Abstract
Background
Iluvien® is a novel, nonbiodegradable, sustained-release drug delivery system (0.2 μg/d fluocinolone acetonide [FAc]) indicated in Europe for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.
Objective
To evaluate the safety and efficacy of 190-μg FAc implant in patients with chronic DME refractory to other medical treatment options in a clinical setting.
Methods
Retrospective registry data were collected by using standard case report forms (CRFs). Prior to intravitreal injection of the FAc implant, all patients were treated either with a vascular endothelial growth factor (VEGF) antagonist and/or a steroid (triamcinolone, dexamethasone implant). Patients were excluded from receiving FAc if they had a known history of elevated intraocular pressure (IOP) following corticosteroid therapy, glaucoma, ocular hypertension, or any contraindications cited in the summary of product characteristics. Best-corrected visual acuity (BCVA) was the main study parameter. Central fovea thickness (CFT) and IOP were measured concurrently. These parameters were recorded prior to and after the injection of the 190-μg FAc implant (between 1 week and 9 months). Injections were performed between May 2013 and March 2014.
Results
Fifteen eyes from ten patients were treated. Thirteen eyes (nine patients) were pseudophakic, and seven eyes (five patients) were vitrectomized prior to receiving therapy. BCVA improved in eleven eyes (73.3%), remained unchanged in two eyes (13.3%), and decreased slightly in two eyes (13.3%) at the last follow-up visit versus baseline levels. IOP increased in two patients and was controlled using fixed-combination of IOP-lowering eyedrops or sectorial cyclocryotherapy (n=1).
Conclusion
The 190-μg FAc implant was efficacious and showed a favorable benefit-to-risk profile in the patient population with chronic DME of this case series that were refractory to other therapies. The longer-term efficacy and safety in a real-life setting is still being assessed in this center. FAc may offer an important treatment option for patients with chronic DME refractory to other treatment options.
Introduction
Diabetic macular edema (DME) is the most common cause of reduced visual acuity in patients with diabetes. It could be shown that 20.1% of patients with type 1 diabetes, 25.4% with type 2 diabetes using insulin, and 13.9% with type 2 diabetes, not using insulin respectively, develop DME within a 10-year time period.Citation1 The global prevalence of diabetic retinopathy among individuals with diabetes is around 35%, with DME present in 6.8%.Citation2 Without treatment, nearly half of these patients developing DME will lose two or more lines of visual acuity within 2 years.Citation3 It is well established that the incidence of DME increases with the duration of diabetes, the severity of retinopathy, and with increasing levels of glycosylated hemoglobin.Citation4–Citation7 Many interrelated pathways are linked to the cellular damage from hyperglycemia and hypoxia affecting the blood–retina barrier (BRB), including angiogenic growth factors and inflammatory cytokines. Glucocorticoids modulate multiple pathways to exert a therapeutic effect in DME. Recently, with the better understanding of the pathophysiology of DME and the discovery of the critical role played by the inflammatory cascade, many physicians have employed the use of corticosteroids to treat this sometimes relapsing or refractory disease.Citation8–Citation11 Corticosteroids have a direct angiostatic effect, suppress group II phospholipase A2, and block the inflammatory response.Citation12,Citation13 Furthermore corticosteroids have been shown to inhibit the expression of vascular endothelial growth factor (VEGF).Citation14–Citation16 By applying a drug directly into the vitreous, the BRB is bypassed and therapeutic intraocular levels can be achieved with doses of corticosteroids even at a submicrogram level.Citation17 Intravitreal injections of corticosteroids, such as triamcinolone acetonide, have been shown to be beneficial in the treatment of patients with DME.Citation18,Citation19 It could be shown that intravitreal triamcinolone (4 mg) appeared to reduce the risk of diabetic retinopathy over a time period of 3 years.Citation20 Furthermore, it could be shown that over a time period of 4 months mean visual acuity was better in patients with DME receiving 4 mg triamcinolone as compared to triamcinolone 1 mg or focal/grid laser photocoagulation. Interestingly visual acuity was better in the laser treatment arm as compared to both medical treatment arms (triamcinolone 1 and 4 mg) after 16 months and up till 2 years of the study extension period.Citation21 Triamcinolone – even if the substance is used for the medical treatment of DME – is not approved and marketed for the treatment of this eye disease. One major disadvantage of applying triamcinolone into the vitreous is that frequent injections are needed.Citation21 To solve this issue, microimplants have been developed, which offer the advantage of a sustained release of the corticosteroid and thus reduce the number of procedures required to maintain therapeutic levels of the drug over a longer time period. Iluvien® (Alimera Sciences Inc., Alpharetta, GA, USA) is an intravitreal, nonbioerodable microimplant containing the corticosteroid fluocinolone acetonide (FAc). This implant is made of polyimide and measures 3.5×0.37 mm. It is administered into the vitreous using a preloaded 25-gauge inserter, and once injected the microimplant continuously releases a low dose of FAc into the vitreous (0.2 μg/d FAc) that lasts for up to 36 months.Citation22 FAc is indicated for the treatment of vision impairment associated with chronic DME, considered insufficiently responsive to available therapies.Citation23 The product is licensed in a number of European countries and was recently approved in the US by the FDA. Campochiaro et alCitation24 reported combined 3-year results of two pivotal studies with FAc in patients with chronic DME. In the FAME (Fluocinolone Acetonide in patients with diabetic Macular Edema) studies they evaluated 953 eyes of patients with persistent DME after one or more laser therapy treatments, randomized 1:2:2 for sham injection (n=185), low-dose FAc implant (0.2 μg/d, n=375), or high-dose FAc implant (0.5 μg/d FAc, n=393). At 36 months, 27.8% (high dose) and 28.7% (low dose) of all implant-treated eyes versus 18.9% of sham eyes demonstrated an improvement of 15 or more letters (P=0.018). In addition, preplanned subgroup analysis showed a significant and increased benefit, especially in patients with chronic DME.Citation25 Chronic DME was defined by a duration of the disease of ≥3 years. In this typically difficult-to-treat patient population, a gain of ≥15 letters was achieved by 34.0% of patients treated with FAc 0.2 μg/d versus 13.4 in the sham group. Known side effects of corticosteroid therapy are cataract formation and an increase of intraocular pressure (IOP). Indeed, in the FAME studies, four out of five patients underwent cataract surgery. Also, a rise in IOP that required IOP lowering drops was observed in 38.4% of the patients and 4.8% of these patients required an incisional IOP lowering procedure. The objective of this study was to collect a series of cases to evaluate the effects of 190-μg FAc implant in a real-life setting. Ten patients with chronic DME refractory to other medical treatment options, such as VEGF antagonists and/or intravitreal corticosteroids (triamcinolone, dexamethasone), were evaluated. Changes in best-corrected visual acuity (BCVA), central fovea thickness (CFT), and morphological improvements of the DME were analyzed. Adverse events (AEs) were assessed and form the basis of this report.
Methods
This was a retrospective registry study involving all eyes of the site (15 eyes from ten patients) treated with the 190-μg FAc implant between May 2013 and March 2014. Thus, this series describes both, early and longer-term results of all patients with DME of the site treated with FAc within this time period. Persistent clinical DME was defined as retinal thickening and hard exudates within 500 μm of the macular center and the occurrence of hard exudates within this area. If spectral domain optical coherence tomography (OCT) data were available, DME was also defined as a CFT of >250 μm. The study was approved by the local ethics committee. Informed consent was gained from all patients, which enabled the collection and evaluation of individual patient data and the publication of anonymized patient data. All data were collected using standardized case report forms (CRFs).
All procedures were performed in an aseptic operating room under topical anesthesia by the same physician. The recommended technique for intraocular injection of the implant is as follows: the patient was prepared in an operating room with the same standards as followed for intraocular procedures. Whilst maintaining aseptic conditions the cap protecting the needle was carefully removed. The intravitreal injection of 190-μg FAc implant was performed by using the preloaded injector with a 25-gauge needle. The implant was injected inferotemporally and inserted into the vitreous of the eye. All patients were examined pre- and postoperatively on day 1, and the following parameters were assessed at each follow-up visit: BCVA, anterior chamber reaction, IOP, and fundus evaluation by indirect ophthalmoscopy. Complete ocular examination was performed at each follow-up visit. Due to the retrospective character of this case series, OCT (Cirrus 1, Carl Zeiss Meditech, Dublin, CA, USA) data were available for most but not all patients. Angiographic examinations were performed in all patients, thus macular edemas were detected either by OCT and/or fluorescence angiography. Determination of BCVA (measured on a decimal scale) was done thoroughly and refraction was done at each visit.
Patients with DME who met the following criteria were included: persistent clinically significant DME involving the center of the fovea, DME refractory to other medical treatment options (intravitreal VEGF antagonists and/or corticosteroid injections), ability to give informed consent.
Exclusion criteria were a history of corticosteroid-responsive IOP increase and the contraindications listed in the summary of product characteristics for Iluvien®.
The main outcome measure was BCVA at all follow-up visits compared to baseline. Secondary outcome measures included change in CFT on OCT, changes in IOP (Goldmann applanation tonometry), evaluation of the fundus by fluorescence angiography (Spectralis, Heidelberg Engineering, Heidelberg, Germany), and development of any side effects resulting from the intravitreal injection of the 190-μg FAc implant.
Results
Patients
Fifteen eyes of nine male and one female patients with refractory chronic DME were treated with 190-μg FAc implant. The mean age of the patients was 69.2 years (range: 52–83 years). Nine patients were pseudophakic at baseline and one patient (patient number 1) suffered from a bilateral cataract which was removed 5–6 weeks after the injection of the FAc implant. Seven eyes (five patients) had a history of vitrectomy. At baseline, BCVA was in the range of 0.1–0.6 and the CFT was between 291 and 729 μm. Baseline IOP ranged between 12 and 19 mmHg (). All patients/eyes had been previously treated with VEGF antagonists (ranibizumab 0.5 mg, bevacizumab 1.25 mg) and/or corticosteroids (triamcinolone 4–25 mg, dexamethasone implant 700 μg) prior to initiation of the treatment with FAc, and had not responded sufficiently. Details for all patients, eyes, and the history of prior medical treatments for DME are shown in as well as the numbers of injections. The follow-up period for all patients/eyes ranged between 2 and 37 weeks (). summarizes the results for the development of BCVA, CFT, and IOP for all patients/eyes showing the baseline values and the last reading for each of these parameters.
Table 1 Patient demographics and baseline values
Table 2 Individual patients: age, sex, best-corrected visual acuity (BCVA) at baseline, and prior medical treatments
Table 3 Individual patients: BCVA, CFT, and IOP pre- and postoperatively
Best-corrected visual acuity (BCVA)
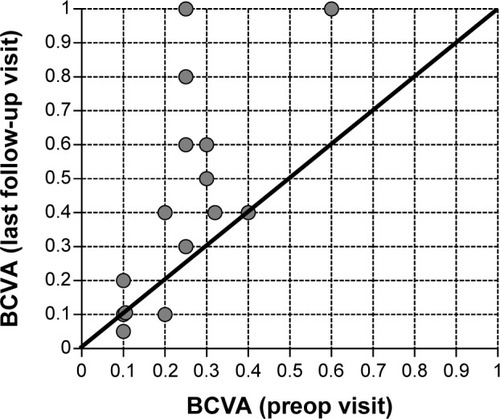
BCVA improved at the last follow-up visit in eleven of 15 eyes (ie, 73.3% of cases) versus baseline. The mean gain in visual acuity, for all 15 eyes treated, was +0.19±0.24 (from a starting value of 0.25±0.13) with a range between −0.1 and +0.75. In three eyes, BCVA was unchanged versus baseline, and in two eyes decreased slightly (−0.1 and +0.05, patient 8 and 10 respectively; ). shows the development of BCVA for each individual patient at baseline versus the last follow-up visit. The gain in visual acuity was independent from the prior treatment, as BCVA improved in cases where patients had been treated previously with VEGF antagonists, corticosteroids, or both ().
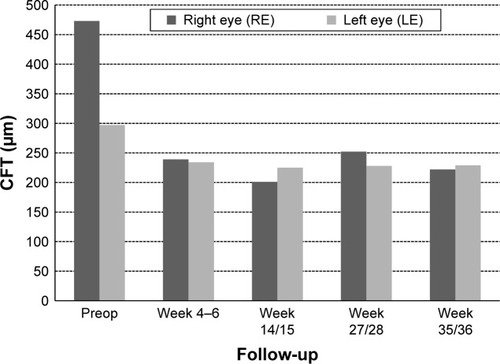
Central fovea thickness (CFT)
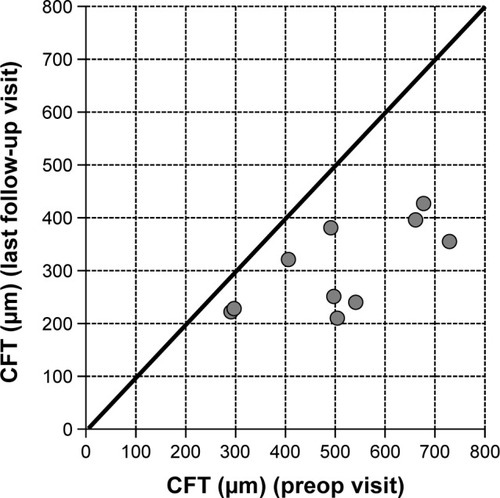
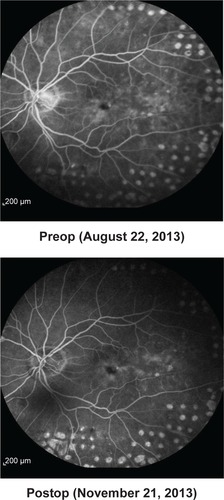
Baseline values for CFT were available for ten of the 15 eyes. CFT at baseline ranged between 291 and 729 μm (mean CFT at baseline ± SD: 509.4±150.7 μm). After initiation of treatment with FAc, CFT declined in all ten eyes on average by −206.3 μm (mean CFT last follow-up visit ± SD: 303.1±82.1 μm), for which CFT data were available. shows the development of CFT for each individual patient at baseline versus the last follow-up visit. Fast improvements were also visible in the fluorescence angiograms. As an example the angiographic findings for patient number 5 (left eye) are shown in pre- and 2 months postinjection of FAc.
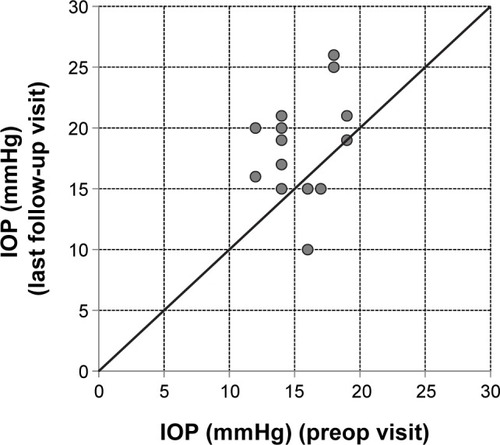
Intraocular pressure (IOP)
Three of the 15 eyes showed an increase of IOP (>7 mmHg) (). In two patients IOP was lowered successfully by a sectorial cyclocryotherapy and/or a medical treatment with a fixed combination of IOP-lowering eyedrops (dorzolamide/timolol or brinzolamide/timolol). Cyclocryotherapy is a routine procedure used at the center for patients that do not respond sufficiently to a fixed-dose combination. The mean change in IOP was 4.1±5.8 mmHg from a mean baseline value of 15.5±2.3 mmHg (individual data are plotted in ). shows the development of IOP for each individual patient at baseline versus the last follow-up visit.
Figure 4 IOP at the preop visit versus the last follow-up visit for all individual eyes.
Abbreviation: IOP, intraocular pressure.
In order to illustrate the overall findings, two patient cases are described more in detail. The selection was based on the length of the follow-up period: One patient (patient number 2) was selected with a short follow-up period and one patient (patient number 3) with a longer follow-up period.
Case reports
Case A – patient number 2
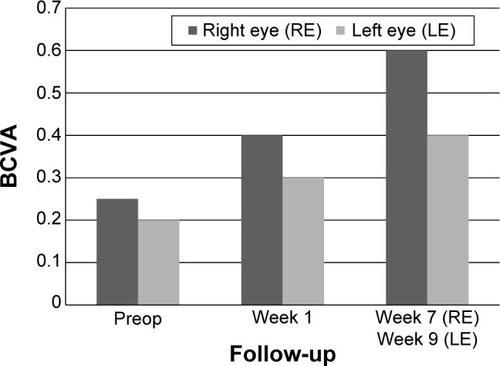
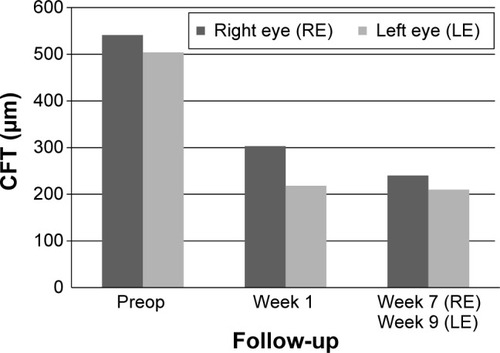
This patient is a 64-year-old female with a history of chronic DME. Systemically, the patient suffers from multiple forms of arthrosis such as gonarthrosis, omarthrosis, and arthrosis of the hands. This patient also had a long history of ophthalmic diseases: diabetic retinopathy, DME, AMD, laser capsulotomy, bilateral pseudophakia, and pars plana vitrectomy. Prior to injection of 190 μg FAc, both eyes of this patient were treated as follows: bevacizumab (four injections, left eye), ranibizumab (two injections, left eye), and bevacizumab (three injections, left eye). BCVA at baseline (last visit before FAc) was 0.25 (right eye) and 0.2 (left eye), CFT was 541 μm (right eye) and 504 μm (left eye). IOP baseline values were 18 mmHg for both eyes. Intravitreous injection of the 190-μg FAc implant was conducted in the right eye on January 15, 2014 and in the left eye on January 28, 2014.
Morphology – OCT findings
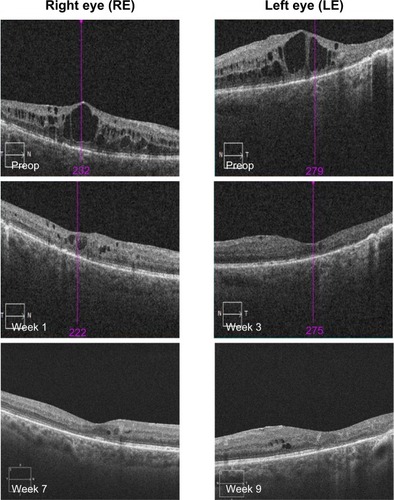
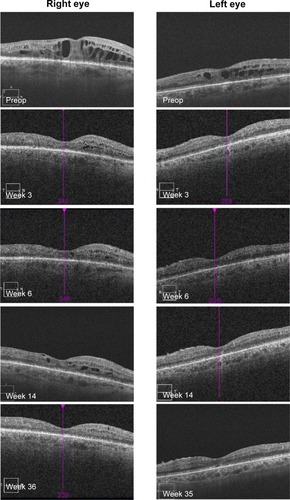
OCT showed a fast morphological improvement of DME in both eyes with a clear reduction of the magnitude of cysts. The improvements for both eyes and all follow-up visits are shown in . Intraretinal cysts in the right eye due to DME were significantly reduced during the 7–9 weeks after injection of the FAc implant.
Best-corrected visual acuity (BCVA)
In both eyes, BCVA showed a rapid gain 1–3 weeks after the procedure: BCVA improved in the right eye from 0.25 to 0.4 and in the left eye from 0.2 to 0.3. A further gain was observed 7–9 weeks after the procedure ().
Central fovea thickness (CFT)
CFT decreased significantly during the follow-up period in the right eye from 541 to 240 μm (−301 μm; week 7) and in the left eye from 504 to 210 μm (−294 μm; week 9) ().
Intraocular pressure (IOP)
IOP increased from 18 mmHg at baseline to 26 mmHg in the right eye at week 7 and from 18 to 25 mmHg in the left eye at week 9. A medical treatment of increased IOP with a fixed combination dorzolamide/timolol (bid) was initiated, which reduced IOP to ≤20 mmHg.
Case B – patient number 3
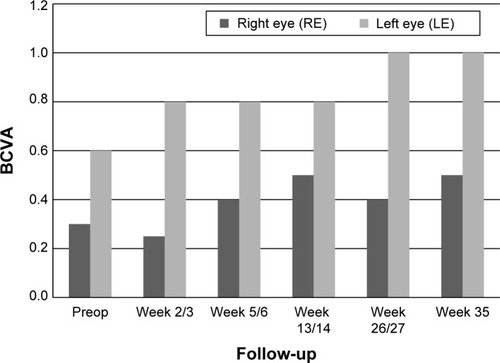
This patient is a 72-year-old male with a long history of DME. Systemically the patient suffers from arterial hypertension and type 2 diabetes. Ophthalmic diseases of this patient included diabetic retinopathy, DME, and bilateral pseudophakia. Prior to injection with FAc, both eyes of this patient were treated as follows: bevacizumab (seven injections, both eyes), triamcinolone 4 mg (one injection, right eye), triamcinolone 10 mg (left eye), and triamcinolone 25 mg (six injections, both eyes). BCVA at baseline (last visit before FAc) was 0.3 (right eye) and 0.6 (left eye), CFT was 473 μm (right eye) and 297 μm (left eye). IOP baseline values were 14 mmHg (right eye) and 12 mmHg (left eye).
Morphology – OCT findings
OCT showed a fast morphological improvement of DME in both eyes with a clear reduction of the magnitude of cysts. The improvements for both eyes and all follow-up visits are shown in . Intraretinal cysts in the right eye due to DME were significantly reduced at the first follow-up visit 3 weeks after injection of the 190-μg FAc implant. The effect was sustained up till the last follow-up visit (week 35).
Best-corrected visual acuity (BCVA)
In both eyes, BCVA showed a rapid gain within 1–3 weeks after the procedure: BCVA improved in the right eye from 0.3 to 0.5 and in the left eye from 0.6 to 1.0 ().
Central fovea thickness (CFT)
CFT decreased significantly during the follow-up period in the right eye from 541 to 222 μm (week 35) and in the left eye from 297 to 210 μm (week 36) ().
Intraocular pressure (IOP)
At week 35, IOP remained almost unchanged in the right eye (+1 mmHg from a baseline value of 14 mmHg). At week 36, IOP had increased slightly in the left eye (+4 mmHg from a baseline value of 12 mmHg). In both eyes IOP was ≤16 mmHg.
Discussion
Beside the description of single patient reports, there is little information available on the outcome of treatment of patients with FAc under “real life” conditions. To the author’s knowledge, this is the first report of a series of cases that were treated with FAc in a routine clinical setting. The safety and efficacy of 190-μg FAc implant in patients with chronic DME refractory to other medical treatment options in a case series in the real world clinical setting was evaluated. In total, 15 eyes were treated from ten subjects (nine male and one female patient). Prior to treatment with FAc, all patients were treated either with a VEGF antagonist and/or a steroid (triamcinolone, dexamethasone implant). BCVA improved in eleven eyes (73.3%; range: 0.05–0.75), remained unchanged in two eyes (13.3%), and decreased slightly in two eyes (13.3%; range: −0.05 to −0.1) at the last follow-up visit in comparison to baseline. Data from randomized, controlled studies have shown that laser photocoagulation,Citation26 and intravitreal injections of VEGF antagonistsCitation27,Citation28 and corticosteroidsCitation8,Citation10 are efficacious and useful in the medical treatment of DME. However, these studies also show that repeated treatments are often required to control macula edema, prevent vision loss, and increase the chance of visual improvement. Furthermore, subanalyses data from two well controlled long-term clinical studies with ranibizumab (RISE and RIDE studies) show that a delayed initiation of medical treatment in chronic DME results in a small gain in visual acuity relative to those patients treated 24 months earlier.Citation29 These data indicate that the number of VEGF antagonist nonresponders (defined as patients with a gain in visual acuity of <10 letters) should be expected in a range between 35.4% and 37.6%Citation30 based on monthly injections. For these patients that do not respond adequately to VEGF antagonists, alternative treatment options, such as FAc, are of great importance. FAc has been recently approved for the treatment of chronic DME in patients refractive to other medical treatments. A study in eyes with persistent DME demonstrated that the FAc microimplant produced improvements in visual acuity, decreased macular thickness, and fluorescein leakage that were sustained for up to 3 years.Citation24 This study confirmed the beneficial effect of FAc on the clinical outcome of DME in a real-life clinical setting. In parallel to the decrease of CFT, a gain in visual acuity was observed in 73.3% of all eyes. All patients of this registry study showed signs of a persistent DME after being treated with either VEGF antagonists and/or intravitreal corticosteroids. Interestingly even most of the latter patients with prior corticosteroid treatment showed an improvement in BCVA and CFT after the treatment with FAc. This finding may, at least in part, be explained by a different release of the active ingredient from the microimplant and different pharmacokinetics such as a sustained and constant release of the active ingredient from the matrix.
One common side effect resulting from the use of corticosteroids for the treatment of DME is the possible rise in IOP. For triamcinolone, a rise of IOP of ≥10 mmHg versus baseline was reported in 16% and 33% of patients treated with 1 and 4 mg respectively.Citation21 In this study, three of the 15 eyes had a rise of IOP of >7 mmHg. The rise in IOP was controlled either by a sectorial cyclocryotherapy and/or a medical treatment with fixed combinations. Another known AE of corticosteroids is cataract formation. Cataract surgery was performed in 23% and 51% of eyes treated with 1 and 4 mg triamcinolone respectively.Citation21 The effect of FAc on cataract formation in this case series could not be evaluated since most of the patients from this patient cohort were pseudophakic (n=13/15 eyes), and because one patient already had an existing cataract with removal planned for after intravitreal injection of 190-μg FAc implant (). Furthermore, the possible differences of FAc on the visual outcomes (BCVA, CFT) in patients with DME with and without prior vitrectomy can only be speculated. First, patients with vitrectomized eyes normally show worse baseline values in both, BCVA and CFT and second, many of the patients from this small patient population were vitrectomized prior to the FAc treatment.
Conclusion
The 190-μg FAc implant appeared efficacious and safe in the treatment of the patients with chronic DME refractive to other medical treatments in this patient population. Visual acuity improved in eleven out of 15 eyes (73.3% of cases). Distinct increases of IOP (>7 mmHg) were observed in three eyes (20% of cases) during the follow-up period which could be controlled by cyclocryotherapy (n=1) and/or medical treatment. The data from this registry study indicate that FAc may be an effective addition in the armamentarium for the medical treatment of chronic DME, especially for those patients refractive to other medical treatments. According to the guidelines of the German Retinological Society, a change of treatment may be considered if the BCVA does not show an improvement of at least one line or a reduction of retina thickness of at least 10% during the last 3 months postinjection of VEGF antagonists. It is necessary to closely monitor the further development of all of these patients during the next several months in order to evaluate the further development of all parameters and be able to collect additional information of the longer-term effects of the 190-μg FAc implant.
Acknowledgments
I acknowledge the support provided by Friedemann Kimmich, PhD (Eyecons, Karlsruhe, Baden-Württemberg, Germany) and Albert Augustin, MD (Augenklinik Städtisches Klinikum, Karlsruhe, Baden-Württemberg, Germany). The publication of this article and editorial assistance was funded by Alimera Sciences Ltd. The views and opinions expressed in the article are those of the authors and not necessarily those of Alimera Sciences Ltd.
Disclosure
The author reports no conflicts of interest in this work.
References
- KleinRKleinBEMossSEThe Wisconsin epidemiologic study of diabetic retinopathy: XV. The long-term incidence of macular edemaOphthalmology199510217167831044
- YauJWRogersSLKawasakiRGlobal prevalence and major risk factors of diabetic retinopathyDiabetes Care20123555656422301125
- FerrisFLIIIPatzAMacular edema. A complication of diabetic retinopathySurv Ophthalmol198428Suppl4524616379946
- BhagatNGrigorianRATutelaAZarbinMADiabetic macular edema: pathogenesis and treatmentSurv Ophthalmol20095413219171208
- MossSEKleinRKleinBEThe 14-year incidence of visual loss in a diabetic populationOphthalmology199810599810039627648
- KleinRKleinBEMossSECruickshanksKJThe Wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetesOphthalmology1998105180118159787347
- Diabetes Control and Complications Trial Research GroupProgression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications TrialOphthalmology19951026476617724182
- MassinPAudrenFHaouchineBIntravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trialOphthalmology20041112218224 discussion 224–22515019365
- TangJKernTSInflammation in diabetic retinopathyProg Retin Eye Res201130534335821635964
- JonasJBKamppeterBAHarderBVossmerbaeumerUSauderGSpandauUHIntravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized studyJ Ocul Pharmacol Ther200622320020716808682
- JonasJBIntravitreal triamcinolone acetonide: a change in a paradigmOphthalmic Res200638421824516763379
- FolkmanJAngiogenesis and apoptosisSemin Cancer Biol200313215916712654259
- NakanoTOharaOTeraokaHAritaHGlucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expressionJ Biol Chem19902652112745127482373711
- LeopoldIHNonsteroidal and steroidal anti-inflammatory agentsSearsMTarkkanenASurgical Pharmacology of the EyeNew York, NYRaven Press198583133
- EdelmanJLLutzDCastroMRCorticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood aqueous barrier breakdownExp Eye Res20058024925815670803
- FunatsuHYamashitaHNomaHMimuraTYamashitaTHoriSIncreased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edemaAm J Ophthalmol2002133707711755841
- CampochiaroPANguyenQDHafizGAqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implantsOphthalmology201312058358723218184
- GilliesMCSutterFKSimpsonJMLarssonJAliHZhuMIntravitreal triamcinolone for refractory diabetic macular edema: two-year results of a doublemasked, placebo-controlled, randomized clinical trialOphthalmology20061131533153816828501
- MartidisADukerJSGreenbergPBIntravitreal triamcinolone for refractory diabetic macular edemaOphthalmology200210992092711986098
- BresslerNMEdwardsARBeckRWExploratory analysis of diabetic retinopathy progression through 3 years in a randomized clinical trial that compares intravitreal triamcinolone acetonide with focal/grid photocoagulationArch Ophthalmol20091271566157120008708
- Diabetic Retinopathy Clinical Research NetworkA randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edemaOphthalmology20081151447144918662829
- CampochiaroPAHafizGShahSMFAMOUS Study GroupSustained ocular delivery of fluocinolone acetonide by an intravitreal insertOphthalmology20101171393139920202684
- ILUVIEN® [summary of product characteristics] Last updated on eMC 18-Feb-2014. Available from: https://www.medicines.org.uk/emc/medicine/27636Accessed July 24, 2014
- CampochiaroPABrownDMPearsonASustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edemaOphthalmology20121192125213222727177
- Cunha-VazJAshtonPIezziRSustained delivery fluocinolone acetonide vitreous implantsOphthalmology20141211892190324935282
- NeubauerASUlbigMWLaser treatment in diabetic retinopathyOphthalmologica20072219510217380063
- NguyenQDShahSMKhwajaAATwo year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) studyOphthalmology20101172146215120855114
- BresslerNMVarmaRSuñerIJVision-related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISEOphthalmology2014121122461247225148789
- BrownDMNguyenQDMarcusDMLong-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDEOphthalmology20131202013202223706949
- NguyenQDBrownDMMarcusDMRanibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDEOphthalmology2012119789801 Supplemental material (Table 6). Available from: http://www.aaojournal.org/cms/attachment/2005446053/2023774732/mmc1.pdfAccessed August 22, 201422330964