Abstract
Purpose
To investigate the clinical findings that characterize exudative age-related macular degeneration (AMD) with choroidal vascular hyperpermeability (CVH).
Design
Retrospective comparative study.
Participants
Forty-eight consecutive patients attending the outpatient clinic of Tokyo University Hospital between May 2013 and July 2013.
Methods
The presence or absence of CVH was determined with indocyanine green angiography performed at the latest visit. When CVH was observed, the eye was categorized as CVH(+) AMD, otherwise it was categorized as CVH(-) AMD. Using high-penetration optical coherence tomography, we measured choroidal thickness at the fovea and at four midperipheral areas (mean choroidal thickness at points on 6- and 9-papilla diameter circles superior, inferior, temporal, and nasal to the fovea). Ultrawide field retinal imaging was used to investigate abnormalities in midperipheral fundus autofluorescence (FAF). Choroidal thickness and the proportion of FAF abnormalities were compared between the CVH(+) AMD and CVH(−) AMD eyes and between eyes with polypoidal choroidal vasculopathy and typical AMD. Multiple regression analysis was used to control for treatment history and other characteristics.
Results
CVH was observed in 17 cases. Choroidal thickness was higher in the CVH(+) AMD eyes than in the CVH(−) AMD eyes at the fovea (325 μm versus 229 μm, respectively; P=0.0010, t-test), superior point (277 μm versus 215 μm, respectively; P=0.0021, t-test), inferior point (225 μm versus 161 μm, respectively; P=0.0002, t-test), and nasal point (202 μm versus 165 μm, respectively; P=0.042, t-test). The significance was maintained after controlling for possible confounders. The choroid was thicker at the fovea and at the inferior point in polypoidal choroidal vasculopathy than in typical AMD. The rate of midperipheral FAF abnormality was significantly higher in the CVH(+) AMD eyes than in the CVH(−) AMD eyes (82% versus 48%, respectively; P=0.031).
Conclusion
AMD with CVH is associated with widespread choroidal thickening and peripheral FAF abnormalities.
Introduction
Choroidal vascular hyperpermeability (CVH), characterized by multifocal choroidal hyperfluorescence on indocyanine green angiography (ICGA), was first described in central serous chorioretinopathy (CSC),Citation1–Citation5 and it has also been reported in eyes with polypoidal choroidal vasculopathy (PCV).Citation6 Since these first reports two decades ago, PCV with CVH has been actively investigated.Citation7–Citation10 In patients with PCV, the subfoveal choroid is thicker in eyes with CVH than in those without.Citation8,Citation9 Some investigators have associated CVH with inferior outcomes after intravitreal ranibizumab (IVR) or bevacizumab treatment for PCV,Citation9,Citation10 but CVH is not unique to CSC and PCV. A previous study showed that even in typical age-related macular degeneration (tAMD) in Japan, the subfoveal choroid was significantly thicker in eyes with CVH than in those without.Citation8 Additionally, a more recent study from Japan examined the characteristics of eyes with choroidal neovascularization (CNV) accompanied by CVH, and reported that type 1 CNV (located beneath the retinal pigment epithelium [RPE]) was more frequent than previously thought, although type 2 CNV (present between the sensory retina and RPE)Citation11 and polypoidal lesions were also observed.Citation12 The study also showed that the genetic background of patients with CVH was different from that previously reported in age-related macular degeneration (AMD) patients, and it was rather similar to that of the general population, suggesting that exudative AMD with CVH is different from that without CVH. However, no other clinical evidence has reported exudative AMD with CVH as a distinct clinical entity.
Against this background, in this study, we employed multimodal imaging using recently developed devices to clarify the characteristics of patients with exudative AMD with CVH. First, we used high-penetration optical coherence tomography (HP-OCT) and examined choroidal thickness over a wide area in patients with AMD with or without CVH. Second, we used wide-field retinal imaging to investigate the association between midperipheral fundus autofluorescence (FAF) abnormalities and CVH in eyes with exudative AMD.
Methods
Subjects
Among consecutive patients with exudative AMD who visited the outpatient macular clinic of the University of Tokyo Hospital (Tokyo, Japan) between May 2013 and July 2013, eyes with a previous history of laser photocoagulation, vitrectomy, or with other pathological conditions such as diabetic retinopathy were excluded. Only those patients with a definite diagnosis of exudative AMD based on fluorescein angiography and ICGA were included in the analysis. Both fluorescein angiography and ICGA were obtained as a part of routine practice for patients with a diagnosis of exudative AMD.
Institutional Review Board approval was obtained from the University of Tokyo for this retrospective study.
Diagnostic criteria
Patients with exudative AMD had been diagnosed by slit-lamp biomicroscopy, fluorescein angiography, and ICGA. ICGA was performed using a confocal scanning laser ophthalmoscope (HRA-2; Heidelberg Engineering, Inc., Heidelberg, Germany). After an intravenous dose of 25 mg of the dye in 2 mL of aqueous solvent, angiograms were obtained. A diagnosis of PCV was made if elevated orange–red lesions were observed during fundus examination, or if the polypoidal structure of the choroidal vessels with or without abnormal vascular networks was observed on ICGA.Citation13–Citation15 Two independent investigators judged cases in a blinded manner. For cases where their judgment differed, they re-examined the images together to reach a consensus.
Choroidal thickness
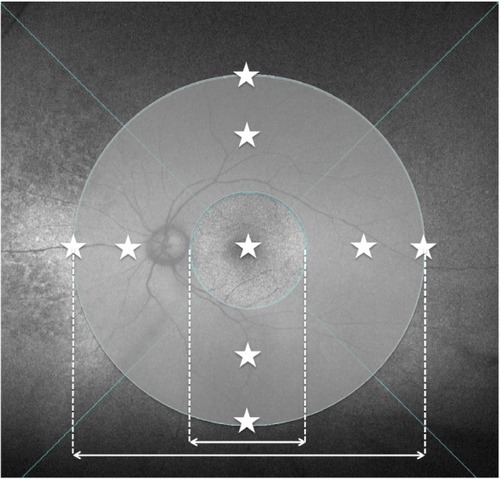
Choroidal thickness was examined with HP-OCT (DRI OCT-1 Atlantis; Topcon Corporation, Tokyo, Japan). A standardized protocol was used for image acquisition. Each patient underwent five examinations while gazing forward, upward, downward, right, and left. Images covering a 9×12 mm area were obtained following the three-dimensional raster scan protocol. An investigator blinded to the clinical diagnosis retrospectively analyzed the HP-OCT findings. Choroidal thickness was defined as the distance between Bruch’s membrane and the chorioscleral border and was measured manually by one investigator (YN) at nine points: at the fovea; and at eight extrafoveal points on 6- and 9-papilla diameter circles superior, inferior, temporal, and nasal to the fovea (). The mean choroidal thickness at the two points superior to the fovea was used as the thickness of the superior point, and thickness was likewise calculated for the inferior, temporal, and nasal points.
Figure 1 Nine measurement points of choroidal thickness and the area between the 3-papilla diameter and 9-papilla diameter circles (midperipheral area) in which abnormal autofluorescence abnormalities were investigated.
Midperipheral fundus autofluorescence abnormalities
Imaging was performed using the Optos 200Tx ultrawide field retinal-imaging device (Optos plc, Dunfermline, UK). A standard acquisition protocol was used to obtain green-light (532 nm) FAF images. As previously described,Citation16 FAF abnormalities were determined by the presence of any area of increased or decreased autofluorescence relative to the homogenous grayish-white background. “Midperipheral” was defined as the zone outside the circle with a 3-papilla diameter and inside the circle with a 9-papilla diameter around the fovea (), and it was measured using software built in-house.
Choroidal vascular hyperpermeability
Similar to previous reports,Citation8–Citation10,Citation12 CVH was defined as the presence within the choroid of multifocal areas of hyperfluorescence with blurred margins identified in the late phase of ICGA. For patients with a history of treatments such as photodynamic therapy (PDT) or IVR injections, ICGA images that had been recorded before and after the treatments were reviewed, and changes in CVH were investigated.
Statistical analysis
Statistical analysis was performed using JMP® software version 10.0 (SAS Institute Inc., Cary, NC, USA). Categorical data were assessed using the chi-squared test, and continuous variables were compared using Student’s t-test. A P-value <0.05 was considered significant. Multiple regression analysis was used to examine the associations between outcomes (ie, choroidal thickness and the proportion of FAF abnormalities) and baseline characteristic factors such as age, axial length, history of IVR injections or PDT, and the presence or absence of CVH.
Results
Patient characteristics
A total of 48 eyes in 48 subjects were assessed, and patient characteristics are summarized in . Sex, age, and axial length did not differ significantly between patients with and without CVH. None of the patients had retinal angiomatous proliferation. The proportion of PCV patients was higher in the CVH-positive (CVH[+]) AMD eyes. Eight patients had a history of CSC, but the proportion of those with such history did not differ between the two groups (). Patients comprised seven treatment-naïve patients, ten patients with a history of PDT, and 40 patients with a history of IVR (Table S1). None of the patients had a history of intravitreal aflibercept. Forty-one patients had a history of either PDT or IVR injections. Among 23 patients whose ICGA images before and after treatment were available, no changes in CVH were seen after treatment. For the remaining 18 patients, ICGA images were only available from before the treatment.
Table 1 Patient demographics
Choroidal thickness
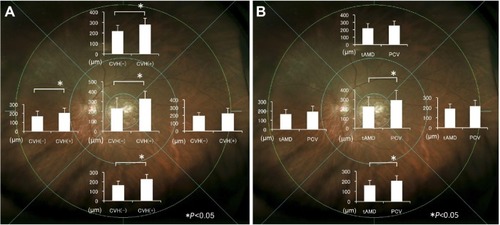
The mean choroidal thickness was greater in the CVH(+) AMD eyes than in the CVH-negative (CVH[−]) AMD eyes at the fovea (325±78 μm versus 229±108 μm, respectively; P=0.0010, t-test), superior point (277±62 μm versus 215±58 μm, respectively; P=0.0021, t-test), inferior point (225±52 μm versus 161±45 μm, respectively; P=0.0002, t-test), and nasal point (202±57 μm versus 165±59 μm, respectively; P=0.042, t-test) (). The choroid was thicker in eyes with PCV than in those with tAMD only at the fovea (290±106 μm versus 225±101 μm, respectively; P=0.037, t-test) and at the inferior point (203±53 μm versus 158±51 μm, respectively; P=0.0054, t-test) ().
Figure 2 Comparison of choroidal thickness.
Abbreviations: CVH(−), choroidal vascular hyperpermeability-negative; CVH(+), choroidal vascular hyperpermeability-positive; tAMD, typical age-related macular degeneration; PCV, polypoidal choroidal vasculopathy; AMD, age-related macular degeneration; SD, standard deviation.
Because the subjects were heterogeneous, we performed multiple regression analysis to assess the association between CVH and baseline characteristics such as age, axial length, and history of PDT and/or IVR. This analysis also showed that the presence of CVH was significantly associated with a thicker choroid at the fovea (P=0.0015), superior point (P=0.0029), inferior point (P<0.0001), and nasal point (P=0.039), independent of age, axial length, and history of PDT or IVR.
Fundus autofluorescence abnormalities
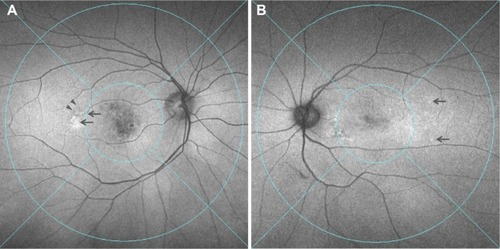
FAF abnormalities in the midperipheral fundus was observed in 48% (15/31) of the CVH(−) AMD eyes and 82% (14/17) of the CVH(+) AMD eyes (P=0.031, chi-squared test) (). Among the 29 eyes presenting with FAF abnormalities, increased granular FAF, decreased mottled FAF, and decreased nummular FAF were observed in nine eyes, two eyes, and 18 eyes, respectively.Citation16 No gravitational tracts were detected in any eyes. Multiple regression showed that the presence of CVH was significantly associated with the presence of FAF abnormalities (P=0.025), independent of age, axial length, and history of PDT or IVR. When AMD was divided into tAMD and PCV, the proportions of FAF abnormalities were 39%, 62%, 100%, and 80% in the CVH(−) tAMD, CVH(−) PCV, CVH(+) tAMD, and CVH(+) PCV eyes, respectively, with no significant differences in the rate of FAF abnormalities between the CVH(+) tAMD and CVH(+) PCV eyes.
Figure 3 Two representative cases of CVH(+) AMD showing FAF abnormalities in the midperipheral fundus.
Abbreviations: CVH(+), choroidal vascular hyperpermeability-positive; AMD, age-related macular degeneration; FAF, fundus autofluorescence.
Discussion
Choroidal thickness in eyes with exudative AMD and CVH
In this study, the choroid was thicker in the CVH(+) AMD eyes not only at the fovea, but also across a wide area of the fundus. To the best of our knowledge, previous studies have only evaluated choroidal thickness at areas close to the fovea in eyes with exudative AMD.Citation8,Citation9,Citation17–Citation19 In the present study, the choroid was thicker in the PCV eyes than in the tAMD eyes at the fovea, similar to the findings of previous studies.Citation17–Citation19 However, the difference in choroidal thickness across a wide area of the choroid was even more apparent between the CVH(+) AMD and CVH(−) AMD eyes. Taken together with previous findings,Citation8,Citation9,Citation12 our findings support the classification of AMD based on the presence of CVH and suggest that CVH(+) AMD is associated with widespread choroidal thickening.
Recently, ultrawide field ICGA revealed extensive dilated choroidal vessels in the early phase of CSC and areas of choroidal hyperpermeability in the late phase.Citation20 Dilation of choroidal vessels extended along the entire course of the vessel back to the vortex vein ampullas, indicating outflow congestion of the draining vortex vein. Additionally, the area with dilated veins corresponded to the areas of hyperpermeability, even outside the macular area. This indicated that the choroid is affected across a wider area than previously thought, and it is likely to be thick across an extensive area in CSC.
Indeed, in the present study, an extensively thick choroid was observed in CVH(+) AMD patients. Although a history of CSC was not confirmed in many of our subjects, we speculate that a similar mechanism, such as outflow congestion, may be involved in AMD with CVH. There was no significant difference in the choroidal thickness at the temporal point. Because there are few draining choroidal vessels in the temporal choroid,Citation21 this finding may further support the theory that congestion of choroidal vessels is the cause of choroidal thickening. The choroidal vessel layer consists of loose connective tissue embedded with numerous large- and medium-sized blood vessels, and the hydrostatic pressure rise due to congestion may diffuse throughout the choroidal layers to the choriocapillaris, where the main leakage occurs.
Midperipheral FAF abnormalities in exudative AMD eyes with CVH
Recently, ultrawide field imaging of eyes with exudative AMD has frequently revealed abnormalities in peripheral FAF,Citation16 suggesting that attention should be paid not only to the clinical findings of the macular area, but also to the peripheral RPE and overlying neurosensory retina when investigating exudative AMD. In the present study, we also frequently noted peripheral FAF abnormalities in AMD eyes with CVH, regardless of the presence of tAMD or PCV. FAF abnormalities are caused by differences in the levels of lipofuscin in the RPE cells.Citation22–Citation24 Lipofuscin contains toxic compounds such as A2E (N-retinylidene-N-retinylethanolamine), and excessive lipofuscin accumulation, which causes increased FAF,Citation22,Citation23 represents a common pathway in various degenerative and monogenetic retinal diseases.Citation23,Citation25,Citation26 The causes of reduced FAF signaling include reduced RPE lipofuscin density and increased RPE melanin content.Citation24 The loss of the RPE also leads to decreased lipofuscin density, making regions hypofluorescent.Citation23,Citation24 Thus, almost all FAF abnormalities represent various degrees of RPE alteration. In the present study, AMD with CVH was frequently accompanied by both alterations in peripheral RPE and widespread changes in choroidal thickness.
Recently, a new clinical entity termed pachychoroid pigment epitheliopathy was reported.Citation27 Patients with pachychoroid pigment epitheliopathy show choroidal thickening and a spectrum of overlying RPE abnormalities around the fovea. This entity was documented as a forme fruste of CSC, but none of the reported 18 eyes had clinically detectable macular edema, subretinal fluid, pigmental epithelium detachment, or CNV during the follow-up period. Given that our CVH(+) AMD patients showed choroidal and RPE changes similar to patients with pachychoroid pigment epitheliopathy in the peripheral fundus, we surmise that pachychoroid pigment epitheliopathy also occurs in the peripheral fundus at least in a subset of CVH(+) AMD cases.
Limitations
There are several limitations in the present study. First, it had a retrospective design and was not based on a pre-established protocol such as that used for ICGA. In our study, ICGA was limited to the posterior 50°, and correspondence between choroidal thickness and hyperpermeability could not be fully identified. Second, all patients were from a single institution and the number of subjects was moderately small. Third, the patients were heterogeneous, including treatment-naïve patients and patients with a history of PDT or multiple IVR injections. It has been reported that subfoveal choroidal thickness decreases by approximately 20 μm by 12 months after IVR in AMDCitation28 and decreases by about 40 μm by 6 months after PDT in PCV.Citation29 However, in the present study, the proportion of the subjects who had a history of PDT or IVR was lower in the CVH(−) group. Even if the treatment had affected choroidal thickness, the choroid before treatment would have been thinner in the CVH(−) group than in the CVH(+) group. Additionally, multiple regression analysis showed that CVH was associated with choroidal thickening and a higher frequency of FAF abnormalities, independent of a history of PDT or IVR treatment. Lastly, no difference was seen in a history of CSC between CVH(+) AMD and CVH(−) AMD patients, which contradicts our previous finding; however, this could be due to recall bias or to the relatively small sample size of the present study.
Conclusion
This study demonstrated that AMD with CVH is characterized by a broadly thickened choroid and is frequently associated with peripheral FAF abnormalities. The majority of CVH(+) AMD patients in this study did not have a history of CSC or clinical findings suggesting a history of CSC (ie, gravitational tract), leading us to conclude that the terminology “CNV secondary to CVH” rather than “CNV secondary to CSC”Citation30 is more appropriate for describing AMD with CVH, because CNV secondary to CSC constitutes only a subset of CNV secondary to CVH. AMD with CVH presents with broad changes to the RPE and choroid, and may have distinguishing clinical characteristics.
Acknowledgments
This study was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Supplementary material
Table S1 Previous treatments of patients
Disclosure
The authors report no conflicts of interest in this work.
References
- PiccolinoFCBorgiaLCentral serous chorioretinopathy and indocyanine green angiographyRetina19941432312427973118
- PrünteCFlammerJChoroidal capillary and venous congestion in central serous chorioretinopathyAm J Ophthalmol1996121126348554078
- SpaideRFCampeasLHaasACentral serous chorioretinopathy in younger and older adultsOphthalmology19961031220702079 ; discussion 2079–2080.9003341
- LafautBASalatiCPriemHDe LaeyJJIndocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patientsGraefes Arch Clin Exp Ophthalmol199823675135219672797
- TsujikawaAOjimaYYamashiroKPunctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiographyRetina201030580180920094008
- SpaideRFYannuzziLASlakterJSSorensonJOrlachDAIndocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathyRetina19951521001107542796
- SasaharaMTsujikawaAMusashiKPolypoidal choroidal vasculopathy with choroidal vascular hyperpermeabilityAm J Ophthalmol2006142460160717011852
- JirarattanasopaPOotoSNakataIChoroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathyInvest Ophthalmol Vis Sci20125373663367222570352
- KoizumiHYamagishiTYamazakiTKinoshitaSRelationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeabilityAm J Ophthalmol20131552305313 e1.23022162
- ChoHJKimHSJangYSEffects of choroidal vascular hyperpermeability on anti-vascular endothelial growth factor treatment for polypoidal choroidal vasculopathyAm J Ophthalmol2013156611921200 e1.24011522
- GassJDBiomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranesAm J Ophthalmol199411832852987521987
- MiyakeMTsujikawaAYamashiroKChoroidal neovascularization in eyes with choroidal vascular hyperpermeabilityInvest Ophthalmol Vis Sci20145553223323024781946
- MarukoIIidaTSaitoMNagayamaDSaitoKClinical characteristics of exudative age-related macular degeneration in Japanese patientsAm J Ophthalmol20071441152217509509
- ObataRYanagiYKamiJTakahashiHInoueYTamakiYPolypoidal choroidal vasculopathy and retinochoroidal anastomosis in Japanese patients eligible for photodynamic therapy for exudative age-related macular degenerationJpn J Ophthalmol200650435436016897221
- ShoKTakahashiKYamadaHPolypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristicsArch Ophthalmol2003121101392139614557174
- TanCSHeussenFSaddaSRPeripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degenerationOphthalmology201312061271127723433790
- ChungSEKangSWLeeJHKimYTChoroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degenerationOphthalmology2011118584084521211846
- KoizumiHYamagishiTYamazakiTKawasakiRKinoshitaSSubfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathyGraefes Arch Clin Exp Ophthalmol201124981123112821274555
- RishiPRishiEMathurGRavalVOcular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normalsEye (Lond)20132791038104323764988
- PangCEShahVPSarrafDFreundKBUltra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathyAm J Ophthalmol20141582362371 e2.24794091
- MoriKGehlbachPLYoneyaSShimizuKAsymmetry of choroidal venous vascular patterns in the human eyeOphthalmology2004111350751215019327
- DeloriFCDoreyCKStaurenghiGArendOGogerDGWeiterJJIn vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristicsInvest Ophthalmol Vis Sci19953637187297890502
- HolzFGBindewald-WittichAFleckensteinMDreyhauptJSchollHPSchmitz-ValckenbergSFAM-Study GroupProgression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degenerationAm J Ophthalmol2007143346347217239336
- NandakumarNBuzneySWeiterJJLipofuscin and the principles of fundus autofluorescence: a reviewSemin Ophthalmol2012275–619720123163276
- BoultonMDayhaw-BarkerPThe role of the retinal pigment epithelium: topographical variation and ageing changesEye (Lond)200115Pt 338438911450762
- SchüttFDaviesSKopitzJHolzFGBoultonMEPhotodamage to human RPE cells by A2-E, a retinoid component of lipofuscinInvest Ophthalmol Vis Sci20004182303230810892877
- WarrowDJHoangQVFreundKBPachychoroid pigment epitheliopathyRetina20133381659167223751942
- YamazakiTKoizumiHYamagishiTKinoshitaSSubfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month resultsOphthalmol2012119816211627
- MarukoIIidaTSuganoYSaitoMSekiryuTSubfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathyAm J Ophthalmol20111514594603 e1.21295766
- FungATYannuzziLAFreundKBType 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degenerationRetina20123291829183722850219
