Abstract
Choroidal neovascularization (CNV) due to age-related macular degeneration (AMD) is an important cause of visual morbidity globally. Modern treatment strategies for neovascular AMD achieve regression of CNV by suppressing the activity of key growth factors that mediate angiogenesis. Vascular endothelial growth factor (VEGF) has been the major target of neovascular AMD therapy for almost two decades, and there have been several intravitreally-administered agents that have enabled anatomical restitution and improvement in visual function with continual dosing. Aflibercept (EYLEA®), initially named VEGF Trap-eye, is the most recent anti-VEGF agent to be granted US Food and Drug Administration approval for the treatment of neovascular AMD. Biologic advantages of aflibercept include its greater binding affinity for VEGF, a longer intravitreal half-life relative to other anti-VEGF agents, and the capacity to antagonize growth factors other than VEGF. This paper provides an up-to-date summary of the molecular mechanisms mediating CNV. The structural, pharmacodynamic, and pharmacokinetic advantages of aflibercept are also reviewed to rationalize the utility of this agent for treating CNV. Results of landmark clinical investigations, including VIEW 1 and 2 trials, and other important studies are then summarized and used to illustrate the efficacy of aflibercept for managing treatment-naïve CNV, recalcitrant CNV, and CNV due to polypoidal choroidal vasculopathy. Safety profile, patient tolerability, and quality of life measures related to aflibercept are also provided. The evidence provided in this paper suggests aflibercept to be a promising agent that can be used to reduce the treatment burden of neovascular AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe visual loss in people over the age of 65 years in the industrialized world.Citation1 It is a bilateral, progressive disease that demonstrates great interindividual variability with respect to the rate of visual loss over time.Citation2 The global burden of AMD is projected to increase over the next two decades, and thus, there is an urgent need to clarify the pathophysiology of this disease and to identify viable treatment strategies that will arrest disease-induced vision loss.Citation3
Choroidal neovascularization (CNV) is a complication that may occur during the natural course of AMD.Citation4 In a study of individuals who were diagnosed with early or intermediate AMD at baseline visit, approximately 10% developed CNV over a median follow-up period of 6.3 years.Citation5 Fortunately, significant progress has been made in the management of neovascular AMD over the past two decades. Treatment paradigms have shifted from observation to laser photocoagulation,Citation6 to photodynamic therapy,Citation7 to submacular surgery,Citation8 and, more recently, to the use of intravitreal vascular endothelial growth factor (VEGF) therapy.Citation9 Regarding the latter therapeutic approach, in 2004 pegaptanib (Macugen®; Eyetech Pharmaceuticals Inc, Palm Beach Gardens, FL, USA and Pfizer Inc, New York, NY, USA) was the first VEGF inhibitor to be approved by the US Food and Drug Administration (FDA) to demonstrate efficacy in clinical trials for treating neovascular AMD.Citation10 However, the use of pegaptanib was quickly surpassed in most settings by newer agents that antagonized a broader range of VEGF isoforms. The MARINACitation11 and ANCHORCitation12 trials demonstrated the utility of ranibizumab (Lucentis; Genentech USA Inc, San Francisco, CA, USA) for managing neovascular AMD, and this agent received approval by the FDA in 2006. Bevacizumab (Avastin, Genentech USA, Inc) has been used off label for many years for treating CNV and was shown to be noninferior to ranibizumab in the Comparison of AMD Treatment Trial (CATT)Citation13 and Inhibition of VEGF in Age-related choroidal Neovascularization (IVAN)Citation14 trial.
Aflibercept (EYLEA®; Regeneron Pharmaceutical Inc, Tarrytown, NY, USA and Bayer Healthcare, Berlin, Germany), initially named VEGF Trap-eye, is the most recent anti-VEGF agent to be granted FDA approval (2011) for treating neovascular AMD. The ocular formulation of aflibercept has been specifically purified and buffered to minimize the risk of eye toxicity when injected intravitreally.Citation15 When administered in an intravenous formulation for oncologic indications, the drug is referred to as ziv-aflibercept (Zaltrap; Regeneron Pharmaceutical Inc). There is compelling evidence from laboratory and clinical research that aflibercept is efficacious for treating neovascular AMD.Citation16 This paper is a systematic discussion of the pathophysiology of CNV, the pharmacokinetic and pharmacodynamic advantages of aflibercept, and the efficacy of this agent for managing neovascular AMD.
Cellular and molecular mechanisms of CNV
Physiological concentrations of growth factors, cytokines, and metabolic substrates are required for normal cellular function in the outer retinal compartment.Citation17 Retinal pigment epithelium (RPE) and glial cells are the predominant cell types to secrete growth factors in the human retina and are therefore critical determinants of retinal health and disease.Citation18,Citation19 Disturbances and imbalances in the concentrations of pro-/antiangiogenic factors can alter the metabolic environment of the outer retina and shift it from one that supports neuronal physiology to one that favors vascular proliferation in the context of neovascularization (NV). This shift can be due to insults that serve to deplete regional RPE and glial cell populations and thereby diminish the global availability of trophic substrates.Citation20 The shift may also be consequent to diseases that modulate the function of a normal population of cells through feedback mechanisms without altering cell structure and density.Citation21 In most chorioretinal diseases, both mechanisms are likely to be involved.
Anatomical disturbances to the outer retina coupled with breakdown in the biological mechanisms that govern vascular function predispose to CNV.Citation22,Citation23 There has been great focus on the role of growth factor-mediated mechanisms in neovascular AMD with less attention paid to the contribution of structural outer retinal changes such as the loss of RPE integrity.Citation24,Citation25 The latter changes have been the focus of novel strategies that are being considered for the management of non-neovascular AMD.Citation26 Specifically, the efficacy of antioxidant supplementation complement inhibitors including intravitreal anti-factor D, ciliary neurotrophic factor, brimonidine tartrate, and visual cycle modulators is currently being investigated for the management of non-neovascular AMD.Citation27
Angiogenic factors that are known to promote vascular proliferation in AMD include VEGF,Citation28 platelet derived growth factor (PDGF),Citation29 transforming growth factor-β1,Citation30 fibroblast growth factor (FGF),Citation31 angiogenin,Citation32 placental growth factor (PlGF),Citation33 and basic FGFs.Citation34 Known inhibitors of vascular proliferation include endostatin,Citation35 thrombospondin,Citation36 and pigment epithelium derived factor-1.Citation37 The putative roles of each of these factors in angiogenesis and NV are summarized in .
Table 1 Growth factors and enzymes involved in angiogenesis
As neoplasms are highly vascularized, metabolically hyperactive tissues, there is significant overlap in the cellular pathways that govern tumor angiogenesis and CNV. Our understanding about the pathophysiology of neovascular AMD has been greatly aided by developments in oncology.Citation38 Current knowledge about neovascular AMD pathogenesis can be summarized with the following sequence of structural and biochemical changes that occur in a stepwise fashion during the process of angiogenesis:Citation39,Citation40
Loss of endothelial cell tight junctions in the native vascular bed.
Increased vascular permeability and extravasation of proteins that over time form a biochemical scaffold and facilitate the migration of capillary buds.
Secretion of proteases by endothelia that degrade the extracellular matrix and propagate new endothelial cell proliferation and migration.
Maturation and organization of the proliferative network with subsequent formation of capillary lumina and envelopment by pericytes.
Establishment of tight junctions and basement membranes in the neovascular bed.
It is important to consider that although the NV occurring in AMD is typically termed “choroidal” neovascularization, it can originate from either the choroidal (type 1 or 2 NV) or retinal (type 3) vascular circulations.Citation41,Citation42 A recent paper by Jung et alCitation43 demonstrated that type 3 NV is the second most common subtype of NV in newly diagnosed, white AMD patients. These findings have major implications for hypotheses regarding the pathogenesis of AMD and suggest that a focused investigation of the retinal circulation may provide novel insights into the biology of NV. These findings also exemplify the importance of understanding the relationship between the deep retinal capillary beds and NV in AMD. This relationship is easily forgotten when old AMD nomenclature, such as “choroidal neovascularization”, is used to denote all subtypes of NV. Fortunately, the definitions proposed by GassCitation44 and Freund et al,Citation45 which have greater relevance for prognosticating disease course and long-term visual potential are increasingly being used in the clinical setting to subclassify NV.
VEGF is upregulated in response to ischemia,Citation46 hypoxia,Citation47 inflammation,Citation48 and trauma.Citation49 In AMD, the initiating factors that induce VEGF upregulation are unclear. However, there is good experimental evidence to demonstrate that complement components of drusen such as C3a and C5a are involved in this process.Citation50 The accumulation of oxidized lipids in Bruch’s membrane is also thought to play an important role in upregulation of VEGF and NV.Citation51 In addition to accumulation of lipid, there is likely to be an ischemic component, due to atrophy of the choriocapillaris, driving VEGF upregulation in AMD.Citation52
As VEGF isoforms mediate many of the steps involved in angiogenesis, nullifying the effects of VEGF has become a powerful way to arrest the process of NV in AMD. This can be done by antagonizing VEGF receptor function or by direct binding to VEGF isoforms to reduce their ability to interact with VEGF receptors. In order to understand why aflibercept is an efficacious therapeutic agent for the management of NV, a detailed understanding of the biological function of VEGF isoforms and receptors is required. These topics will be covered in the subsequent sections.
VEGF isoforms and VEGF receptors
The VEGF–PDGF supergene family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and PlGF.Citation53 Of these, VEGF-A is the best-characterized member of the VEGF–PDGF family. Molecules in the VEGF–PDGF family are structurally different but share varying degrees of homology with VEGF-A.Citation54 Furthermore, members of the VEGF–PDGF family are distinguished by their affinity for various VEGF receptors ().Citation55 As VEGF-A is most closely implicated in the biology of CNV, it will be the focus of this review.
Table 2 VEGF molecules, isoforms and their receptor targets on the endothelial surface
The VEGF gene is located on chromosome 6 and is encoded by 8 exons and separated by 7 introns.Citation56 Alternative splicing of the VEGF-A gene generates five dominant VEGF-A isoforms (VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A189, and VEGF-A206) that are differentiated by amino acid number, molecular weight, and receptor binding domains.Citation55,Citation57,Citation58 In addition to the five major isoforms, other VEGF variants are also generated by alternative splicing of the VEGF gene, but the role of these variants in the pathophysiology of AMD is unclear. The five major isoforms have differing solubility; VEGF-A121 is highly diffusible while VEGF-A189 and VEGF-A201 are tissue bound with poor solubility.Citation28 VEGF-A165 is postulated to be the major isoform involved in NV in the human eye and has intermediate solubility compared to the other isoforms.
VEGF receptors populate the surface of endothelial cells.Citation58 VEGF can bind to three related receptor tyrosine kinases: VEGFR-1, VEGFR-2, and VEGFR-3. VEGF-A binds to VEGFR-1 and VEGFR-2 but not VEGFR-3. The latter receptor binds only to VEGF-C and VEGF-D.Citation59 The extracellular domain of VEGFR-1 and -2 contain seven immunoglobulin-like domains. These receptors also have a transmembrane domain and a consensus tyrosine kinase sequence that is interrupted by a kinase-insert domain. VEGF-A also interacts with coreceptors heparin sulfate proteoglycans, neuropilin-1, and neuropilin-2, which are also located on the surface of endothelial cells.Citation55 Coreceptors modulate the activity of VEGFR-1 and VEGFR-2.Citation60 For example, neuropilin-2 receptor presents VEGF165 to VEGFR-2 in a manner that enhances the effectiveness of VEGFR-2 signal transduction. VEGF coreceptors do not exert any activity on cell function if they do not interact with VEGFR-1 or -2.Citation61
The process of VEGF receptor signaling is initiated by the binding of covalently linked VEGF dimers to the extracellular receptor domain.Citation55 This results in the dimerization of two receptor monomers with resultant phosphorylation of tyrosine kinases in both the intracellular juxtamembrane domain, the tail of the receptor, and the kinase insert domain. The net effect of these biochemical changes is the recruitment of signaling molecules that activate various cellular pathways involved in angiogenesis.Citation62
There are marked differences in the biological effects of VEGFR-1 and VEGFR-2 activation, and the functions of these two receptors are dichotomously opposed in many ways.Citation58,Citation62 There is good evidence to suggest that VEGFR-2 mediates most of the cellular responses underlying VEGF-driven angiogenesis including mitogenesis, vascular hyperpermeability, and microvascular remodeling. The function of VEGFR-1 is less well understood. However, it is known to behave as a decoy receptor that sequesters VEGF from VEGFR-2.Citation58 By doing so, VEGFR-1 has the capacity to modulate the function of VEGFR-2. The differential function of VEGFR-1 and -2 is further supported by studies that have shown that these receptors are not equally upregulated following insults such as ischemia and hypoxia.Citation63 Investigating the patterns of VEGFR-1 and -2 expression in various biological environments can therefore aid our understanding the role of VEGF isoforms in vascular homeostasis and proliferative vascular disease.
Pharmacology of aflibercept
Aflibercept is a fully humanized recombinant protein that is constructed from portions of the human VEGFR.Citation64 It functions as a soluble decoy receptor that is 115 kDa in size and is made by fusing the Fc region of human IgG1 to the second binding domain of VEGFR-1 and third binding domain of VEGFR-2 ().Citation65 The configuration of aflibercept facilitates binding to VEGF isoforms with greater affinity than the binding of VEGF to VEGFR-1 and VEGFR-2. The dissociation constant [Kd] of aflibercept for VEGF165 is 0.49 pmol/L,Citation66 and the Kd of VEGF165 for VEGFR-1 and VEGFR-2 are 9.33 and 88.8 pmol/L, respectively.Citation67 In addition to blocking VEGF-A isoforms, aflibercept has binding affinities to VEGF-B (Kd =352 pmol/L) and PlGF-2 (Kd =17.5 pmol/L).Citation66
Figure 1 VEGF receptors and the structure of aflibercept.
Abbreviation: VEGF, vascular endothelial growth factor; Ig, immunoglobulin; IgG1, immunoglobulin G1; Fc, fragment crystallizable.
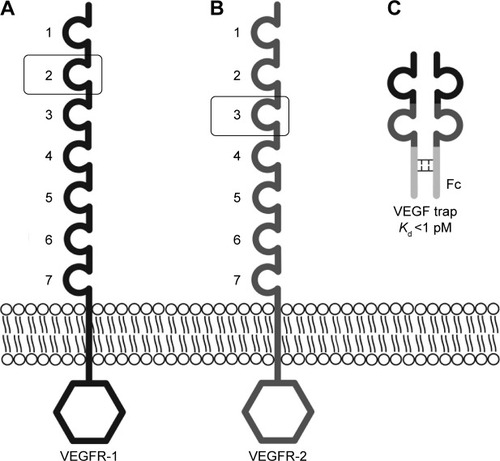
A comparison of the structural and biochemical properties of aflibercept, ranibizumab, bevacizumab, and pegaptanib are provided in . A major functional distinction between aflibercept and other anti-VEGF agents is that it blocks VEGF-B, PlGF1, and PlGF-2 in addition to VEGF-A isoforms. Aflibercept antagonizes a broader spectrum of growth factors, and the potency of aflibercept for blocking VEGF-mediated signaling by VEGF121 and VEGF165 is greater than ranibizumab and bevacizumab by several orders of magnitude.Citation66 The affinity of aflibercept for VEGF-A165 is 94 times greater than ranibizumab and approximately 120 times greater than bevacizumab.Citation66
Table 3 Structural, pharmacodynamic, and pharmacokinetic properties of different anti-VEGF agents used in the clinical management of neovascular AMD
The intravitreal half-life of aflibercept (4.7 days) is greater than ranibizumab, bevacizumab, and pegaptanib.Citation68–Citation71 Mathematical modeling has estimated that the intravitreal VEGF binding capacity of aflibercept (1.15 mg) 79 days after injection is equivalent to the binding capacity of ranibizumab 30 days after injection.Citation68 However, it is important to remember that these values were derived from experimental studies involving nonprimate animals (mainly rabbits) and so the results could be different if the experiments were performed in the human eye. To our knowledge, the half-life of aflibercept in the human eye has not been determined.
Intravitreally administered drugs are cleared by two main mechanisms:Citation72
Anterior elimination pathway via counterdirectional aqueous flow;
Posterior elimination pathway via vitreoretinochoroidal bulk flow due to hydrostatic and osmotic pressure gradients in the posterior segment.
In order to reach the site of NV, intravitreal agents need to penetrate the different layers of the retina to enter the subretinal, sub-RPE, and intraretinal compartments. Fortunately, tight junctions are not a major anatomical feature of most retinal layers,Citation73 and the vast majority of molecules experience an unhindered passage through the retina to reach the desired area of pathology. The major determinants of drug penetration include molecular size, charge, and lipid solubility. Large cationic molecules are most resistant to permeating the retina.Citation74 Full thickness retinal penetration has been demonstrated following intravitreal injection of bevacizumab, the largest of the anti-VEGF molecules in the rabbit eye.Citation75 Similar studies have not been performed with aflibercept, but given the evidence that this agent is highly efficacious for treating type 1 NV (as discussed in the subsequent sections), it is likely that aflibercept achieves high levels of transretinal penetration in human eyes with AMD.
The systemic half-life of aflibercept is approximately 1.5 days and is greater than that of ranibizumab (6 hours) and less than that of bevacizumab (20 days).Citation68 Maximum systemic concentrations of aflibercept are achieved approximately 2–3 days after intravitreal injection, and these maximal concentrations are estimated to be approximately 200-fold less than the concentration required for maximal VEGF binding.Citation76,Citation77 Therefore, it is unlikely that aflibercept will reduce systemic VEGF concentrations to a level that will disrupt key homeostatic mechanisms required for normal cardiovascular function. Additionally, aflibercept has not been detected in the systemic circulation 2 weeks after intravitreal administration.Citation78
Data from oncology trials have shown that systemic clearance of aflibercept occurs by two major pathways.Citation15 The first pathway involves renal clearance following binding of VEGF, and this occurs when serum concentrations of aflibercept are relatively low. The second pathway occurs during states of relatively greater aflibercept concentration and involves pinocytic-mediated mechanisms and elimination by proteolysis.
Alfibercept for the management of neovascular AMD
Preclinical trials
The unique structural, pharmacodynamic, and pharmacokinetic properties of aflibercept make it ideally suited for treating neovascular AMD. One of the earliest studies to demonstrate the clinical efficacy of aflibercept examined its application for the management of laser-induced CNV and subretinal NV in transgenic mice.Citation79 This study showed that suppression of choroidal and subretinal neovascularization could be achieved by subconjunctival and intravitreal administration of this agent. Many years later, the efficacy of aflibercept for treating laser-induced CNV in nonhuman primates was demonstrated by Nork et al.Citation80
Clinical trials
Results of the first randomized, multicenter, placebo-controlled Phase I trial to examine the efficacy of aflibercept for treating CNV due to AMD were reported by Nguyen et alCitation81 for the CLEAR (Clinical Evaluation of Antiangiogenesis in the Retina) Study Group. This trial evaluated the effects of intravenous administration of aflibercept in eyes with neovascular AMD. In this study, comparisons between 19 patients treated with intravenous aflibercept and 6 patients who received placebo showed that those eyes that received treatment with either single or multiple administrations of aflibercept experienced an average reduction of 60% excess retinal thickness. The maximum tolerated intravenous dose of aflibercept in the study group was deemed to be 1.0 mg/kg with the major causes of dose-limiting toxicity being hypertension and proteinuria. The anatomical improvement in retinal thickness following intravenous administration of aflibercept provided the impetus to explore the utility of intravitreal delivery even though a significant change in visual acuity was not noted after intravenous administration.
A subsequent dose escalation study was performed by the same investigators to determine the safety, tolerability, maximum tolerated dose, and bioactivity of intravitreal aflibercept.Citation82 A total of 21 patients were enrolled in this study and received a single dose of either 0.05, 0.15, 0.5, 1, 2, or 4 mg of aflibercept. The primary end point was 6 weeks, although some patients were followed for 12 weeks. The main outcome measures were related to safety, and there were no identifiable cases of intraocular inflammation or serious adverse effects in any of the subjects reported. The side effects of hypertension and proteinuria were also not encountered in study patients who received intravitreal therapy. Ninety-five percent of patients in the study demonstrated stable or improved visual acuity at 6 weeks (mean gain of 4.43 letters) after intravitreal aflibercept regardless of the dose that was administered. The greatest visual gains were identified in those who received a dose of 1.0 mg or greater (mean increase of 13.5 letters when 2 and 4 mg groups were combined).
After the safety profile of intravitreal aflibercept for treating CNV was established, a Phase II clinical trial was undertaken by the CLEAR-IT 2 investigators.Citation83 One hundred fifty-nine patients with subfoveal CNV were randomized into one of five groups. The first two groups received 0.5 or 2 mg of aflibercept every 4 weeks for a total of 12 weeks. The other three groups received a dose of 0.5, 2, or 4 mg of aflibercept at day 1 and 12 weeks (a total of two injections during the study period). The primary endpoint was change from baseline in central retinal thickness and lesion thickness at week 12. Best corrected visual acuity (BCVA) and other numeric changes in visual acuity were secondary outcome measures. At the 12-week endpoint, the groups that received therapy every 4 weeks showed the greatest improvement in visual acuity and reduction in retinal thickness. Interestingly, BCVA at the 8-week visit was not significantly different between the group receiving 2 mg aflibercept every 4 weeks and the group receiving 2 mg of aflibercept every 12 weeks. This finding led the authors to suggest that a dose of 2 mg every 8 weeks may be as effective as dosing every 4 weeks. Furthermore, results at week 52 of the CLEAR-IT 2 study showed that pro re nata (PRN) dosing maintained the significant anatomic and vision improvements established during the 12-week fixed dosing period.Citation83,Citation84
VIEW 1 and VIEW 2 clinical trials
The “VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD” studies (VIEW 1 and VIEW 2) were two Phase III double-masked, multinational, parallel-group, active-controlled clinical trials that evaluated the clinical efficacy of aflibercept in a prospective fashion. The VIEW studies were the largest controlled trials of anti-VEGF agents in neovascular AMD ever performed.Citation78,Citation85 Both trials were similarly designed. Patients in VIEW 1 were randomized at 154 centers in the United States and Canada (n=1,217). Patients in VIEW 2 were randomized at 172 centers in Europe, Middle East, Asia-Pacific, Australia, and Latin America (n=1,240). Only one eye from each patient was included in the study. The pertinent inclusion/exclusion criteria and major design features of the VIEW studies on enrollment are summarized in . Eligibility was determined by neovascular lesion characteristics based on fluorescein angiography.
Table 4 Key features of the study criteria for VIEW 1 and 2 clinical trials
There were 4 arms/treatment regimens evaluated in each of the VIEW studies. One regimen consisted of patients who were administered 0.5 mg ranibizumab injections every 4 weeks (Rq4). Subjects in the other three treatment regimens received 0.5 mg aflibercept every 4 weeks (0.5q4), 2 mg aflibercept every 4 weeks (2q4), or 2 mg aflibercept every 8 weeks (2q8) following 3 injections, each given every 4 weeks. The primary endpoint of the VIEW 1 and 2 studies was non-inferiority of aflibercept regimens to ranibizumab in patients who maintained vision at 52 weeks. This was defined as losing less than 15 letters of visual acuity on the chart used in the Early Treatment Diabetic Retinopathy Study (ETDRS).Citation78 Secondary measures included the change in BCVA, retinal thickness and fluid as judged using time-domain optical coherence tomography (OCT).
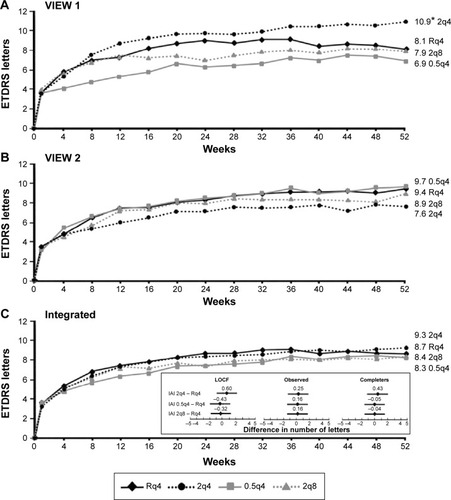
Integrated analysis of data from both VIEW studies at the 52-week visit showed non-inferiority of all three aflibercept treatment regimens compared to the ranibizumab regimen ().Citation78 All treatment regimens demonstrated a rapid increase in BCVA following the first injection, after which there were small and sustained increases in BCVA that persisted until week 52. Interestingly, the integrated data from both VIEW studies revealed that the mean visual acuity of all four treatment regimens was within 1 letter of each other at the 52-week visit. Mean change in BCVA from baseline to week 52 in the individual VIEW studies and in the integrated analysis is provided in . Importantly, the mean visual acuity of the aflibercept group that was dosed every 8 weeks was within 0.3 letters of the ranibizumab group receiving dosing every 4 weeks. Regarding secondary outcome measures, all the aflibercept regimens were comparable to the monthly ranbizumab regimen in terms of reduction in retinal thickness and fluid.
Figure 2 VIEW 1 and VIEW 2 primary endpoint: maintenance of vision at week 52.
Abbreviations: VIEW, VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD; RBZ, ranibizumab; VEGF, vascular endothelial growth factor; Rq4, 0.5 mg ranibizumab every 4 weeks; 2q4, 2 mg IAI every 4 weeks; IAI, intravitreal aflibercept injection; 0.5q4, 0.5 mg IAI every 4 weeks; 2q8, 2 mg IAI every 8 weeks after three initial 4-week doses.
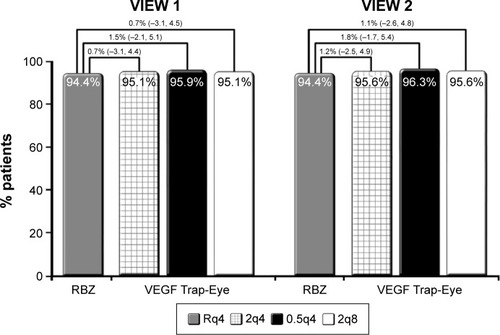
Figure 3 Mean change in BCVA from baseline to week 52 in the (A) VIEW 1 study. (B) VIEW 2 study. (C) Integrated analysis.
Abbreviations: VIEW, VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD; Rq4, 0.5 mg ranibizumab every 4 weeks; 0.5q4, 0.5 mg IAI every 4 weeks; IAI, intravitreal aflibercept injection; 2q4, 2 mg IAI every 4 weeks; 2q8, 2 mg IAI every 8 weeks after three initial 4-week doses; ETDRS, Early Treatment Diabetic Retinopathy Study; BCVA, best-corrected visual acuity; CI, confidence interval; LOCF, last observation carried forward.
From weeks 52 to 96, the dosing schedule was changed to mandatory quarterly dosing with examination-guided interim injections that were also referred to as “capped-PRN dosing”. Specifically, patients continued to be evaluated every 4 weeks. Mandatory treatment with the same dose of drug was administered every 12 weeks with treatment provided sooner if any one of the following criteria were identified during the 4-week review:
New or persistent fluid on OCT
Increase in central retinal thickness by 100 μm or more on OCT
Loss of 5 or more letters on the ETDRS chart with recurrence of fluid on OCT
New onset classic CNV or new or persistent leakage on fluorescein angiography
New macular hemorrhage.
Ninety-one percent of the original cohort of subjects from VIEW 1 and 2 entered the second year of study. Eighty-four percent of the patients who enrolled in the study completed week 96. It was found that the mean increase in BCVA between baseline and the 96-week time point was comparable between the four treatment groups. At 96 weeks, the mean increase in BCVA across all four groups was approximately 7 letters on the ETDRS chart. The number of patients who gained 15 letters from baseline to 96 weeks was also similar across all groups. Post hoc comparisons of the number of injections administered between 52 and 96 weeks showed that the 2q4 (4.1±1.8 injections) and 2q8 (4.2±1.7 injections) groups received significantly fewer injections than the Rq4 (4.7±2.2 injections) group. The lower number of injections administered in the capped-PRN phase of the VIEW studies may explain why there was a 1–2 letter loss in all groups between 52 and 96 weeks. It may also explain why the percentage of patients with no retinal fluid decreased from week 52 to 96 in all treatment groups.
The VIEW studies successfully highlighted the pharmacodynamic advantages of aflibercept in the clinical management of CNV due to AMD. The finding that patients in the 2q8 group achieved visual and anatomic outcomes that were comparable to the Rq4 and 2q4 groups (with a mean of 5 fewer injections over 2 years) suggested that the use of aflibercept for the management of neovascular AMD could potentially be associated with fewer patient clinic visits. The other major finding to arise from VIEW studies was that switching from a fixed dosing to a capped-PRN protocol is likely to result in small losses of visual acuity as well as recurrence of anatomical changes such as fluid on OCT. The latter findings are similar to what was shown in the CATT trial. In the CATT trial, at the 2-year visit, the mean visual gain was significantly greater in patients treated with fixed dosing relative to the as-needed regimen.Citation86
Efficacy of aflibercept in nonresponders
The characteristics of neovascular lesions with respect to cellular composition, expression of VEGF receptors, and, ultimately, response to antiangiogenic therapy are markedly heterogeneous.Citation87 It is known that a distinct subset of neovascular lesions neither respond to anti-VEGF therapy in a predictable manner nor respond in a way that is consistent with the clinical course reported in large-scale trials. In the ophthalmic literature, these eyes are often denoted as “non-responders” or eyes that have “failed therapy”.Citation88
A number of studies have evaluated the rate of nonresponse to bevacizumab and ranibizumab therapy. The published estimates have varied from 10.1% to 45%.Citation89–Citation91 These estimates are, to a great extent, influenced by the definition of nonresponse, a nonstandardized term that varies significantly between different studies. Commonly used measures of nonresponse include decrease in BCVA, worsening of or new exudative findings, and the need for a greater frequency of injections to prevent progression of disease. Persistence or worsening of pigment epithelial detachment (PED), subretinal fluid, macular edema, hemorrhage, and increase/no change in central retinal thickness are other measures of nonresponse. Increase in lesion size and leakage on dye angiography have also been described as failed therapy in some studies.
It is important to note that the terms refractory, treatment-resistant, and nonresponse are frequently used interchangeably in the ophthalmic literature. Recently, the expert panel review by Amoaku et alCitation92 proposed a set of standardized nomenclature to define treatment response to anti-VEGF therapy in neovascular AMD. In that report, “nonresponse” was defined as those eyes that demonstrated a >−5 letter decline in Snellen visual acuity from baseline after the third injection in the initiation schedule of anti-VEGF therapy. It was also recommended that unchanging or increasing central retinal thickness, subretinal fluid, intraretinal fluid, and/or PED compared to the baseline visit should also be defined as nonresponse.Citation92
One proposed reason for treatment nonresponse to anti-VEGF therapy is the time-dependent development of tachyphylaxis. Several biological mechanisms have been linked to the development of tachyphylaxis,Citation93 and these include a compensatory upregulation of VEGF expression by macrophages at the site of CNV in response to repeated anti-VEGF therapy. Chronic changes to the vascular walls of CNV can also result in increased permeability to fluid and exudate and decreased response to anti-VEGF therapy. The development of neutralizing antibodies to therapeutic humanized monoclonal antibodies is also thought to contribute to the development of tachyphylaxis.
Clinical features that are thought to be predictive of non-response to anti-VEGF therapy include angiographic patterns that are consistent with occult NV, type 1 NV, occurrence of a fibrovascular PED, relatively greater area of NV as determined using dye angiography, and poor reading ability at baseline visit.Citation90 Age and number of injections were not significantly associated with treatment failure in the study by Ehlken et al.Citation94 Treatment failure in that study was defined as no improvement or deterioration in visual acuity and retinal morphology, as seen on OCT, including an increase in intraretinal fluid and/or subretinal fluid.
The greater affinity of aflibercept for VEGF isoforms, its ability to antagonize the effects of PlGF and VEGF-B, and its longer intravitreal half-life are expected to confer unique biological advantages relative to other anti-VEGF agents. It is therefore plausible that aflibercept will have greater efficacy in the management of neovascular lesions when other anti-VEGF agents are deemed to have failed. Several studies have examined the role of aflibercept in the management of recurrent or refractory NV, and these are summarized in .Citation95–Citation105 Most of these were retrospective studies that lacked a control group. There was also great variation between studies in the size of the treatment group and the treatment history prior to switching to aflibercept. Other variables that were not standardized between studies included the duration of follow-up and the indications for treatment switch. An exception is the prospective study by Chang et alCitation100 that evaluated the effectiveness of intravitreal aflibercept in patients with treatment-resistant AMD. Treatment resistance in this study was defined as persistent intraretinal or subretinal fluid, as seen on spectral domain-optical coherence tomography, despite at least four injections of anti-VEGF agents in the past 6 months. BCVA was also required to be within the range of 35 and 90 ETDRS chart letters for inclusion in this trial. Forty-nine patients were recruited and followed for a 24-week period after switching to aflibercept. The investigators acknowledged that a limitation of this study was the lack of a control group.
Table 5 Summary of clinical studies that have evaluated the efficacy of aflibercept for treating non-responders to other anti-VEGF therapies
All of the reviewed studies demonstrated significant anatomical improvement following the switch to aflibercept. OCT-based anatomical features that were shown to improve included a reduction in retinal thickness, macular volume, intraretinal fluid, subretinal fluid, or dimensions of the PED. With respect to BCVA, most studies demonstrated stabilization of visual acuity with the use of aflibercept, but only three studiesCitation98,Citation100,Citation104 demonstrated improvement in visual acuity. One of these three studies was a small case series that did not apply statistical techniques to validate the improvement in BCVA.Citation104 The frequent dissociation between anatomic recovery and improvement in BCVA in nonresponders highlights the structure–function disconnect in AMD pathophysiology.
An editorial by SchachatCitation106 discussed the limitations and advantages of many studies that have aimed at evaluating the efficacy of anti-VEGF treatment changes in presumed nonresponders. An important issue that was raised in this editorial is our current inability to predict the eyes that require a relatively longer course of treatment before a therapeutic response is seen. Specifically, SchachatCitation106 suggested that it is possible for presumed nonresponders to improve over the course of time if the same drug was continued. Therefore, a switch may implicate an agent as being efficacious when really it was introduced at a point in a recalcitrant disease course at which improvement was looming. This is an important point that cannot be resolved with current clinical data. Large, randomized, prospective trials with control arms are therefore needed. Although there is broad clinical evidence to suggest that aflibercept has efficacy in the management of NV in nonresponders, these findings need to be validated using clinical trials with robust design and standardized inclusion/exclusion criteria.
Aflibercept in PCV
Polypoidal choroidal vasculopathy (PCV) has, for many years, been considered a variant of AMD. First described by Yannuzzi et al,Citation107 PCV was characterized by serosanguineous neovascular complications that were on the extreme scale of what was ordinarily encountered in AMD. Evaluation of eyes with PCV using indocyanine green angiography also identified polyps which were thought to be another clinical feature that distinguished PCV from AMD.Citation108 The predilection for pigmented patients and those of Asian heritage also implicated a degree of inherited predisposition for PCV.Citation109,Citation110
Our understanding of the pathophysiology of PCV is currently limited. However, there is good evidence to suggest that the biological mechanisms driving angiogenesis and NV in PCV may be different from AMD. Eyes with PCV often do not manifest drusen, an important mediator of NV in AMD. There is also a greater likelihood for eyes with PCV to manifest a unique phenotypic choroidal manifestation, known as pachychoroid, which is structurally and functionally different from the normal human choroid.Citation111–Citation113 The recent shift to utilizing multimodal imaging to categorize degenerative chorioretinal diseases has refined our understanding of the pachychoroid phenotype. This definition of pachychoroid currently includes one or more of the following features:
Absolute increase in choroidal thickness.
Disproportional concentration of large, dilated pachyvessels at sites of pathology.
Diminution of the choriocapillaris layer in the setting of normal choroidal thickness.
Several studies have examined the efficacy of aflibercept for PCV. The study by Hosokawa et alCitation114 showed that 6 months of intravitreal aflibercept achieved total resolution of polypoidal lesions in 77.7% of eyes and total resolution of retinal exudative changes in 94.4% of eyes. A significant decrease in central retinal thickness and improvements in BCVA were also achieved after 6 months of therapy. Similar results were also reported by Ijiri and SugiyamaCitation115 after 3 months of aflibercept monotherapy. Recently, Yamamoto et alCitation116 published the results of a larger retrospective study and provided the outcomes following 1 year of aflibercept therapy. One year of therapy resulted in similar improvements in BCVA and retinal thickness measurements as 3 and 6 months of treatment reported in other studies. However, this report demonstrated complete resolution of polypoidal lesions in only 55.4% of eyes. Furthermore, only 13.4% of eyes showed a decrease in the size of branching vascular networks after 1 year of treatment. Two different reports evaluated the efficacy of aflibercept in PCV subjects that did not respond to ranibizumab therapy.Citation117,Citation118 Both studies demonstrated significant improvements in BCVA and retinal thickness when treatment was switched to aflibercept.
As discussed, neovascular lesions in AMD and PCV may respond differently to anti-VEGF therapy because of important distinctions in the pathogenic mechanisms mediating the two diseases. The recent publication by Koizumi et alCitation119 demonstrated that aflibercept therapy for PCV improved BCVA and induced a significant decrease in subfoveal choroidal thickness. Although their study was not designed to evaluate the relationship between BCVA and choroidal thickness, their findings, by extension, suggest that aflibercept may improve BCVA in PCV by addressing the spectrum of pachychoroid abnormalities. There is increasing evidence to suggest that PCV is best managed using a combination of photodynamic therapy (PDT) and intravitreal anti-VEGF.Citation120 These findings further exemplify the biological differences between PCV and AMD; the latter infrequently requiring PDT for management.
Safety and tolerability of aflibercept
Intravitreal injection, of any therapeutic agent, is associated with risk of ocular adverse events that range from mild, self-limiting disease to serious complications that result in irrecoverable vision loss. The adverse events associated with intravitreal ranibizumab and bevacizumab have been provided in reports of large-scale clinical trials,Citation13 and the most comprehensive documentation of adverse events related to intravitreal aflibercept use is provided in the VIEW studies.Citation78,Citation85,Citation121 Integrated results from over 2,000 subjects at the 96-week visit documented the frequency of several adverse events including conjunctival hemorrhage (range: 23.7%–29.9%), retinal hemorrhage (range: 13.6%–16.2%), reduced visual acuity (range: 11.3%–13.0%), eye pain (range: 8.9%–12.1%), vitreous detachment (range: 7.7%–10.0%), and increased intraocular pressure (IOP; range: 6.2%–10.8%).Citation85 Only five cases of endophthalmitis were recorded from all aflibercept regimens from both VIEW studies. The same number was identified in the ranibizumab arm of both studies.
A number of reports have demonstrated an association between repeat intravitreal bevacizumab/ranibizumab injection and IOP elevation.Citation122,Citation123 Recently, Freund et alCitation124 evaluated data from 2,457 patients from the VIEW studies to evaluate the relationship between IOP changes and intravitreal aflibercept use. Several metrics were used to study IOP changes including the following: 1) prevalence of IOP >21 mmHg through week 96 and 2) prevalence of IOP change from baseline of ≥10 mmHg through week 96. Their analysis revealed that at the week-96 visit the incidence of patients with an IOP change ≥10 mmHg in the 2q4, 2q8, and 0.5q4 groups was 2.9%, 3.1%, and 3.8%, respectively. At the same visit, the incidence of patients with an IOP >21 mmHg in the 2q4, 2q8, and 0.5q4 groups was 14.2%, 12.1%, and 12.5%, respectively. Interestingly, their analysis demonstrated that the incidence of elevated IOP in all aflibercept regimens was significantly lower than the ranibizumab regimen. The reason for the different degree of IOP changes observed in ranibizumab versus aflibercept treated eyes was unknown, but it was speculated that glycosylation of aflibercept may improve solubility in the vitreous cavity and reduce protein accumulation in the trabecular meshwork. Intravitreal aflbercept may therefore be preferable for managing neovascular AMD in patients with IOP concerns such as subjects with glaucoma, ocular hypertension, or a family history of glaucoma.
Systemic adverse effects following intravitreal anti-VEGF therapy are uncommon. There has been some speculation about the association between anti-VEGF therapy and the occurrence of stroke, myocardial infarction, and bleeding. However, the retrospective cohort study of 146,942 Medicare beneficiaries did not demonstrate a significant risk of these complications or mortality between subjects receiving ranibizumab/bevacizumab and those receiving pegaptanib/PDT.Citation125 Data from the VIEW studies demonstrated that the incidence of Antiplatelet Trialists’ Collaboration-defined arterial thromboembolic events was 3.3% for pooled data from all aflibercept regimens at the 96-week visit and 3.2% for the ranibizumab treatment arm.Citation85 The incidence of these complications was not significantly different between treatment groups. The percentage of deaths in the Rq4, 2q4, 0.5q4, and 2q8 groups was 2.7%, 2.1%, 3.2%, and 3.3%, respectively. The incidence of death was also not different between treatment groups. Collectively, these results suggest that intravitreal administration has an acceptable safety profile and is well-tolerated by patients.
In recent times, there has been great concern about the increased risk of thromboembolic events in patients receiving intravitreal anti-VEGF therapy. There is now good evidence to show that the risk of these complications does not differ between the various anti-VEGF agents.Citation126 There is also good evidence to show that the risk of arterial thromboembolic events in patients receiving long-term anti-VEGF therapy is not different to the elderly population not receiving anti-VEGF therapy. Long-term results of PIER, IVAN, CATT, MARINA, ANCHOR, HORIZON, SECURE, and VIEW studies reported a rate of arterial thrombotic events between 3% and 5.6%.Citation126 In comparison, the crude incidence of myocardial infarction, an arterial thrombotic event, was determined to be 5.2 per 1,000 person-years following the evaluation of 26,185 subjects not receiving anti-VEGF therapy in the Tronso study.Citation127
Patient-focused perspectives
Assessment of health-related quality of life outcome measures provides practical information about the effects of visual disability on a patient with eye disease. Such information is typically used to quantify the magnitude of visual loss as a result of disease. The 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25) is a self-reported measure that has been utilized in anti-VEGF clinical trials to assess changes in vision-related function.Citation128 It is a standardized, reliable, and reproducible measure of vision-related function that can be used to infer knowledge about a patient’s capacity to satisfactorily complete activities of daily living.
Yuzawa et alCitation129 recently published the analysis of NEI VFQ-25 questionnaires from VIEW studies up to and including the 52-week visit. Vision-related quality of life improvement was identified in six subscales including mental health, general vision, near activities, role difficulties, distance activities, and dependency. Wijeyakumar et alCitation130 reported similar findings in a smaller cohort of subjects receiving aflibercept for neovascular AMD. In their study, they showed that patients with greater visual gain experienced a greater improvement in vision-related quality of life.
These findings have major implications as they demonstrate that aflibercept therapy improves an individual’s capacity to function. An improvement in distance activities is likely to indicate decreased risk of falls due to improved mobility. An improvement in mental health may suggest a lower risk of depression. Yuzawa et alCitation129 concluded that these benefits are likely to translate to less need for informal care giving, professional in-home help, and medical care transportation. It may also result in improved productivity in paid work or volunteer activities.
Analysis of VIEW trial data showed that there were minimal differences in subscale data between the group receiving aflibercept every 8 weeks and the group receiving ranibizumab every 4 weeks. In the VIEW trials, clinic visits were scheduled for assessment of visual acuity, OCT, and other clinical measures every 4 weeks for all groups even if therapy was not administered. By extension, this suggests that patients receiving aflibercept in routine clinical settings, where clinic visits could be scheduled every 8 weeks, would experience greater improvement in vision-related quality of life than what is provided from the VIEW trials.
Conclusion
There is strong evidence to demonstrate that intravitreal aflibercept is an efficacious therapeutic agent for managing treatment-naïve NV and recalcitrant NV due to AMD. Studies have shown that aflibercept is also effective in treating NV due to PCV.Citation117,Citation118 The unique pharmacodynamic and pharmacokinetic properties of aflibercept confer several biologic advantages in the management of CNV. A major advantage of aflibercept, as demonstrated in several trials, is the requirement for less frequent dosing to achieve comparable visual and anatomical improvements as other agents. This is expected to translate to fewer clinic visits and potential cost-savings to the patient and the health-care system.
Integrated data from landmark clinical trials have shown that frequent intravitreal administration of aflibercept has an acceptable safety profile and is well-tolerated by patients. Visual gains as a result of aflibercept use are also associated with improvements in key measures of quality of life. For these reasons, aflibercept appears to be a suitable first-line therapy for neovascular AMD.
A number of new clinical trials including the Perseus-IT trial (A prospective Non-Interventional Study to Assess the Effectiveness of Aflibercept [Eyelea®]) in Routine Clinical Practice in Patients With Wet Age-related Macular Degeneration), the DRAW study (A Pharmacokinetic Study of Intravitreal Aflibercept in Vitrectomized and Non-vitrectomized Eyes with Wet Age-related Macular Degeneration), the RIVAL trial (A comparison of Ranibizumab and Aflibercept for the Development of Geographic Atrophy in [Wet] AMD Patients), and the SHIFT-2 trial (Intravitreal Aflibercept in Wet Age Related Macular Degeneration Patients With an Incomplete Response to Monthly Ranibizumab Injections) are designed to provide new, evidence-based information about the pharmacology and efficacy of aflibercept in various, atypical AMD settings. Results from these studies are expected to refine our knowledge about the role of aflibercept in AMD management.
Acknowledgments
LuEsther T. Mertz Retinal Research Center, Manhattan Eye, Ear and Throat Hospital/North Shore Long Island Jewish Hospital, New York, NY, USA, and The Macula Foundation, Inc., New York, NY, USA. The funding organizations had no role in the design or conduct of this research.
Disclosure
KB Freund is a consultant to Genentech, ThromboGenics, Ohr Pharmaceutical, Optos, Optovue, and Heidelberg Engineering (honorarium for each). The authors report no other conflicts of interest in this work.
References
- FriedmanDSO’ColmainBJMunozBEye Diseases Prevalence Research GroupPrevalence of age-related macular degeneration in the United StatesArch Ophthalmol2004122456457215078675
- van LeeuwenRKlaverCCVingerlingJRHofmanAde JongPTThe risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam studyArch Ophthalmol2003121451952612695249
- WongWLSuXLiXGlobal prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysisLancet Glob Health201422e106e11625104651
- BarbazettoIASarojNShapiroHWongPHoACFreundKBIncidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trialsAm J Ophthalmol20101496939946.e93120378094
- ClemonsTEMiltonRCKleinRSeddonJMFerrisFL3rdAge-Related Eye Disease Study Research GroupRisk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19Ophthalmology2005112453353915808240
- VirgiliGBiniALaser photocoagulation for neovascular age-related macular degenerationCochrane Database Syst Rev20073CD00476317636773
- Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study GroupPhotodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP reportArch Ophthalmol1999117101329134510532441
- EandiCMGiansantiFVirgiliGMacular translocation for neovascular age-related macular degenerationCochrane Database Syst Rev20084CD00692818843739
- SolomonSDLindsleyKVedulaSSKrzystolikMGHawkinsBSAnti-vascular endothelial growth factor for neovascular age-related macular degenerationCochrane Database Syst Rev20148CD00513925170575
- SingermanLJMasonsonHPatelMPegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trialBr J Ophthalmol200892121606161118614570
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- BrownDMKaiserPKMichelsMRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- GroupCRMartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- ChakravarthyUHardingSPRogersCAIVAN Study InvestigatorsAlternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trialLancet201338299001258126723870813
- DixonJAOliverSCOlsonJLMandavaNVEGF trap-eye for the treatment of neovascular age-related macular degenerationExpert Opin Investig Drugs2009181015731580
- SemeraroFMorescalchiFDuseSParmeggianiFGambicortiECostagliolaCAflibercept in wet AMD: specific role and optimal useDrug Des Devel Ther20137711722
- BazanNGHomeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor LectureInvest Ophthalmol Vis Sci2007481148664881 biography 4864–486517962433
- Saint-GeniezMKuriharaTSekiyamaEMaldonadoAED’AmorePAAn essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillarisProc Natl Acad Sci U S A200910644187511875619841260
- BaiYMaJXGuoJMuller cell-derived VEGF is a significant contributor to retinal neovascularizationJ Pathol2009219444645419768732
- AblonczyZDahroujMMarnerosAGProgressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levelsFASEB J20142852369237924558195
- YangXMYafaiYWiedemannPHypoxia-induced upregulation of pigment epithelium-derived factor by retinal glial (Muller) cellsJ Neurosci Res201290125726621922517
- ShirinifardAGlazierJASwatMAdhesion failures determine the pattern of choroidal neovascularization in the eye: a computer simulation studyPLoS Comput Biol201285e100244022570603
- CampochiaroPASolowayPRyanSJMillerJWThe pathogenesis of choroidal neovascularization in patients with age-related macular degenerationMol Vis199953410562658
- FunkMKarlDGeorgopoulosMNeovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumabOphthalmology2009116122393239919815292
- MuetherPSNeuhannIBuhlCHermannMMKirchhofBFauserSIntraocular growth factors and cytokines in patients with dry and neovascular age-related macular degenerationRetina20133391809181423492946
- GirmensJFSahelJAMarazovaKDry age-related macular degeneration: a currently unmet clinical needIntractable Rare Dis Res20121310311425343081
- SingerMAdvances in the management of macular degenerationF1000Prime Rep201462924860651
- GrisantiSTatarOThe role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degenerationProg Retin Eye Res200827437239018621565
- JoNMailhosCJuMInhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularizationAm J Pathol200616862036205316723717
- FerrariGCookBDTerushkinVPintucciGMignattiPTransforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosisJ Cell Physiol2009219244945819180561
- OgataNMatsushimaMTakadaYExpression of basic fibroblast growth factor mRNA in developing choroidal neovascularizationCurr Eye Res19961510100810188921239
- SkeieJMZengSFaidleyEAMullinsRFAngiogenin in age-related macular degenerationMol Vis20111757658221364907
- RakicJMLambertVDevyLPlacental growth factor, a member of the VEGF family, contributes to the development of choroidal neo-vascularizationInvest Ophthalmol Vis Sci20034473186319312824270
- FrankRNAminRHEliottDPuklinJEAbramsGWBasic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranesAm J Ophthalmol199612233934038794712
- TatarOShinodaKAdamAExpression of endostatin in human choroidal neovascular membranes secondary to age-related macular degenerationExp Eye Res200683232933816584730
- WangSSorensonCMSheibaniNLack of thrombospondin 1 and exacerbation of choroidal neovascularizationArch Ophthalmol2012130561562022232368
- MoriKDuhEGehlbachPPigment epithelium-derived factor inhibits retinal and choroidal neovascularizationJ Cell Physiol2001188225326311424092
- GundaVSudhakarYARegulation of tumor angiogenesis and choroidal neovascularization by endogenous angioinhibitorsJ Cancer Sci Ther201351241742625258675
- DasAMcGuirePGRetinal and choroidal angiogenesis: pathophysiology and strategies for inhibitionProg Retin Eye Res200322672174814575722
- WeisSMChereshDATumor angiogenesis: molecular pathways and therapeutic targetsNat Med201117111359137022064426
- YannuzziLANegraoSIidaTRetinal angiomatous proliferation in age-related macular degeneration. 2001Retina201232Suppl 141643422451953
- YannuzziLAFreundKBTakahashiBSReview of retinal angiomatous proliferation or type 3 neovascularizationRetina200828337538418327130
- JungJJChenCYMrejenSThe incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degenerationAm J Ophthalmol20141584769779.e76225034111
- GassJDStereoscopic Atlas of Macular Diseases4th edSt Louis, MOMosby1997
- FreundKBZweifelSAEngelbertMDo we need a new classification for choroidal neovascularization in age-related macular degeneration?Retina20103091333134920924258
- VinoresSAYoussriAILunaJDUpregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal diseaseHistol Histopathol1997121991099046048
- Pe’erJFolbergRItinAGnessinHHemoIKeshetEVascular endothelial growth factor upregulation in human central retinal vein occlusionOphthalmology199810534124169499769
- WeissKSteinbruggerIWegerMIntravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumabEye (Lond)20092391812181819169227
- SkoldMKvon GerttenCSandberg-NordqvistACMathiesenTHolminSVEGF and VEGF receptor expression after experimental brain contusion in ratJ Neurotrauma200522335336715785231
- NozakiMRaislerBJSakuraiEDrusen complement components C3a and C5a promote choroidal neovascularizationProc Natl Acad Sci U S A200610372328233316452172
- SpaideRFHo-SpaideWCBrowneRWArmstrongDCharacterization of peroxidized lipids in Bruch’s membraneRetina199919214114710213241
- McLeodDSGrebeRBhuttoIMergesCBabaTLuttyGARelationship between RPE and choriocapillaris in age-related macular degenerationInvest Ophthalmol Vis Sci200950104982499119357355
- HolmesDIZacharyIThe vascular endothelial growth factor (VEGF) family: angiogenic factors in health and diseaseGenome Biol20056220915693956
- IyerSAcharyaKRTying the knot: the cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokinesFEBS J2011278224304432221917115
- NeufeldGCohenTGengrinovitchSPoltorakZVascular endothelial growth factor (VEGF) and its receptorsFASEB J19991319229872925
- WeiMHPopescuNCLermanMIMerrillMJZimonjicDBLocalization of the human vascular endothelial growth factor gene, VEGF, at chromosome 6p12Hum Genet19969767947978641698
- TischerEMitchellRHartmanTThe human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicingJ Biol Chem19912661811947119541711045
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- KarkkainenMJMakinenTAlitaloKLymphatic endothelium: a new frontier of metastasis researchNat Cell Biol200241E2E511780131
- OlssonAKDimbergAKreugerJClaesson-WelshLVEGF receptor signalling – in control of vascular functionNat Rev Mol Cell Biol20067535937116633338
- DjordjevicSDriscollPCTargeting VEGF signalling via the neuropilin co-receptorDrug Discov Today2013189–1044745523228652
- Cebe-SuarezSZehnder-FjallmanABallmer-HoferKThe role of VEGF receptors in angiogenesis; complex partnershipsCell Mol Life Sci200663560161516465447
- UlyattCWalkerJPonnambalamSHypoxia differentially regulates VEGFR1 and VEGFR2 levels and alters intracellular signaling and cell migration in endothelial cellsBiochem Biophys Res Commun2011404377477921168388
- Aflibercept: AVE 0005, AVE 005, AVE0005, VEGF Trap – Regeneron, VEGF Trap (R1R2), VEGF Trap-EyeDrugs R D20089426126918588357
- EconomidesANCarpenterLRRudgeJSCytokine traps: multi-component, high-affinity blockers of cytokine actionNat Med200391475212483208
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- HolashJDavisSPapadopoulosNVEGF-trap: a VEGF blocker with potent antitumor effectsProc Natl Acad Sci U S A20029917113931139812177445
- StewartMWWhat are the half-lives of ranibizumab and aflibercept (VEGF trap-eye) in human eyes? Calculations with a mathematical modelEye Rep20111e5
- ChristoforidisJBCarltonMMKnoppMVHinkleGHPET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit modelInvest Ophthalmol Vis Sci20115285899590321685343
- BakriSJSnyderMRReidJMPulidoJSEzzatMKSinghRJPharmacokinetics of intravitreal ranibizumab (Lucentis)Ophthalmology2007114122179218218054637
- BakriSJSnyderMRReidJMPulidoJSSinghRJPharmacokinetics of intravitreal bevacizumab (Avastin)Ophthalmology2007114585585917467524
- EdelhauserHFRowe-RendlemanCLRobinsonMROphthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applicationsInvest Ophthalmol Vis Sci201051115403542020980702
- SmelserGKIshikawaTPeiYFElectron Microscopic Studies of Intra-retinal Spaces: Diffusion of Particulate MaterialsIIStuggart, GermanySchattauer-Verlag1965
- TornquistPAlmABillAPermeability of ocular vessels and transport across the blood-retinal-barrierEye (Lond)19904Pt 23033092199237
- GaudreaultJFeiDBeyerJCPharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbitsRetina20072791260126618046235
- AveryRLCastellarinAASteinleNCSystemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMDBr J Ophthalmol201498121636164125001321
- EYLEA™ (aflibercept) injection [US prescribing information]Tarrytown, NYRegeneron Pharmaceuticals, Inc2012
- HeierJSBrownDMChongVIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- SaishinYSaishinYTakahashiKVEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrierJ Cell Physiol2003195224124812652651
- NorkTMDubielzigRRChristianBJPrevention of experimental choroidal neovascularization and resolution of active lesions by VEGF trap in nonhuman primatesArch Ophthalmol201112981042105221825187
- NguyenQDShahSMHafizGCLEAR-AMD 1 Study GroupA phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degenerationOphthalmology200611391522.e11522.e1416876249
- NguyenQDShahSMBrowningDJA phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degenerationOphthalmology20091161121412148.e214119700196
- HeierJSBoyerDNguyenQDCLEAR-IT 2 InvestigatorsThe 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosingOphthalmology201111861098110621640258
- BrownDMHeierJSCiullaTCLEAR-IT 2 InvestigatorsPrimary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degenerationOphthalmology201111861089109721640257
- Schmidt-ErfurthUKaiserPKKorobelnikJFIntravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studiesOphthalmology2014121119320124084500
- Comparison of Age-related Macular Degeneration Treatments Trials Research Group; MartinDFMaguireMGFineSLRanibi-zumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- ParkUCShinJYMcCarthyLCPharmacogenetic associations with long-term response to anti-vascular endothelial growth factor treatment in neovascular AMD patientsMol Vis2014201680169425558172
- KrebsIGlittenbergCAnsari-ShahrezaeiSHagenSSteinerIBinderSNon-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degenerationBr J Ophthalmol201397111443144623966368
- LuxALlacerHHeussenFMJoussenAMNon-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesionsBr J Ophthalmol200791101318132217537784
- SuzukiMNagaiNIzumi-NagaiKPredictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degenerationBr J Ophthalmol20149891186119124711658
- OtsujiTNagaiYShoKInitial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD)Clin Ophthalmol201371487149023901256
- AmoakuWMChakravarthyUGaleRDefining response to anti-VEGF therapies in neovascular AMDEye (Lond)201529101397139826446737
- ForooghianFCukrasCMeyerleCBChewEYWongWTTachyphylaxis after intravitreal bevacizumab for exudative age-related macular degenerationRetina200929672373119516114
- EhlkenCJungmannSBohringerDAgostiniHTJunkerBPielenASwitch of anti-VEGF agents is an option for nonresponders in the treatment of AMDEye (Lond)201428553854524722504
- YonekawaYAndreoliCMillerJBConversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degenerationAm J Ophthalmol201315612935.e2223668679
- BakallBFolkJCBoldtHCAflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumabAm J Ophthalmol201315611522.e1123706500
- HoVYYehSOlsenTWShort-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitorsAm J Ophthalmol201315612328.e2223664153
- KumarNMarsigliaMMrejenSVisual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degenerationRetina20133381605161223549101
- ChoHWeberMLShahCPHeierJSInitial utilization of aflibercept in exudative age-related macular degenerationEur J Ophthalmol201424457658124706352
- ChangAALiHBroadheadGKIntravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationOphthalmology2014121118819224144450
- GharbiyaMIannettiLParisiFDe VicoUMungoMLMarencoMVisual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationBiomed Res Int2014201427375424895562
- GriffinDRRichmondPPOlsonJCIntravitreal aflibercept outcomes in patients with persistent macular exudate previously treated with bevacizumab and/or ranibizumab for neovascular age-related macular degenerationJ Ophthalmol2014201449717825505976
- BroadheadGKHongTZhuMResponse of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degenerationRetina201535597598125627086
- PatelKHChowCCRathodRRapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumabEye (Lond)2013275663667 quiz 66823558214
- GrewalDSGillMKSarezkyDLyonATMirzaRGVisual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month resultsEye (Lond)201428789589924833178
- SchachatAPSwitching anti-vascular endothelial growth factor therapy for neovascular age-related macular degenerationAm J Ophthalmol2013156112.e123791369
- YannuzziLASorensonJSpaideRFLipsonBIdiopathic polypoidal choroidal vasculopathy (IPCV). 1990Retina201232Suppl 118
- SpaideRFYannuzziLASlakterJSSorensonJOrlachDAIndocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathyRetina19951521001107542796
- WenFChenCWuDLiHPolypoidal choroidal vasculopathy in elderly Chinese patientsGraefes Arch Clin Exp Ophthalmol2004242862562915257461
- CiardellaAPDonsoffIMHuangSJCostaDLYannuzziLAPolypoidal choroidal vasculopathySurv Ophthalmol2004491253714711438
- DansinganiKNaysanJBalaratnasingamCFreundKEn Face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomographyRetina Epub8122015
- BalaratnasingamCLeeWKKoizumiHDansinganiKKInoueMFreundKBPolypoidal choroidal vasculopathy: a distinct disease or manifestation of many?Retina Epub9232015
- Gallego-PinazoRDolz-MarcoRGomez-UllaFMrejenSFreundKBPachychoroid diseases of the maculaMed Hypothesis Discov Innov Ophthalmol20143411111525756060
- HosokawaMShiragaFYamashitaASix-month results of intravitreal aflibercept injections for patients with polypoidal choroidal vasculopathyBr J Ophthalmol20159981087109125712826
- IjiriSSugiyamaKShort-term efficacy of intravitreal aflibercept for patients with treatment-naive polypoidal choroidal vasculopathyGraefes Arch Clin Exp Ophthalmol2015253335135725023147
- YamamotoAOkadaAAKanoMOne-year results of intravitreal aflibercept for polypoidal choroidal vasculopathyOphthalmology Epub9162015
- YamashitaMNishiTHasegawaTOgataNResponse of serous retinal pigment epithelial detachments to intravitreal aflibercept in polypoidal choroidal vasculopathy refractory to ranibizumabClin Ophthalmol2014834334624591809
- MiuraMIwasakiTGotoHIntravitreal aflibercept for polypoidal choroidal vasculopathy after developing ranibizumab tachyphylaxisClin Ophthalmol201371591159523966764
- KoizumiHKanoMYamamotoAShort-term changes in chor-oidal thickness after aflibercept therapy for neovascular age-related macular degenerationAm J Ophthalmol2015159462763325555799
- RuamviboonsukPTadaratiMVanichvaranontSHanutsahaPPokawattanaNPhotodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary studyBr J Ophthalmol20109481045105120530656
- OguraYTerasakiHGomiFVIEW 2 InvestigatorsEfficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 studyBr J Ophthalmol2015991929725107900
- HoangQVMendoncaLSDella TorreKEJungJJTsuangAJFreundKBEffect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injectionsOphthalmology2012119232132622054994
- HoangQVTsuangAJGelmanRClinical predictors of sustained intraocular pressure elevation due to intravitreal anti-vascular endothelial growth factor therapyRetina201333117918722990314
- FreundKBHoangQVSarojNThompsonDIntraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumabOphthalmology201512291802181026025097
- CurtisLHHammillBGSchulmanKACousinsSWRisks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degenerationArch Ophthalmol2010128101273127920937996
- SemeraroFMorescalchiFDuseSGambicortiERomanoMRCostagliolaCSystemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an overviewExpert Opin Drug Saf201413678580224809388
- BraekkanSKHaldEMMathiesenEBCompeting risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: the Tromso studyArterioscler Thromb Vasc Biol201232248749122075253
- MangioneCMLeePPGutierrezPRSpritzerKBerrySHays RD; National Eye Institute Visual Function Questionnaire Field TestIDevelopment of the 25-item National Eye Institute Visual Function QuestionnaireArch Ophthalmol200111971050105811448327
- YuzawaMFujitaKWittrup-JensenKUImprovement in vision-related function with intravitreal aflibercept: data from phase 3 studies in wet age-related macular degenerationOphthalmology2015122357157825439429
- WijeyakumarWHongTBroadheadTLiHZhuMChangAAChanges in quality of life among patients treated with aflibercept for neovascular age-related macular degeneration (nAMD)Poster presented at: ARVO 2014 Annual Meeting AbstractsMay 6, 2014Orlando, FL
- VEGF Trap-Eye (aflibercept ophthalmic solution) Briefing Document Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM259143.pdfAccessed November 17, 2015
