Abstract
The main objective of the study was to quantify serum levels of nicotinamide phosphoribosyltransferase (Nampt/pre-B-Cell colony-enhancing factor 1/visfatin) in subjects with a history of retinal vascular occlusions (RVOs), disease conditions characterized by pronounced ischemia, and metabolic energy deficits. A case–control study of 18 subjects with a history of RVO as well as six healthy volunteers is presented. Serum Nampt levels were quantified using a commercially available enzyme-linked immunosorbent assay kit. Serum Nampt levels were 79% lower in patients with a history of RVO compared with that in healthy volunteers (P<0.05). There was no statistically significant difference among the types of RVOs, specifically branch retinal vein occlusions (n=7), central retinal vein occlusions (n=5), hemiretinal vein occlusions (n=3), and central retinal artery occlusions (n=3; P=0.69). Further studies are needed to establish the temporal kinetics of Nampt expression and to determine whether Nampt may represent a novel biomarker to identify at-risk populations, or whether it is a druggable target with the potential to ameliorate the long-term complications associated with the condition, ie, macular edema, macular ischemia, neovascularization, and permanent loss of vision.
Introduction
Retinal vascular occlusions (RVOs) can present as retinal vein occlusions or retinal artery occlusions and are common causes of irreversible vision loss.Citation1 These occlusions can include either the central retinal vein or artery (CRVO or CRAO) or a branch vessel (BRVO or BRAO).Citation1 Of these, retinal vein occlusions are the most common and also are a common cause of retinal vascular disease, second only to diabetic retinopathy.Citation2 RVOs are prevalent in about 0.5% of the population, with an increased prevalence with increasing age, and some variance in terms of race and ethnicity.Citation3 About half of these cases occur in patients older than 65 years of age, and more than half of these cases are found in patients with cardiovascular diseases such as hypertension, diabetes mellitus, and dyslipidemia. Compromised retinal venous outflow due to intraocular pressure, such as seen in open-angle glaucoma, can produce stasis, predisposing to RVOs.Citation4
Vision loss in RVOs is most commonly caused by macular edema.Citation5 Obstruction within blood vessels leads to serious exudation and, subsequently, macular edema. Permanent edema can lead to degeneration and changes such as subsequent macular edema and epiretinal membranes, ultimately resulting in vision loss.Citation2 Similarly, retinal ischemia increases the release of vascular endothelial growth factors (VEGFs), causing retinal neovascularization, which may in turn lead to retinal hemorrhage or glaucoma secondary to neovascularization.Citation6,Citation7
Various treatment options have been explored; however, outcomes remain unsatisfactory. The current “gold standard” of treatment for BRVO is laser photocoagulation, which has been shown to reduce the risk of vision loss and to improve vision in up to two-thirds of patients with macular edema due to BRVO.Citation8 Various newer treatment options include dexamethasone implants and anti-VEGF therapy, which help decrease central macular thickness, thereby promoting a clinically significant increase in visual acuity.Citation9
However, currently there are no clinically or diagnostically relevant biomarkers that help predict an individual’s risk for developing an RVO or even for disease and therapy outcome. In a first step to identifying novel predictive and diagnostic biomarkers and potential drug targets, we herein quantified the serum levels of Nampt in subjects with RVO and in healthy volunteers.
Nampt as nicotinamide phosphoribosyltransferase functions as an enzyme of critical importance for cellular energy metabolism. In addition, Nampt has been characterized as a proinflammatory cytokine that also possesses neuroprotective properties.Citation10 Besides these physiological functions, Nampt has been correlated with a number of pathological states, including acute lung injury, rheumatoid arthritis, Leber congenital amaurosis,Citation11 and possibly diabetes mellitus (for review, see Sun et alCitation10).
Nampt is the rate-limiting enzyme that catalyzes the first step in the biosynthesis of nicotinamide adenine dinucleotide from nicotinamide (for review, see ImaiCitation12). Given the fact that the retina is one of the tissues with the highest energy demand in the body, with most energy derived from oxidative metabolism,Citation13,Citation14 Nampt is a critical metabolic enzyme required for an adequate energy supply. Inhibiting Nampt activity results in detrimental consequences on retinal energy metabolism and in retinal toxicity,Citation11 analogously to mutations in nuclear nicotinamide mononucleotide adenyltransferase (Nampt) in Leber congenital amaurosis.Citation11,Citation15,Citation16
Our results indicate significantly lower circulating Nampt levels in patients with fully resolved RVO compared with the same in healthy volunteers. Our data provide a strong rationale for performing future studies investigating the temporal kinetic profile of Nampt expression, from the acute phase of the RVO through the treatment and resolution phase, to determine the feasibility of using low serum Nampt levels as a novel biomarker for RVOs.
Methods
Subjects
The clinical research presented herein adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board. Subjects were identified in the Eye Clinic at Truman Medical Centers (Kansas City, MO, USA) based on the diagnosis of RVO. We enrolled 18 subjects with a previous diagnosis of RVO and six healthy, age-matched volunteers into our study. The patient demographics are shown in , and clinical parameters are presented in . All patients had fully resolved RVO; average time between RVO event and biomarker assessment was 6±2 months. None of the patients had a history of ophthalmological problems before the RVO.
Table 1 Patient demographics (18 eyes of 18 patients)
Table 2 Clinical parameters of subjects with RVOs
Sample processing
For quantification of serum Nampt levels, 13 mL of blood was collected by venipuncture in a BD Vacutainer® venous blood collection tube with clot activator and gel for serum separation (BD, Franklin Lakes, NJ, USA). Serum was prepared according to the manufacturer’s recommendations of inversion and centrifugation. Serum was aliquoted and stored at −80°C until use in experiments.
Enzyme-linked immunosorbent assay
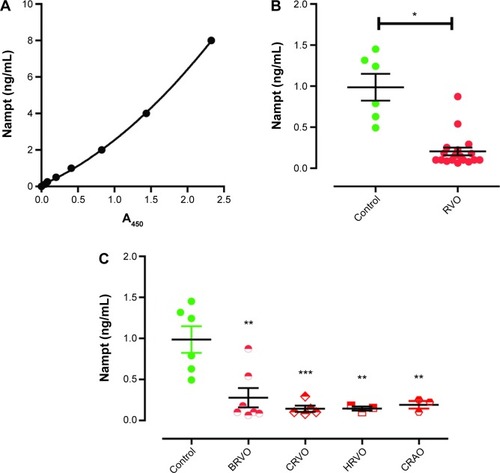
Serum Nampt levels were quantified from subjects’ sera using a commercially available and previously validated assay (Adipogen, San Diego, CA, USA),Citation17 according to the manufacturer’s instructions and using a Synergy H1 plate reader (Biotek, Winooski, VT, USA). Serum samples were tested in triplicates, and the results were validated by quantification using three separate enzyme-linked immunosorbent assay (ELISA) plates. The standard curve was established using a second-order polynomial (quadratic) fit in Prism software v5 (GraphPad Inc, La Jolla, CA, USA), according to the manufacturer’s recommendations, which yielded a goodness of fit of RCitation2=0.9996 ().
Figure 1 Serum Nampt levels are lower in subjects with RVOs.
Abbreviations: A450, absorbance at 450 nm; BRVO, branch retinal vein occlusion; CRAO, central retinal artery occlusion; CRVO, central retinal vein occlusion; ELISA, enzyme-linked immunosorbent assay; HRVO, hemiretinal vein occlusion; Nampt, nicotinamide phosphoribosyltransferase; RVO, retinal vascular occlusion; SEM, standard error of the mean.
Extraction of genomic DNA and single-nucleotide polymorphism analysis
Genomic DNA was extracted from the blood clots remaining after serum separation, essentially as described previously,Citation18 in combination with a commercial DNA extraction kit (QIAamp DNA Blood Maxi Kit, Qiagen Inc, Germantown, MD, USA).
Purity and concentration of genomic DNA were assessed using absorbance at 260, 280, and 320 nm using a NanoVue™ spectrophotometer (GE Healthcare, Little Chalfont, Buckinghamshire, UK). We used molecular beacon technology for the custom designing of single-nucleotide polymorphism (SNP) genotyping probes (sequences are listed in ; Sigma-Aldrich Inc, St Louis, MO, USA) to determine the presence of T1001G and C1535T polymorphisms in the PBEF1 gene. Molecular beacons were labeled with either 6-carboxyfluorescein (6FAM) or hexachloro-6-carboxyfluorescein (HEX) at the 5′-end and with the quencher Black Hole Quencher 1 (BHQ1®) at the 3′-end (). SNP genotyping was performed using Taqman Universal Mastermix and a StepOne Plus real-time polymerase chain reaction machine (both Applied Biosystems; Life Technologies Inc, Foster City, CA, USA), using 54.5°C as the annealing temperature. Analysis was performed using the SDS software v4 (Applied Biosystems). The probes were validated using a restriction digest approach, as described in Ye et alCitation19 (data not shown). The polymorphisms T1001G and C1535T (originally described as C1543TCitation19) had been identified as predisposing individuals to acute lung injury associated with sepsis.Citation19
Table 3 SNP determination
Statistics
Data are presented as individual data points on scatter plots; error bars represent the standard error of the mean. Nampt levels were compared between healthy volunteers and subjects with a history of RVO using the Student’s t-test. To compare subtypes of RVOs, we performed one-way analysis of variance (ANOVA) with Tukey’s post hoc test. For correlation analysis, we calculated a Pearson product–moment correlation coefficient (r) to test the strength of the association. All statistical analysis was done in Prism software v5 (GraphPad Inc).
Results
Serum Nampt levels are reduced in RVO
Nampt levels in the sera of control subjects were 0.97±0.16 ng/mL (n=6; , ), in accordance with the published literature.Citation17 In contrast, subjects with a history of RVO had statistically significant reduced Nampt levels (0.20±0.05 ng/mL, n=18, unpaired t-test: P<0.05; , ).
We tested whether different types of RVOs were associated with different Nampt levels. However, in our cohort, we did not identify any statistically significant differences in serum Nampt levels among patients manifesting with hemiretinal vein occlusion (HRVO), BRVO, CRVO, or CRAO (n=19, P=0.69; ). However, each type of RVO was significantly different from controls (ANOVA P<0.001; HRVO: n=3, P<0.01; BRVO: n=7, P<0.001; CRVO: n=6, P<0.01; CRAO: n=2, P<0.01; ).
When we compared the Nampt levels between the sexes, we did not find any differences between females and males (P=0.11), in accordance with the published literature.Citation20
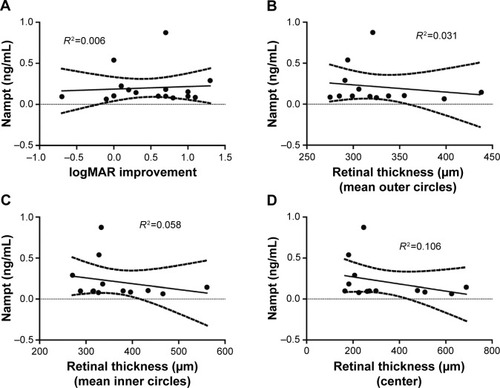
Furthermore, we correlated the serum Nampt levels with the improvement of visual acuity after the RVO event (), as well as the clinical measurements of retinal thickness derived from spectral-domain optical coherence tomography data () and did not identify any statistically significant associations. There was no statistically significant association between the time since the RVO event and serum Nampt levels (data not shown).
Figure 2 Serum Nampt levels do not correlate with visual acuity or SD-OCT findings.
Abbreviations: logMAR, logarithm of the minimum angle of resolution; Nampt, nicotinamide phosphoribosyltransferase; SD-OCT, spectral-domain optical coherence tomography.
Known confounding factors affecting serum Nampt levels are absent in RVO patients
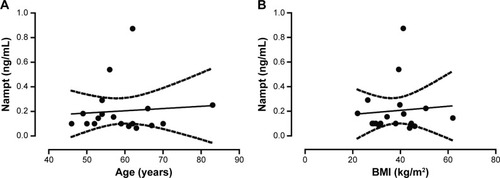
There are several disease conditions and biometric parameters, including stroke, hepatitis C, and hyperthyroidism, reported in the scientific and medical literature, which show potential correlations with Nampt levels. We, therefore, systematically assessed them within our patient population as potential confounding factors. To determine whether Nampt levels were correlated with age or weight, we calculated a Pearson product–moment correlation coefficient for age and body mass index (BMI). In our cohort, serum PBEF levels did not correlate with age (P=0.75, RCitation2=0.006; ) or BMI (P=0.75, RCitation2=0.007; ). Similarly, we did not detect any differences among ethnicities (data not shown). For four conditions, namely acute lung injury,Citation19 chronic kidney disease,Citation21 and rheumatoid arthritisCitation22 as well as during chronic hemodialysis,Citation20 serum Nampt levels are being discussed as a reflection of a patient’s inflammation status rather than as a specifically disease-related change. None of the patients in our study suffered from these or related inflammatory disease conditions.
Figure 3 Serum Nampt levels do not correlate with age or BMI.
Abbreviations: BMI, body mass index; Nampt, nicotinamide phosphoribosyltransferase.
Additional studies have linked SNPs in the PBEF1 gene with a predisposition for worse disease and therapy outcomes in acute lung injury and sepsis.Citation19,Citation23–Citation25 Regarding the disease conditions and biometric parameters affecting serum Nampt levels, we tested the patients’ DNA samples for two known polymorphisms.Citation19 We did not detect the presence of the T1001G or the C1535T polymorphisms in any of our patients ().
Discussion
We herein describe low serum Nampt levels in subjects with a history of RVO. To our knowledge, this is the first report of Nampt levels being associated with this disease condition and it represents the identification of the first potential biomarker for RVOs.
The level of serum Nampt in healthy volunteers was in good agreement with the concentrations previously reported.Citation17 Currently, there is significant discrepancy among the different methodologies used to detect Nampt, as described in detail by Korner et al.Citation17 Therefore, we chose a commercially available ELISA assay that has previously been shown to detect Nampt accurately and reproducibly from human serum samples with respect to both epitope specificity and the ability to quantify Nampt over a wide range of concentrations.Citation17 Experimental parameters that we took into consideration were specificity of the antibody, the effect of diluted vs undiluted serum samples, and the fit of the standard curve. Specifically, we validated the Nampt standard using Western blotting and the validated antibody used by Ye et alCitation19 (data not shown). Furthermore, using an in-house-generated recombinant human Nampt protein in the commercial ELISA yielded comparable serum concentrations that were not statistically significantly different from those reported herein using the provided protein standard (data not shown). Diluting the serum sample did not have any effect on quantitative analysis, further indicating the specificity of the antibody. For quantification, we used a second-order polynomial fit, as recommended by the manufacturer. In addition, we ensured that data points fell on the linear portion of the standard curve. Performing linear regression analysis yielded comparable serum Nampt concentrations (data not shown). Overall, our data provide evidence that the ELISA used in the present study resulted in not only high reproducibility but also high specificity for accurately detecting Nampt levels in human serum samples.
To exclude known confounding factors affecting serum Nampt levels from our study’s RVO patient population, we assessed the patients’ medical history, current health status, biometric measures, and genetically predisposing factors associated with the PBEF1 gene. Our experiments did not reveal any differences in Nampt levels between females and males, in accordance with the published literature.Citation20 Furthermore, the role of Nampt in obesity remains unclear. Several studies suggest a direct correlation and role of the protein in obesity development, whereas other studies could not identify any correlation (Stastny et alCitation26 and references therein). The discrepancy among these studies may result from the chosen method for detecting visfatin,Citation17 as well as differences in clinical trial design and statistical power.
Previous studies have identified increases in Nampt serum levels to be associated with acute lung injury,Citation19 chronic kidney disease,Citation21 and rheumatoid arthritis,Citation22 as well as during chronic hemodialysis,Citation20 and it has been suggested that the serum Nampt level in these conditions may reflect the inflammation status rather than a disease-related change.Citation20 In Leber congenital amaurosis, mutations in the nicotinamide adenine dinucleotide (NAD) biosynthesis pathway result in effects reminiscent of decreased Nampt activity.Citation11 Furthermore, SNPs in the PBEF1 gene have been identified as predisposing individuals to worse outcomes after acute lung injury and sepsis.Citation19,Citation23–Citation25 We herein ruled out the two clinically significant polymorphisms, T1001G and C1535T,Citation19 as contributing and/or confounding factors in our patient population.
In the present study, we identified a lower serum Nampt level in subjects with resolved RVOs compared with those in healthy volunteers and healthy control subjects reported in the literature.Citation20
Through its enzymatic activity, Nampt has been shown to exert potent protective effects both in vitro, in cellular models using neurons and cardiac myocytes,Citation27,Citation28 and in vivo, in a middle cerebral artery occlusion model for cerebral ischemia–reperfusion injury and stroke.Citation27–Citation29 Experimental downregulation of Nampt resulted in lower levels of autophagy in animals with cerebral ischemia–reperfusion injury and subsequent poorer outcomes had no effect, however, in the sham condition.Citation29 It is thus perceivable that circulating levels of Nampt correlate with its cellular concentration and thereby its Nampt enzymatic activity level in cells. Similarly, in vitro studies showed that Nampt protected neurons from energy deprivation in neurons using an oxygen–glucose deprivation model.Citation28 Accordingly, small interfering RNA-mediated knockdown of the PBEF gene resulted in increased levels of apoptosis of cardiac monocytes in vitro following glucose deprivation.Citation27
Thus, it can be speculated that subjects with lower systemic levels of Nampt would experience lower levels of cellular Nampt enzyme activity, resulting in reduced endogenous protection from ischemic events that could predispose to and increase the risk for RVOs. Alternatively, the RVO event may prevent normal release of Nampt into the blood stream. Given the different pathophysiology states of retinal vein vs retinal artery occlusions,Citation1 it is notable that patients with both types of occlusion had significantly lower serum Nampt levels that healthy volunteers.
Our results provide an important basis for future studies aimed at identifying whether serum Nampt levels represent a novel risk factor, and hence a potential diagnostic biomarker, for RVOs. Such studies will require a temporal kinetic analysis of serum Nampt levels. Furthermore, our data provide a rationale for devising neuroprotective pharmaceutical strategies targeting an increase in or induction of higher Nampt levels to increase endogenous protection of cells in general, and neurons in particular, in subjects with low circulating Nampt levels, and potentially other at-risk populations, to prevent subsequent deleterious effects on the retina and other metabolically active organs. Such strategies will probably need to consider multiple components of the NAD pathway, given the challenges faced by previous gene therapy approaches targeting NMNAT expression in Leber congenital amaurosis.Citation30
Acknowledgments
The authors thank Dr Mahendra Rupani for contributing his clinical expertise to this project. The authors thank Margaret, Richard, and Sara Koulen for generous support and encouragement.
Funding
Research reported in this publication was supported by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), and the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, a Challenge Grant from Research to Prevent Blindness, and the Vision Research Foundation of Kansas City is gratefully acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
- MirshahiAFeltgenNHansenLLHattenbachLORetinal vascular occlusions: an interdisciplinary challengeDtsch Arztebl Int20081052647447919626196
- KeanePASaddaSRRetinal vein occlusion and macular edema – critical evaluation of the clinical value of ranibizumabClin Ophthalmol2011577178121750610
- SartoriMTBarbarSDonàARisk factors, antithrombotic treatment and outcome in retinal vein occlusion: an age-related prospective cohort studyEur J Haematol201390542643323461717
- YauJWLeePWongTYBestJJenkinsARetinal vein occlusion: an approach to diagnosis, systemic risk factors and managementIntern Med J2008381290491019120547
- SaddaSDanisRPPappuruRRVascular changes in eyes treated with dexamethasone intravitreal implant for macular edema after retinal vein occlusionOphthalmology201312071423143123499064
- RogersSLMcIntoshRLLimLNatural history of branch retinal vein occlusion: an evidence-based systematic reviewOphthalmology201011761094.e1101.e20430447
- HeierJSCampochiaroPAYauLRanibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trialOphthalmology2012119480280922301066
- MitryDBunceCCharterisDAnti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusionCochrane Database Syst Rev20131CD00951023440840
- MerkoudisNGranstamETreatment of macular edema associated with retinal vein occlusion using sustained-release dexamethasone implants in a clinical settingEur J Ophthalmol201323455856323483492
- SunZLeiHZhangZPre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functionsCytokine Growth Factor Rev201325443344223787158
- ZabkaTSSinghJDhawanPRetinal toxicity, in vivo and in vitro, associated with inhibition of nicotinamide phosphoribosyltransferaseToxicol Sci2015144116317225505128
- ImaiSNicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseasesCurr Pharm Des2009151202819149599
- NivenJELaughlinSBEnergy limitation as a selective pressure on the evolution of sensory systemsJ Exp Biol2008211pt 111792180418490395
- Wong-RileyMTEnergy metabolism of the visual systemEye Brain201029911623226947
- FalkMJZhangQNakamaru-OgisoENMNAT1 mutations cause Leber congenital amaurosisNat Genet20124491040104522842227
- PerraultIHaneinSZanlonghiXMutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophyNat Genet201244997597722842229
- KornerAGartenABluherMTauscherRKratzschJKiessWMolecular characteristics of serum visfatin and differential detection by immunoassaysJ Clin Endocrinol Metab200792124783479117878256
- Se Fum WongSKueiJJPrasadNA simple method for DNA isolation from clotted blood extricated rapidly from serum separator tubesClin Chem200753352252417234731
- YeSQSimonBAMaloneyJPPre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injuryAm J Respir Crit Care Med2005171436137015579727
- KatoAOdamakiMIshidaJHishidaARelationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patientsAm J Nephrol2009291313518663287
- AxelssonJWitaspACarreroJJCirculating levels of visfatin/pre-B-cell colony-enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKDAm J Kidney Dis200749223724417261426
- SenoltLKryštůfkováOHulejováHThe level of serum visfatin (PBEF) is associated with total number of B cells in patients with rheumatoid arthritis and decreases following B cell depletion therapyCytokine201155111612121524918
- BajwaEKYuCLGongMNThompsonBTChristianiDCPre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndromeCrit Care Med20073551290129517414088
- LiuYShaoYYuBSunLLvFAssociation of PBEF gene polymorphisms with acute lung injury, sepsis, and pneumonia in a northeastern Chinese populationClin Chem Lab Med201250111917192223093105
- LeeKHuhJWLimCMKohYHongSBClinical role of serum pre-B cell colony-enhancing factor in ventilated patients with sepsis and acute respiratory distress syndromeScand J Infect Dis2013451076076523746338
- StastnyJBienertova-VaskuJVaskuAVisfatin and its role in obesity developmentDiabetes Metab Syndr20126212012423153983
- HsuCPOkaSShaoDHariharanNSadoshimaJNicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytesCirc Res2009105548149119661458
- BiJLiHYeSQDingSPre-B-cell colony-enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic modelsJ Neurochem2012120233434622044451
- WangPGuanYFDuHZhaiQWSuDFMiaoCYInduction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemiaAutophagy201281778722113203
- CideciyanAVJacobsonSGBeltranWAHuman retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvementProc Natl Acad Sci U S A20131106E517E52523341635
- HolladayJTProper method for calculating average visual acuityJ Refract Surg19971343883919268940
