctival staining, and adverse events.
Results
OSDI scores and dry eye symptoms showed a rapid and sustained reduction from baseline in each group. Both CHO-1 and CHO-2 met the primary efficacy endpoint of noninferiority to RT in day 90 OSDI score change from baseline. OSDI ocular symptoms subscale improved more with CHO-1 than CHO-2 (P=0.048). In subjects with clinically relevant baseline ocular surface staining (>14 total score of a maximum of 55), day 90 improvements were greater with CHO-1 and CHO-2 than RT (P≤0.044). Day 90 improvements in OSDI ocular symptoms subscale scores were also greater with CHO-1 than RT (P<0.007) in subjects with clinically relevant ocular staining. All treatments were well tolerated.
Conclusion
Both combination artificial tear formulations were efficacious and well tolerated in subjects with dry eye. CHO-1 demonstrated the best performance in improving ocular symptoms and reducing ocular staining in this heterogeneous study population.
Introduction
Dry eye is a highly prevalent, multifactorial, symptomatic disease that results in ocular discomfort and visual disturbance,Citation1,Citation2 diminishes quality of life, and is associated with limitations in several ordinary activities, including reading, driving, computer use, and professional work.Citation3 More than 321 million patients worldwide have been estimated to experience severe, moderate, or episodic dry eye symptoms, and the number is expected to keep increasing because of a general increase in life expectancy, improving access to health care in rapidly developing nations, and changes in overall activity patterns that continue to involve more intense visual tasks with computers, smart phones, and other devices.Citation2,Citation4
Historically, ocular lubricants (artificial tears) have been widely used as primary therapy in mild dry eye and as an adjunct to more advanced therapies in moderate and severe disease.Citation5 They typically include one or more water soluble polymers, such as carmellose, polyvinyl alcohol, or polyethylene glycol, to improve retention on the ocular surface and increase duration of action, lubrication, and hydration of the ocular surface. Carmellose sodium, the most well-established active ingredient in artificial tear preparations with efficacy in the treatment of dry eye,Citation6,Citation7 has been shown to bind to ocular surface cellsCitation8,Citation9 and accelerate wound healing in model systems.Citation10 In the development of current dry eye formulations, the viscosity has been varied to influence ocular surface retention time.Citation11 Nonetheless, there is a paucity of well-controlled clinical studies evaluating efficacy and safety of these standard therapies in the treatment and management of dry eye.Citation12
More recently, the roles of tears, tear film osmolarity, and ocular surface inflammation in dry eye disease is beginning to be elucidated.Citation13–Citation16 Newer ocular lubricants are being developed following a strategy that focuses on interaction with ocular surface cells, enhancing protection by preventing cell volume loss, cellular stress, and inflammatory reactions, while simultaneously eliminating damaging preservatives. An example is a combination of the polymers, carmellose sodium and hyaluronic acid (HA), for which a widely available artificial tear has not previously been available. The glycosaminoglycan HA, a natural component of the tear film (average molecular weight, 2.3 million Da), represents a distinct class of polymeric agent owing to its intrinsic properties of water retention, viscoelasticity, and promotion of corneal epithelial wound healing.Citation17 Similar to other soluble polymers used in artificial tears, HA increases viscosity and hydrates and lubricates the ocular surface. It has also been demonstrated to improve corneal and conjunctival staining in patients with dry eye.Citation18–Citation20
In vitro studies have shown potential synergistic effects of combining carmellose and HA polymers in a single formulation. These effects can lead to polymer entanglement and increased viscosity under low shear conditions, which are characteristic of the tear film between blinks. Increased viscosity promotes stabilization of the tear film and retention of the eye drop in the eye, thus, helping to optimize ocular hydration. In contrast, at high shear, such as exists during blinking, the polymer combination produces reduced viscosity (shear thinning), which should improve ocular comfort and reduce stickiness and blur found with some tear products.Citation21
The present study evaluates the efficacy, safety, and acceptability of two novel multi-ingredient artificial tear formulations (CHO-1 and CHO-2) in subjects with signs and symptoms of dry eye disease. CHO-1 (recently introduced as Optive Fusion; Allergan, Inc., Irvine, CA, USA) contains 0.5% carmellose sodium and 0.1% HA, and CHO-2 contains 0.5% carmellose sodium and 0.15% HA. The increased HA content of CHO-2 results in a higher viscosity formulation. Both contain salts and osmoprotectants (glycerin and erythritol) and are preserved with stabilized oxychloro complex (SOC) (Purite™; Allergan, Inc., Irvine, CA, USA). Osmoprotectants function as compatible solutes, accumulating in ocular surface cells and protecting them from cell volume loss, cellular stress, and induction of an inflammatory response under hyperosmotic conditions.Citation13,Citation22,Citation23
Subjects and methods
Study design
This 3-month, multicenter, randomized, double-masked, parallel-group clinical comparison study evaluated CHO-1 and CHO-2 compared with RT (containing 0.5% carmellose sodium, salts, and SOC) in subjects with dry eye. The study (ClinicalTrials.gov identifier NCT01294384) was conducted from May 2011 to September 2012 at 18 sites in Canada and 4 sites in Australia in compliance with the International Conference on Harmonisation guidelines for Good Clinical Practice. Research ethics committee approval was obtained at each study site and all subjects provided written informed consent.
Study population
The primary eligibility criteria are listed in . The participants were adults with mild-to-severe symptoms and mild-to-moderate signs of dry eye, an OSDI score of 18–65, tear breakup time (TBUT) less than 10 seconds, and currently used artificial tears. Key exclusion criteria were a corneal or conjunctival staining score of 5 in any area of either eye, a Schirmer’s test result of less than 3 mm/5 min, recent history of anterior segment surgery, contact lens wear, and use of certain medications that could affect dry eye or the interpretation of the study results.
Table 1 Key eligibility criteria for study participation
Treatment
At the baseline/screening (day 1) study visit, subjects were randomized in a 1:1:1 ratio to treatment with CHO-1 (carmellose sodium 0.5%/HA 0.1%), CHO-2 (carmellose sodium 0.5%/HA 0.15%), or RT based on enrollment order at the study site and a computer-generated random allocation scheme. In addition, subjects were stratified into mild/moderate (combined corneal/conjunctival staining score ≤14 at baseline) and clinically relevant (combined corneal/conjunctival staining score >14 or corneal staining ≥7 at baseline) based upon prior reports.Citation19 To duplicate patient management in the clinical setting, there was no washout from previous artificial tear preparations. Treatment kits were dispensed to subjects as directed by an automated response system that paired subject identification numbers with kit numbers. Subjects were instructed to administer the eye drops in both eyes as needed but at least twice daily for 3 months. To maintain subject and investigator masking, the artificial tears were provided in virtually identical 15-mL bottles and cartons; labels were nonbranded and did not list ingredients. Follow-up study visits were scheduled at days 7, 30, 60, and 90.
Outcome measures
A total of 22 investigators participated in the study and each subject was evaluated by the same investigator throughout the study. In instances when this was not possible, evaluations conducted by 2 investigators overlapped for at least 1 visit to ensure assessment procedures were performed consistently. The primary efficacy measure was the change in ocular surface disease index (OSDI) score from baseline, at day 90. The OSDI score is based on a scale of 0–100, where 100 corresponds to complete disability (a response of “all of the time” to all questions answered).Citation24 A negative change from baseline indicates improvement. Secondary measures included TBUT, which was assessed during the 2-minute waiting period following the instillation of sodium fluorescein using Amcon Dry Eye Test (DET) Strips (Amcon Laboratories, St Louis, MO, USA)Citation25 for corneal staining; three consecutive measurements (timed using a stopwatch provided for the study) were performed in each eye by the same examiner. Corneal staining was evaluated using a slit lamp at 16× magnification and a yellow filter to enhance contrast, and rated based on the modified National Eye Institute (NEI) grid, which assesses five areas of the cornea on a scale of 0–5.Citation26 After corneal staining, conjunctival staining with lissamine green was performed immediately after administration of the dye in each eye (under low-to-moderate white light illumination and without flushing of residual fluorescein), and rated per the modified NEI grid 0–5 scale.
After all other ophthalmic evaluations were completed, standard Schirmer’s tests with anesthesia were performed in each eye (in a dimly lit room) while the subject was looking upward. Testing was initiated 4 minutes after instillation of the anesthetic and the amount of wetting of test strips was measured 5 minutes later. Near visual acuity with habitual correction was measured using near low-contrast (10%) and high-contrast logarithmic visual acuity charts for testing at 40 cm. Other key efficacy measures included symptoms of burning/stinging, grittiness/foreign body sensation, dryness, and eye ache/pain graded on a visual analog scale of 0 (none) to 100 (maximum). Product usage and visual disturbance upon product instillation were evaluated with questionnaires, and reading speed was evaluated with the Minnesota Low-Vision Test (MNREAD).Citation27
Safety measures included adverse events (AEs), distance visual acuity, and biomicroscopy (without pupil dilation). Eyelid margins were assessed for edema/erythema on a scale of 0 (no edema/erythema) to 3 (severe, diffuse swelling/reddish color of lid margins, and superior and/or inferior eyelid).
Data analysis
A sample size of 89 subjects who completed the study and comprised the intent-to-treat (ITT) population for each group (267 total subjects), was estimated to give 80% power to determine noninferiority of CHO to RT in OSDI score change from baseline at day 90.
The primary efficacy analysis used the ITT population of all randomized subjects and last observation carried forward (LOCF) for missing values. Sensitivity analyses used observed values in the per-protocol (PP) population of randomized subjects with no major protocol violations. Safety analyses used all treated subjects based on the treatment received.
Change from baseline in OSDI scores, TBUT, corneal and conjunctival staining scores, Schirmer’s test scores, and symptom scores were analyzed with analysis of variance with a fixed effect of treatment. Least-squares differences between groups and 95% confidence intervals (CI) of the differences were obtained from the models. Noninferiority to RT in change from baseline OSDI score was declared if the upper limit of the two-sided 95% CI of the between-group difference (CHO minus RT) was less than 7.3 at day 90.Citation28 Within-group changes from baseline were evaluated with paired t-tests. Wilcoxon rank-sum tests were used in post hoc analysis to evaluate changes in OSDI subscale scores from baseline. Additional post hoc subgroup analyses of combined corneal/conjunctival staining scores were conducted in subjects with mild/moderate and clinically relevant baseline staining (total score ≤14 and >14, respectively) using LOCF for missing values. Additional analyses of the clinically relevant group for corneal staining alone (score ≥7) and changes in OSDI subscale scores were also performed. Nominal variables were analyzed with Pearson chi-square tests. Statistical tests used an alpha level of 0.05.
Results
Subjects and disposition
A total of 305 subjects were randomized to treatment and 286 (93.8%) completed the study (). There were no significant differences among treatment groups in baseline demographics or signs of dry eye (). During the week prior to the 90-day follow-up visit, subjects reported that they used CHO-1, CHO-2, and RT a mean of 4.3, 3.9, and 3.8 times/day, respectively (median of three times daily).
Figure 1 Subject flow through the study.
Abbreviations: ITT, intent-to-treat; PP, per-protocol; RT, Refresh Tears.
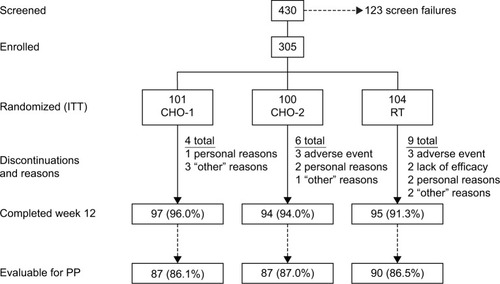
Table 2 Subject characteristics at study entry
Efficacy evaluation
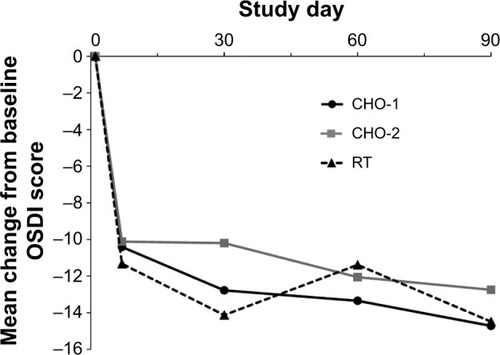
OSDI scores improved significantly from baseline in each treatment group from day 7 through day 90 (P≤0.001). There were no statistically significant differences between groups in mean OSDI score change from baseline at any follow-up visit (). At day 90, the mean change ± standard deviation (SD) from baseline OSDI score was CHO-1, −14.7±16.3; CHO-2, −12.8±17.6; and RT, −14.5±16.4, with no statistically significant difference between CHO-1 or CHO-2 versus RT, respectively, or between CHO-1 and CHO-2 (). Both CHO-1 and CHO-2 met the primary efficacy endpoint and were noninferior to RT in change in OSDI score from baseline at day 90 (CHO-1/RT difference: −0.22, 95% CI: −4.85 to 4.41; CHO-2/RT difference: 1.74, 95% CI: −2.89 to 6.37). Results in the PP population (86.6% of randomized subjects) were similar and confirmed noninferiority of CHO-1 and CHO-2 to RT.
Figure 2 Mean change in Ocular Surface Disease Index (OSDI) score from baseline (intent-to-treat population).
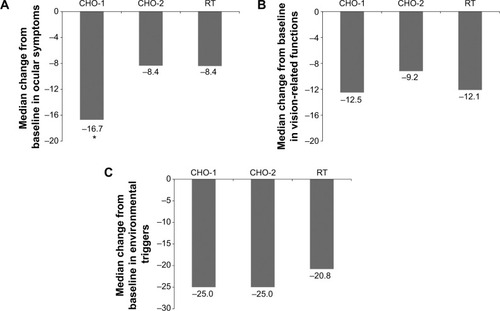
OSDI was further analyzed in the following three subscales: ocular symptoms, vision-related functions, and environmental triggers (). Scores on the ocular symptoms subscale for CHO-1 improved significantly more than for CHO-2 (P=0.048), and directionally more than for RT (P=0.057).
Figure 3 Median change in score from baseline at day 90 for (A) ocular symptoms, (B) vision-related functions, and (C) environmental triggers subscales of the Ocular Surface Disease Index (intent-to-treat population).
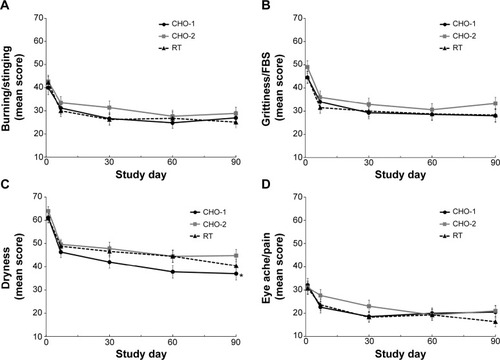
Dry eye symptoms of burning/stinging, grittiness/foreign body sensation, dryness, and eye ache/pain reported by subjects using a visual analog scale also showed significant improvement from baseline in each group at all follow-up time points (P≤0.002), with the exception of eye ache/pain in the CHO-2 group at day 7. Mean symptom scores at each visit were generally similar among groups (). A significant between-group difference was observed for dryness at day 90 in the CHO-1 group compared with the CHO-2 group (37.0 vs 44.8, P=0.044).
Figure 4 Mean symptom scores on visual analog scales for (A) burning/stinging, (B) grittiness/foreign body sensation, (C) dryness, and (D) eye ache/pain. Notes: Error bars indicate standard error of the mean (intent-to-treat population). *P=0.044 for CHO-1 versus CHO-2.
In the overall study population, corneal staining scores, conjunctival staining scores, and combined corneal/conjunctival staining scores were significantly decreased from baseline in each treatment group at each follow-up visit (P≤0.003), with the exception of corneal staining scores at day 7 in the RT group (P=0.053) (). Overall, the change from baseline corneal staining score was greater in the CHO-1 group than in the RT group at day 7 (−1.6 vs −0.7, P=0.036) and day 90 (−2.3 vs −1.2, P=0.015) (). There were no significant differences between treatment groups in the change in conjunctival staining or combined corneal/conjunctival staining from baseline ().
Figure 5 Mean changes in (A) corneal, (B) conjunctival, and (C) combined corneal/conjunctival staining scores from baseline (intent-to-treat population).
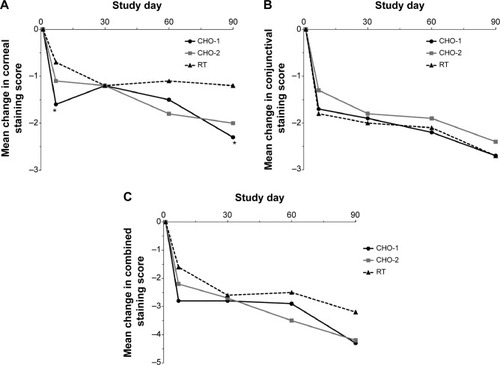
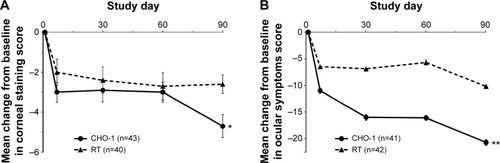
Subgroup analysis based on baseline stratification into either mild/moderate or clinically relevant staining groups demonstrated no differences between treatment groups for the mild/moderate population, but significant differences between CHO-1 and CHO-2 versus RT in the clinically relevant population (mean change ± SD at day 90: −7.1±8.8 and −7.2±6.2 vs −3.8±6.6, P≤0.044) (). Additionally, in the population with clinically relevant combined corneal/conjunctival staining at baseline, CHO-1 demonstrated greater improvements than RT in corneal staining (mean change ± SD: −4.7±3.78 vs −2.6±2.96, P=0.009) and the ocular symptoms subscale of the OSDI (mean change ± SD: −20.7±3.5 vs −10.2±2.9, P=0.007) ().
Figure 6 Mean changes in combined corneal/conjunctival staining scores from baseline in subjects stratified by the severity of baseline staining (per-protocol population). (A) Subjects with combined corneal/conjunctival staining score of ≤14 at baseline. Baseline mean scores were CHO-1, 7.7; CHO-2, 7.7; and RT, 7.8. (B) Subjects with combined corneal/conjunctival staining score of >14 at baseline. Baseline mean scores were CHO-1, 23.4; CHO-2, 21.3; and RT, 22.1.
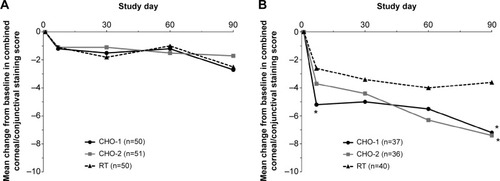
Figure 7 Mean changes in (A) corneal staining scores and (B) ocular symptoms subscale scores of the Ocular Surface Disease Index in subjects with clinically relevant staining at baseline.
On the visual disturbance questionnaire, most subjects in each group were satisfied with the viscosity of their drops and reported no blurring in their vision after drop administration (). However, responses were less favorable with CHO-2, which caused more blur than RT, especially when first applied.
Table 3 Visual disturbance questionnaire results
Improvements in TBUT from baseline were observed in each group at each follow-up visit (P<0.001) with no statistically significant differences between groups. Schirmer’s tests showed significant improvement in tear production only at day 7 in the CHO-1 group and at days 7 and 90 in the CHO-2 group (P≤0.027). At day 7, the increase in tear production was greater in the CHO-2 group than the RT group (1.6 vs 0 mm/5 min, P=0.023). There were no significant between-group differences in change from baseline tear production at days 30, 60, and 90.
There were no significant differences between groups in the frequency distribution of changes from baseline in high-contrast or low-contrast near visual acuity. MNREAD findings at day 90 showed significant improvement from baseline in mean maximum reading speed only in the CHO-1 group (from 169 to 173 words per minute, P=0.049).
Safety evaluation
The overall incidence of treatment-related AEs was low in all groups (CHO-1, 3.9%; CHO-2, 7.9%; and RT, 5.8%). The most common ocular AE was eye irritation, which was reported in 9 subjects (incidence of 0% with CHO-1, 5.0% with CHO-2, and 3.9% with RT). Blurred vision was reported in 2 subjects (2.0%) in the CHO-2 group. The rate of discontinuations due to AEs was 0% in the CHO-1 group, 3.0% in the CHO-2 group (corneal staining/eyelid edema; eye irritation/vision blurred; eye irritation/eye pain/eye discharge), and 2.9% in the RT group (blepharitis; instillation site erythema/irritation/lacrimation; conjunctival hyperemia/eye irritation).
At day 90, distance visual acuity was similar to that recorded at baseline, and there were no differences between groups in the distribution of better, no change, or worse visual outcomes (P≥0.389 for between-group comparisons). At day 90, greater than 84% of subjects in each group had no change in visual acuity or performed better than at baseline, and only 1 subject (in the CHO-1 group) was observed to have a 2-line (10 letters or more) decrease from baseline. In addition, the incidence and severity of biomicroscopy findings was similar in each group.
Discussion
In this study, both CHO formulations met the primary efficacy endpoint and were noninferior to RT in OSDI score change from baseline at day 90. A noninferiority margin of 7.3 was used as it represents the minimal clinically important difference in OSDI score for subjects with severe dry eye symptoms.Citation28 In noninferiority studies, evaluation of both the PP and ITT populations is confirmatory.Citation29 Results of analysis of the PP population were similar to those in the ITT population, with each CHO formulation demonstrating noninferiority to RT.
TBUT and conjunctival staining scores improved similarly in each group, but the CHO-1 formulation improved corneal staining in all subjects, as well as combined corneal and corneal/conjunctival staining in subjects who had clinically relevant staining at baseline, significantly more than RT. Both CHO formulations were well tolerated and had a safety profile similar to RT. Treatment-related AEs and discontinuations due to AEs were low in all groups and lowest with CHO-1.
Comparing the two formulations (CHO-1 and CHO-2) in this study allowed evaluation of a dose response to HA. Reports of visual disturbance were highest in the CHO-2 group, and responses regarding eye drop thickness and initial blur after application were more favorable with CHO-1 and consistent with its lower viscosity. TBUT was not significantly improved by the higher viscosity of CHO-2 versus CHO-1 or RT. For symptoms and ocular surface staining, CHO-1 performed as well or better than CHO-2 despite its lower HA concentration.
HA is a naturally occurring linear biopolymer of glycosaminoglycan disaccharides with notable hygroscopic capacity.Citation30 It provides great resistance to evaporation,Citation30,Citation31 which can help stabilizing the tear film. HA has been shown to be effective in treating severe dry eye,Citation32,Citation33 and studies have also demonstrated that artificial tears containing HA may reduce damage to the ocular surface in patients with dry eye disease.Citation13,Citation18–Citation20,Citation33–Citation35 Carmellose sodium is an anionic cellulose polymer with bioadhesive properties, available in several viscosities corresponding to different molecular weights.Citation30 It has been shown to bind to the corneal surface (including extracellular matrix proteins), which may increase retention time and promote corneal wound healing.Citation8–Citation11,Citation36,Citation37 Carmellose sodium has been reported to provide a temporary but significant improvement in visual acuity in patients with symptomatic or asymptomatic dry eye.Citation38 Inclusion of osmoprotectants in artificial tears may also be potentially valuable for reducing ocular surface damage and visual disturbance.Citation19,Citation39
In the present study, a difference in combined corneal/conjunctival staining and ocular symptom OSDI subscale score between the CHO formulations and RT became particularly evident when the analysis was stratified by the severity of staining at baseline. The results suggest that with little surface staining, low-viscosity tears such as RT may have an adequate effect. In more advanced disease when there is pronounced ocular surface damage, carmellose sodium/HA combinations such as CHO-1 may be a superior therapeutic option.
This initial evaluation of combination carmellose sodium/HA formulations also included a comparison with a standard carmellose sodium-containing eye drop. Prior reports comparing carmellose sodium-based eye drops to drops containing HA only have shown similarity in overall efficacy and safetyCitation19,Citation40 or superior reduction in conjunctival staining.Citation41–Citation43 These reports, together with the present data, suggest that the combination of carmellose sodium and HA may provide a superior level of therapeutic benefit than either polymer alone. However, further work involving direct comparison with other HA-only preparations is indicated.
The present study enrolled a heterogeneous population with respect to the etiology of dry eye, reflecting the dry eye population presenting to the various study centers. The role of deficiencies in tear production versus excessive tear evaporation in producing the signs and symptoms of dry eye, and possibly the therapeutic value of various components of artificial tears, could vary greatly among the study participants. Further study in specific subpopulations of patients with dry eye (eg, patients with more severe clinical signs or meibomian gland dysfunction) is warranted to assist eye care practitioners in treatment decisions. Specific studies utilizing new methods to assess clinical signs are also indicated,Citation44 and the study of the optimum frequency of eye drop instillation is also warranted.
Overall, the results of this study indicate that combining carmellose sodium and HA polymers in an osmoprotective formulation provides a potentially beneficial option for the management of dry eye.
Author contributions
This study was sponsored by Allergan, Inc., Irvine, CA, USA. The study sponsor was involved in the design of the study, data analysis and interpretation, the development of the manuscript, and the decision to submit for publication. All authors participated in the design of the study or in the analysis and interpretation of the study data, and all were involved in developing the manuscript and approved the final version for submission.
Acknowledgments
Writing and editorial assistance were provided to the authors by Kate Ivins, PhD, of Evidence Scientific Solutions and funded by Allergan, Inc., Irvine, CA, USA. The authors would like to thank Genming Shi, PhD and Ru Chen, PhD (former employees of Allergan, Inc.) for their contribution to the data analysis and development of the manuscript, and the participating investigators who provided and cared for the study subjects. Canada: Dr Stephen Baker, Dr Stephanie Baxter, Dr Hélène Boisjoly, Dr Jean Carruthers, Dr Jeffrey Chambers, Dr Jean Deschenes, Dr Marilyn Ekins, Dr Howard Gimbel, Dr Simon Holland, Dr Chris Jackman, Dr Hugh G Jellie, Dr Baseer Khan, Dr Christoph Kranemann, Dr William G Macrae, Dr Roberto LG Piemontesi, Dr Rookaya Mather, Dr Rusty Ritenour, and Dr Kevin Wade. Australia: Dr Minas Coroneo, Dr Colin Chan, Dr Geoffrey Cohn, and Dr Mark Daniell.
Disclosure
The authors are employees of Allergan, Inc., Irvine, CA, USA. The formulations used in this study are investigational or marketed products of Allergan, Inc. The authors report no other conflicts of interest.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf200752759217508116
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- MiljanovićBDanaRSullivanDASchaumbergDAImpact of dry eye syndrome on vision-related quality of lifeAm J Ophthalmol2007143340941517317388
- MarketScopeLLC2013Comprehensive Report on the Global Dry Eye Products Market Available from: http://market-scope.com/products-page/other-reports/2013-comprehensive-report-on-the-global-dry-eye-market/Accessed February 1, 2013
- BehrensADoyleJJSternLthe Dysfunctional Tear Syndrome Study GroupDysfunctional tear syndrome: a Delphi approach to treatment recommendationsCornea200625890090717102664
- GreneRBLankstonPMordauntJHarroldMGwonAJonesRUnpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivitis siccaCornea19921142943011424648
- SimmonsPAVehigeJGClinical performance of a mid-viscosity artificial tear for dry eye treatmentCornea200726329430217413956
- GarrettQSimmonsPAXuSCarboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healingInvest Ophthalmol Vis Sci20074841559156717389485
- NapoliPECoronellaFSattaGMZuccaIAFossarelloMA novel OCT technique to measure in vivo the corneal adhesiveness for sodium carboxymethylcellulose in humans and its validity in the diagnosis of dry eyeInvest Ophthalmol Vis Sci20145553179318524764061
- GarrettQXuSSimmonsPACarboxymethyl cellulose stimulates rabbit corneal epithelial wound healingCurr Eye Res200833756757318600489
- PaughJRNguyenALKetelsonHAChristensenMTMeadowsDLPrecorneal residence time of artificial tears measured in dry eye subjectsOptom Vis Sci200885872573118677236
- NapoliPESattaGMCoronellaFFossarelloMSpectral-domain optical coherence tomography study on dynamic changes of human tears after instillation of artificial tearsInvest Ophthalmol Vis Sci20145574533454024985473
- BaudouinCAragonaPMessmerEMRole of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meetingOcul Surf201311424625824112228
- StahlUWillcoxMStapletonFOsmolality and tear film dynamicsClin Exp Optom201295131122022802
- PflugfelderSCCorralesRMde PaivaCST helper cytokines in dry eye diseaseExp Eye Res201311711812524012834
- StevensonWChauhanSKDanaRDry eye disease: an immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- GomesJAAmankwahRPowell-RichardsADuaHSSodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitroBr J Ophthalmol200488682182515148219
- BaeyensVBronABaudouinCEfficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye diseaseJ Fr Ophtalmol201235641241922483761
- BaudouinCCochenerBPisellaPJRandomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye diseaseEur J Ophthalmol201222575176122287172
- VogelRCrockettRSOdenNLaliberteTWMolinaLDemonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena)Am J Ophthalmol2010149459460120346777
- SimmonsPABeardBJVehigeJGOptimizing viscosity of ophthalmic solutions with the combination of two polymers Available from: http://www.tfos2013.org/program.htmlAccessed December 20, 2013
- De PaivaCSCorralesRMVillarrealALCorticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eyeExp Eye Res200683352653516643899
- CorralesRMLuoLChangEYPflugfelderSCEffects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cellsCornea200827557457918520508
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the ocular surface disease indexArch Ophthalmol2000118561562110815152
- KorbDRGreinerJVHermanJComparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the Dry Eye Test (DET) methodCornea200120881181511685057
- De PaivaCSPflugfelderSCCorneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulationAm J Ophthalmol2004137110911514700652
- LeggeGERossJALuebkerALaMayJMPsychophysics of reading. VIII. The Minnesota low-vision reading testOptom Vis Sci198966128438532626251
- MillerKLWaltJGMinkDRMinimal clinically important difference for the ocular surface disease indexArch Ophthalmol201012819410120065224
- Le HenanffAGiraudeauBBaronGRavaudPQuality of reporting of noninferiority and equivalence randomized trialsJAMA2006295101147115116522835
- ZhengXGotoTOhashiYComparison of in vivo efficacy of different ocular lubricants in dry eye animal modelsInvest Ophthalmol Vis Sci20145563454346024781937
- ZhengXGotoTShiraishiAOhashiYIn vitro efficacy of ocular surface lubricants against dehydrationCornea20133291260126423860431
- CondonPIMcEwenCGWrightMMackintoshGPrescottRJMcDonaldCDouble blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndromeBr J Ophthalmol199983101121112410502570
- McDonaldCCKayeSBFigueiredoFCMacintoshGLockettCA randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndromeEye (Lond)200216560160712194076
- AragonaPPapaVMicaliASantoconoMMilazzoGLong term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eyeBr J Ophthalmol200286218118411815344
- YokoiNKomuroANishidaKKinoshitaSEffectiveness of hyaluronan on corneal epithelial barrier function in dry eyeBr J Ophthalmol19978175335369290362
- LentonLMAlbietzJMEffect of carmellose-based artificial tears on the ocular surface in eyes after laser in situ keratomileusisJ Refract Surg1999152 SupplS227S23110202728
- AlbietzJMLentonLMMcLennanSGEarlMLA comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patientsCLAO J20022829610012054380
- NilforoushanMRLatkanyRASpeakerMGEffect of artificial tears on visual acuityAm J Ophthalmol2005140583083516310460
- MonacoGCacioppoVConsonniDTroianoPEffects of osmoprotection on symptoms, ocular surface damage, and tear film modifications caused by glaucoma therapyEur J Ophthalmol201121324325020872359
- LeeJHAhnHSKimEKKimTIEfficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye diseaseCornea201130217517921045674
- GuillonMMaissaCHoSEvaluation of the effects on conjunctival tissues of Optive eyedrops over one month usageCont Lens Anterior Eye2010332939920227636
- SanchezMATorralbo-JimenezPGironNComparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric studyCornea201029216717120023577
- BrignoleFPisellaPJDupasBBaeyensVBaudouinCEfficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitisGraefes Arch Clin Exp Ophthalmol2005243653153815965673
- NapoliPECoronellaFSattaGMFossarelloMA novel technique of contrast-enhanced optical coherence tomography imaging in evaluation of clearance of lipids in human tearsPLoS One2014911e10984325369027
