Abstract
Background
Patients with various retinal diseases and patients who have undergone retinal procedures and surgeries have an increased risk of developing ocular hypertension and glaucoma. Little is known about the epidemiology of comorbid retinal diseases in glaucoma patients. This study evaluated the prevalence of retinal comorbidities in a population of patients with five types of glaucoma.
Methods
A longitudinal, retrospective study was conducted using International Classification of Disease (ICD-9) billing records from 2003 to 2010 at an academic medical center. Patients were classified as having primary open-angle glaucoma (POAG), low tension open-angle glaucoma (NTG), pigmentary open-angle glaucoma, chronic-angle closure glaucoma (CACG), or pseudoexfoliation glaucoma (PXG) if they had at least three clinic visits with the same ICD-9 code. Patients were classified as having a retinal comorbidity if they had two visits with the same code. Variables were analyzed with the independent t-test, χ2 test, analysis of variance, or Fisher’s exact test.
Results
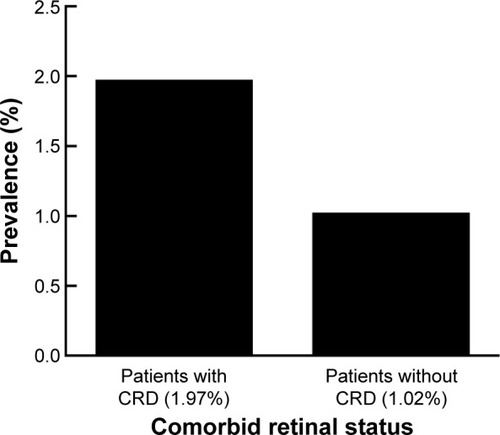
A total of 5,154 patients had glaucoma, and 14.8% of these had a retinal comorbidity. The prevalence of comorbid retinal disease was higher in patients with POAG (15.7%) than in those with NTG (10.7%), PXG (10.1%), or pigmentary open-angle glaucoma (3.7%; P<0.05). Two hundred and two patients had diabetic retinopathy, with POAG patients (4.5%) having a higher prevalence than those with CACG (1.4%) or PXG (0.6%; P<0.001). There were 297 patients who had macular degeneration and both POAG (2.0%) and PXG patients (2.9%) had a higher prevalence of nonexudative macular degeneration than those with CACG (0%; P<0.01). Patients with comorbid retinal disease had a higher prevalence of blindness and low vision than those without comorbid retinal disease (1.97% versus 1.02%, P=0.02).
Conclusion
The high prevalence of comorbid retinal disease and the nearly twofold increase in blindness and low vision in this population demonstrate the need for ophthalmologists to determine if patients have multiple etiologies for their vision loss. The higher prevalence of certain retinal diseases in POAG patients may reflect common pathophysiological processes that warrant further investigation.
Introduction
Glaucoma and various retinal diseases are leading causes of vision loss worldwide. Numerous retinal diseases are associated with and lead to various types of glaucoma. Ischemic conditions such as central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO), central or branch retinal artery occlusion, malignancy, and proliferative diabetic retinopathy are associated with neovascular glaucoma.Citation1 Various types of uveitis,Citation2–Citation5 including Behçet’s disease,Citation6 sarcoidosis,Citation4 syphilis,Citation4 Fuchs’ iridocyclitis,Citation4 and juvenile rheumatoid arthritisCitation4 are causes of secondary glaucoma. Retinitis pigmentosa is associated with primary open-angle and primary-angle closure glaucoma.Citation7 Patients with retinal detachments have higher rates of both ocular hypertension and glaucoma than people in the general public.Citation8
Patients undergoing retinal procedures and retinal surgeries are at increased risk for elevated intraocular pressure (IOP). Intravitreal steroid injections, especially intravitreal triamcinolone acetonide, are associated with elevations in IOP in approximately 50% of patients, which can require treatment with topical medications and, occasionally, glaucoma surgery.Citation9,Citation10 Panretinal photocoagulation is associated with elevated IOPCitation11 and acute-angle closure glaucoma.Citation12,Citation13 Studies on intravitreal anti-vascular endothelial growth factor injections show an association with sustained IOP elevationCitation14 requiring topical medications and, occasionally, glaucoma surgery.Citation15 Vitreoretinal surgeriesCitation16 including both simple vitrectomyCitation17 (eg, vitrectomy without gas, scleral buckle, or silicon oil) and complex vitrectomy,Citation17,Citation18 are associated with IOP elevation during the first 24 hours after the operation. In addition, vitrectomy with silicon oilCitation19 and vitrectomy without silicon oilCitation20,Citation21 are both associated with the development of glaucoma.
Studies have documented the risk of elevated IOP and glaucoma in patients with certain retinal diseases and in certain postoperative or post-procedure retina patients. Little is known about the prevalence of comorbid retinal disease in patients with glaucoma. The prevalence of comorbid retinal diseases may help clinicians consider whether a patient’s vision loss is due to glaucoma or whether it is multifactorial with other disease processes involved. This study examines the prevalence of various types of retinal disease in a group of patients with five different types of glaucoma at a single academic medical center.
Materials and methods
A longitudinal, retrospective study was conducted at a single academic medical center using International Classification of Disease (ICD-9) and Current Procedure Terminology billing records from June 1, 2003 to November 30, 2010. All patients were aged 40 years or older at the time of their last clinical examination and were classified using ICD-9 billing records as having primary open-angle glaucoma (POAG [365.11]), low tension open-angle glaucoma (NTG [365.12]), pigmentary open-angle glaucoma (365.13), chronic-angle closure glaucoma (CACG [365.23]), or pseudoexfoliation glaucoma (PXG [365.52]). This study did not include patients who were glaucoma suspects (365.0–365.04) in order to focus on patients with known glaucomatous damage. Patients with neovascular glaucoma (365.63) were excluded because there are well-established associations between neovascular glaucoma and diabetic retinopathy, CRVO, various ocular malignancies, and ocular ischemic syndrome.Citation22 Patients were similarly classified as having a retinal comorbidity based on ICD-9 codes for retinal detachments and defects (361.0–361.9), other retinal disorders (362.0–362.9), or chorioretinal inflammation, scars, and other disorders of the choroid (363.0–363.9). The Human Subject Research Office of University of Miami Institutional Review Boards approved this study. The study was conducted in accordance with the Declaration of Helsinki.
Patients were included in the study if they had had at least three clinical examinations with the same glaucoma diagnosis ICD-9 code, and had received follow-up care in ophthalmology clinics over at least 2 years. Each patient was categorized as having one type of glaucoma to ensure that each glaucoma subset was composed of unique patients. Patients were excluded if they met the criteria for more than one glaucoma diagnosis (at least three clinical examinations over 2 years for each glaucoma diagnosis).
Patients were categorized as having a retinal comorbidity if they had at least two visits on different dates with the same retinal ICD-9 code. We did not differentiate the type of visit (clinical visit, ophthalmology emergency room visit, surgery visit, or procedure visit). Patients were categorized as having blindness and low vision (ICD-9 369) if they had two visits with ICD-9 code 369. Unlike with the comorbid retina population, we did not require patients to have two visits associated with the same specific (two decimal place) ICD-9 code for blindness and low vision.
Some vitreoretinal ICD-9 codes were excluded because a preliminary analysis revealed that there were no patients with any of the following diagnoses: vitreous abscess (360.04), pan uveitis (360.12), or papilledema (377.00–377.04). Four patients with optic neuritis (377.3), eleven patients with optic atrophy (377.1), and eleven patients with other disorders of the optic nerve (377.4) were similarly excluded.
Statistical analysis was performed using JMP version 10 (SAS Institute Inc, Cary, NC, USA). Categorical variables were analyzed with the χ2 test or Fisher’s exact test. Continuous variables were analyzed with the independent t-test, analysis of variance (ANOVA), or least significant difference post hoc t-test.
Results
A total of 5,154 patients met the criteria for having glaucoma, with the majority of these patients having POAG (n=4,171, 80.9%) and 2,142 (41.6%) seen for at least 5 years. Seven hundred and sixty of these glaucoma patients had a retinal diagnosis, and 35 of these had multiple categories of retinal diagnoses (eg, both a diagnosis within retinal detachments and also within other retinal disorders). Overall, the prevalence of comorbid retinal disease in our glaucoma patient population was 14.8% (760/5,154). The prevalence of retinal detachment was 0.99% (ICD-9 361.xx, 51/5,154), the prevalence of other retinal disorder was 13.3% (ICD-9 362.xx, 684/5,154), the prevalence of other disorders of the choroid was 0.33% (ICD-9 363.xx, 17/5,154), and the prevalence of disorders of the vitreous body was 0.83% (ICD-9 379.2x, 43/5,154).
Characteristics of glaucoma patients with and without retinal comorbidities are listed in . Overall, there were more females (2,906, 56.3%) than males in this study (2,248, 43.7%). There was no difference in rates of comorbid retinal disease between males and females (P=0.29, χ2 test). Glaucoma patients with a comorbid retinal disease were older than glaucoma patients without a retinal disease (mean age 75.4 years versus 73.1 years, P<0.0001, independent t-test). Of patients with retinal comorbidities, PXG patients were older than those with POAG, NTG, or CACG (mean age 83.1 years versus 73.7–75.2 years, P<0.05, ANOVA and least significant difference post hoc t-tests). There was no difference in mean age between patients with PXG and those with pigmentary open-angle glaucoma (83.1 years versus 73.8 years, P>0.05, Fisher’s exact test). There were no differences in mean age between POAG, NTG, pigmentary open-angle glaucoma, and CACG patients with regard to retinal comorbidities (P>0.05, ANOVA).
Table 1 Age and sex of patients with and without comorbid retinal disease
We next asked what types of glaucoma patients are most likely to have comorbid retinal disease. and list the proportions of patients with comorbid retinal disease by glaucoma type. There was a larger proportion of POAG patients with comorbid retinal disease (15.7%) than NTG patients (10.7%, P=0.014, χ2 test), PXG patients (10.1%, P=0.008, χ2 test), and pigmentary open-angle glaucoma patients (3.7%, P=0.013, Fisher’s exact test). There were no other differences in the proportions of comorbid retinal disease between type of glaucoma patients (P>0.05, χ2 test and Fisher’s exact test).
Figure 1 Prevalence of comorbid retinal disease by glaucoma type. POAG patients (15.7%) had a higher prevalence of comorbid retinal disease than NTG (10.7%), PXG (10.1%), and PG (3.7%) patients.
Abbreviations: POAG, primary open-angle glaucoma; NTG, low tension open-angle glaucoma; PG, pigmentary open-angle glaucoma; CACG, chronic angle-closure glaucoma; PXG, pseudoexfoliation glaucoma.
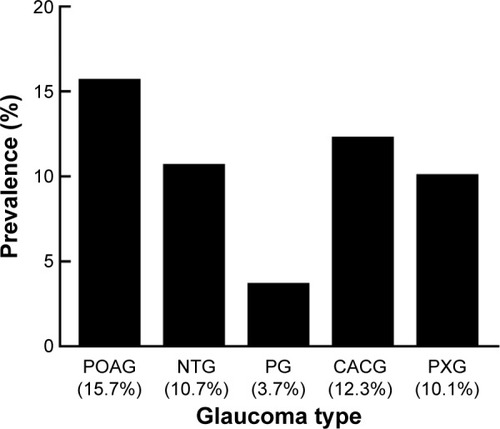
Table 2 Comorbid retina prevalence by glaucoma type
We next examined what types of retinal disease are most common in glaucoma patients. – list the numbers of patients with each type of diagnosis. The most common diagnoses included cystoid macular degeneration (125), nonexudative senile macular degeneration (94), unspecified senile macular degeneration (89), background diabetic retinopathy (82), and proliferative diabetic retinopathy (78), as shown in .
Figure 2 Most common comorbid retinal diagnoses. Of patients with glaucoma, the five most common retinal diagnoses were cystoid MD, nonexudative MD, unspecified MD, background DR, and proliferative DR.
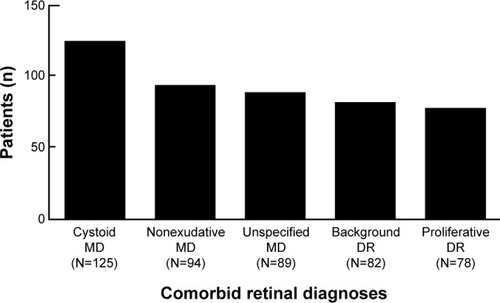
Table 3 Patients with ICD-9 361 diagnoses: retinal detachments and defects (n=51)
Table 4 Patients with ICD-9 362 diagnoses: other retinal disorders (n=684)
Table 5 Patients with ICD-9 363 diagnoses: chorioretinal inflammation, scars and other disorders of the choroid (n=171)
Table 6 Patients with ICD-9 379.2 diagnoses: disorders of the vitreous body (n=43)
We then asked whether there is a relationship between glaucoma subtype and diabetic retinopathy, age-related macular degeneration (AMD), and retinal vascular occlusions, which are three of the most common retinal comorbid diagnoses. Two hundred and thirty-one glaucoma patients had diabetes mellitus with ophthalmic manifestations (ICD-9 250.5–250.53, 362.01–362.07), of which 202 had diabetes-related retinal disease (ICD-9 362.01–362.07). The prevalence of diabetes-related retinal disease among glaucoma patients in this study was 3.92% (202/5,154). Fifty-six patients had visits for just proliferative diabetic retinopathy and 139 patients had visits for nonproliferative or background diabetic retinopathy. Twenty-two had visits for both of these categories. POAG patients had a higher prevalence of comorbid diabetic retinopathy (4.5%) than CACG patients (1.4%, P=0.013, Fisher’s exact test) or PXG patients (0.6%, P<0.001, Fisher’s exact test). There was no difference in mean age between POAG, CACG, and PXG patients with diabetic retinopathy (P>0.05, ANOVA).
One hundred and twenty-eight glaucoma patients were diagnosed with a retinal vascular occlusion, with four patients having a central or branch artery occlusion, 124 patients having a CRVO or BRVO, and two patients having both an arterial and venous occlusion. There was no difference in the prevalence of retinal vascular occlusions according to type of glaucoma (0%–2.9%, P>0.05, Fisher’s exact test). Similarly, there was no difference in prevalence of retinal vein occlusion according to type of glaucoma (0%–2.9%, P>0.05, Fisher’s exact test).
A total of 297 glaucoma patients were diagnosed with macular degeneration (exudative, nonexudative, unspecified, or cystoid macular degeneration), with 84 patients having just nonexudative AMD, 37 patients having just exudative AMD, and ten patients having both exudative and nonexudative AMD. The prevalence of just nonexudative AMD was 1.6% (84/5,154) and the prevalence of exudative AMD was 0.91% (47/5,154). Patients with both exudative and nonexudative AMD were included in the prevalence calculations for exudative AMD because having both diagnoses may represent progression from nonexudative AMD to exudative AMD. POAG patients and PXG patients had a higher prevalence of nonexudative AMD than CACG patients (2.0% versus 0%, P=0.009 and 2.9% versus 0%, P=0.004, Fisher’s exact test). There was no difference in mean age between POAG and PXG patients with nonexudative AMD (84.9 versus 86.3, P>0.05, t-test). There were no differences in the prevalence of exudative AMD according to type of glaucoma (0%–1.5%, P>0.05, Fisher’s exact test).
Lastly, we examined blindness and low vision to determine if glaucoma patients with comorbid retinal disease have a higher prevalence of severe vision loss. Glaucoma patients with comorbid retinal disease had a higher prevalence of severe vision loss than those without retinal disease (1.97% versus 1.02%, P=0.02, χ2 test, ).
Discussion
The purpose of this study was to estimate the prevalence of retinal disease in a population of long-term glaucoma patients at a single academic medical center in the USA and to determine if there are associations between specific types of glaucoma and the most common comorbid retinal diseases. This was a billing-based study and not a chart review, so clinical and laboratory data such as IOP, refraction, body mass index, and glycated hemoglobin were not available. The strengths of the current study include the large number of glaucoma patients who all had long-term follow-up. We required a minimum of 2 years of follow-up to be included in the study, and 41.6% of patients were seen for at least 5 years. We utilized a dataset from a single, tertiary care medical center in order to capture patients who visited the same center for both retinal and glaucoma care although prevalence rates may reflect referral bias. Weaknesses of the current study include the retrospective design, reliance on ICD-9 codes without a chart review to confirm patient diagnoses, lack of a control group, and inclusion of patients with only five types of glaucoma. Patients who were seen for glaucoma at this institution and saw an outside doctor for any retinal disease were not captured in our analysis. Patients with CACG and narrow-angle glaucoma may be at higher risk for complications from dilation, and fundus examinations in these patients may be suboptimal. It is possible that the risk of dilation may have decreased the diagnosis of comorbid retinal disease in this glaucoma population. Further studies are needed to evaluate national prevalence rates among subtypes of glaucoma patients and to evaluate associations between POAG and specific retinal diseases compared with age-matched and sex-matched controls. Detailed chart reviews can be utilized to confirm the results of billing database studies and can also include clinical data such as IOP.
We selected POAG, NTG, CACG, pigmentary open-angle glaucoma, and PXG because these were the adult glaucoma patients most commonly seen at our academic medical center. We excluded neovascular glaucoma because of the well-established associations with proliferative diabetic retinopathy and ocular ischemic syndrome.Citation22 We identified patients who had at least three clinical examinations over the course of 2 years with the same glaucoma diagnosis. We chose these inclusion criteria in order to maximize the accuracy of glaucoma diagnosis, to identify a glaucoma population with multiple years of follow-up care, and to minimize the inclusion of glaucoma patients who were seen at this academic medical center for just a second opinion. Retina patients were required to have the same diagnosis associated with two visits of any type (clinic, procedure, or emergency room). The requirement for at least two visits with the same retinal diagnosis may be expected to reduce false positive (inclusion) diagnostic inaccuracy arising from single coding errors or from evaluation visits found to be negative for a referral question. In addition, glaucoma diagnoses tend to be more consistent over time because patients with retinal disease may have disease progression and therefore have a change in diagnosis (eg, from nonproliferative diabetic retinopathy to proliferative diabetic retinopathy). We selected less stringent criteria for blindness and low vision (two visits with any ICD-9 code 369) because this diagnosis is relatively rare, and we were concerned in this study with whether a patient develops severe vision loss and not the specific level of severe vision loss (eg, total, near-total, profound).
Previous studies have determined that patients with specific retinal diseases, retinal surgeries, and retinal procedures have an increased incidence of ocular hypertension and glaucoma; however, there are no publications to our knowledge that estimate the prevalence of retinal comorbidities in a population of glaucoma patients. We found significant rates of comorbid retinal disease (14.8%) in this glaucoma population. Glaucoma patients with comorbid disease have a higher mean age than those without comorbid retinal disease. The higher mean age may be because increasing age is a risk factor for many retinal diseases. There was no association between sex and rates of comorbid retinal disease.
There are no previous studies on the rates of comorbid retinal disease in patients with glaucoma. Epidemiology studies, however, can provide information on rates of certain diseases in the general population. These studies are drawn from different populations and have different designs, so comparisons are limited. Rates of diabetic retinopathy in this study are consistent with epidemiologic studies in the ophthalmology literature. The results indicating that 3.4% of glaucoma patients have diabetic retinopathy and 3.9% of patients have either diabetic retinopathy or diabetes with ophthalmic manifestations are consistent with previous studies which estimated that the prevalence of diabetic retinopathy is 3.4% among Americans over 40 years of age.Citation23 Thus, using historical controls derived from epidemiological studies, the prevalence of diabetic retinopathy appears to be no different in glaucoma patients than in community-based screening samples.
In contrast, rates of vascular occlusions in this study are higher than other prevalence estimates. We combined BRVO and CRVO for a combined prevalence of 2.4%. Globally, the prevalence is estimated to be 3.77 per 1,000 for BRVO and 0.65 per 1,000 for CRVO.Citation24 Our higher prevalence rates may reflect referral bias or may demonstrate that glaucoma patients have a higher prevalence than the general public. Open-angle glaucoma has long been a recognized risk factor for both BRVO and CRVO.Citation25,Citation26 Ischemic CRVO can lead to neovascular glaucoma and CACG, but this study did not include neovascular glaucoma, and there were no statistically significant differences in rates of vein occlusions or vascular occlusions between the glaucoma types studied here. It is possible that CACG was underdiagnosed in our study if patients did not undergo gonioscopy. The small numbers of patients with a vascular occlusion also limited the statistical power of this study. Of all study patients, only three PXG, five NTG, eight CACG, and 112 POAG patients had a vascular occlusion.
On the other end of the spectrum, our results for the prevalence of macular degeneration in glaucoma patients are lower than prevalence rates from previous studies. A 2004 meta-analysis estimated the prevalence of AMD to be 6.12% for early AMD and 1.02% for neovascular AMD,Citation27 which is higher than our estimates of 2.5% for either exudative or nonexudative AMD, 1.6% for just nonexudative AMD, and 0.91% for exudative AMD. In a 2008 study of Finnish patients with POAG and PXG, the prevalence of AMD was 4.0%.Citation28 The definition of AMD is a key determinant of how prevalence rates are calculated, and the 2004 meta-analysis defined early AMD as having at least one drusen of 125 µm or larger.Citation27 The lower rates of AMD in this study may reflect the limitation of using ICD-9 codes without a chart review. For instance, the proportion of nonexudative AMD patients to exudative AMD patients in this study is 2.5% to 0.91%, which differs from well-established estimates that nonexudative AMD represents 85%–90% of all AMD patients. Together these data may reflect an ascertainment bias where, in the absence of formal prospective study, drusen reflecting early AMD may not have been detected or recorded, or may not have led to referral to retina specialists more likely to code these diseases. Another explanation for the low rates of AMD is that this study population may not be generalizable to a community-based epidemiological study. While we do not have race or ethnicity information on study participants, this academic medical center is located in a county that is 64.3% Hispanic or Latino according to the US Census Bureau.Citation29 It has long been established that Caucasians have higher rates of AMD than African Americans, and a recent study suggests that Hispanics have lower rates of AMD than Caucasians.Citation30 Finally, the lower rates of AMD could suggest a hypothesis that glaucoma may be a protective factor against the development of AMD, although this hypothesis would need considerable additional study as well as a plausible biological mechanism.
When examining the differences in prevalence of comorbid retinal disease between types of glaucoma, we also evaluated differences in mean age between glaucoma types, as age could be a confounder given that it is a known risk factor for both glaucoma and many retinal diseases such as AMD, atherosclerosis and retinal artery occlusive disease, retinal venous occlusive disease, and epiretinal membrane.Citation31 Of all the patients with comorbid retinal disease, PXG patients were older than those with POAG, NTG, and CACG, and there were no other differences between glaucoma types. For instance, POAG patients with comorbid retinal disease were not older than patients with other types of glaucoma.
The nearly twofold increased risk of severe vision loss in patients with comorbid retinal disease highlights the need for physicians to include a careful retinal examination, and to consider multiple etiologies if visual field results or optic nerve assessments do not correlate with the level of vision loss. A multidisciplinary, team-based approach to patient care with more frequent monitoring is clearly indicated for patients at increased risk for vision loss. Understanding the prevalence of comorbid retinal disease in patients with certain types of glaucoma may lead to increased awareness among eye care professionals. Further studies are needed to evaluate the care of glaucoma patients with comorbid retinal disease.
Acknowledgments
The authors gratefully acknowledge the National Eye Institute (P30s EY022589 and EY014801) and an unrestricted grant from Research to Prevent Blindness Inc.
Disclosure
JFG: Pfizer, Inc. (Ownership). The preliminary data from this study were presented as a poster at the Association for Research in Vision and Ophthalmology national meeting at Orlando, FL, USA, in May 2014. The authors report no conflicts of interest in this work.
References
- RitchRShieldsMThe Secondary GlaucomasSt Louis, MOCV Mosby1982
- PanekWCHollandGNLeeDAChristensenREGlaucoma in patients with uveitisBr J Ophthalmol19907442232272337547
- NeriPAzuara-BlancoAForresterJVIncidence of glaucoma in patients with uveitisJ Glaucoma200413646146515534470
- Merayo-LlovesJPowerWJRodriguezAPedroza-SeresMFosterCSSecondary glaucoma in patients with uveitisOphthalmologica1999213530030410516518
- SiddiqueSSSuelvesAMBahetiUFosterCSGlaucoma and uveitisSurv Ophthalmol201358111023217584
- ElginUBerkerNBatmanAIncidence of secondary glaucoma in Behçet diseaseJ Glaucoma200413644144415534466
- BadeebOTropeGMusarellaMPrimary angle closure glaucoma and retinitis pigmentosaActa Ophthalmol19937167277328154244
- PhelpsCDBurtonTCGlaucoma and retinal detachmentArch Ophthalmol1977953418422843271
- KocaboraMSYilmazliCTaskapiliMGulkilikGDurmazSDevelopment of ocular hypertension and persistent glaucoma after intravitreal injection of triamcinoloneClin Ophthalmol20082116717119668401
- JonesR3rdRheeDJCorticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literatureCurr Opin Ophthalmol200617216316716552251
- TsaiJCLeeMBWudunnDDaceyMPChoiJCMincklerDSIncidence of acute intraocular pressure elevation after panretinal photocoagulationJ Glaucoma199541454819920637
- AielloLMPerspectives on diabetic retinopathyAm J Ophthalmol2003136112213512834680
- BlondeauPPavanPRPhelpsCDAcute pressure elevation following panretinal photocoagulationArch Ophthalmol1981997123912417196215
- HoangQVMendoncaLSDella TorreKEJungJJTsuangAJFreundKBEffect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injectionsOphthalmology2012119232132622054994
- TsengJJVanceSKDella TorreKESustained increased intraocular pressure related to intravitreal antivascular endothelial growth factor therapy for neovascular age-related macular degenerationJ Glaucoma201221424124721423038
- AndersonNGFinemanMSBrownGCIncidence of intraocular pressure spike and other adverse events after vitreoretinal surgeryOphthalmology20061131424716360213
- DesaiURAlhalelAASchiffmanRMCampenTJSundarGMuhichAIntraocular pressure elevation after simple pars plana vitrectomyOphthalmology199710457817869160023
- MuetherPSHoersterRKirchhofBFauserSCourse of intraocular pressure after vitreoretinal surgery: is early postoperative intraocular pressure elevation predictable?Retina20113181545155221610561
- HonavarSGGoyalMMajjiABSenPKNaduvilathTDandonaLGlaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachmentsOphthalmology199910611691769917800
- KoreenLYoshidaNEscariaoPIncidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomyRetina201232116016721765372
- ChangSLXII Edward Jackson lecture: open angle glaucoma after vitrectomyAm J Ophthalmol200614161033104316765671
- ShazlyTALatinaMANeovascular glaucoma: etiology, diagnosis and prognosisSemin Ophthalmol200924211312119373696
- KempenJHO’ColmainBJLeskeMCThe prevalence of diabetic retinopathy among adults in the United StatesArch Ophthalmol2004122455256315078674
- RogersSMcIntoshRLCheungNThe prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and AustraliaOphthalmology20101172313319.e120022117
- No authors listedRisk factors for central retinal vein occlusion. The Eye Disease Case-Control Study GroupArch Ophthalmol199611455455548619763
- No authors listedRisk factors for branch retinal vein occlusion. The Eye Disease Case-control Study GroupAm J Ophthalmol199311632862968357052
- FriedmanDSO’ColmainBJMunozBPrevalence of age-related macular degeneration in the United StatesArch Ophthalmol2004122456457215078675
- TarkkanenAReunanenAKivelaTFrequency of age-related macular degeneration among patients with primary chronic open-angle glaucoma and exfoliation glaucomaActa Ophthalmol200886669769818547286
- US Census BureauState and County QuickFactsMiami-DadeFlorida Available from: http://quickfacts.census.gov/qfd/states/12/12086.htmlAccessed April 28, 2014
- VanderbeekBLZacksDNTalwarNNanBMuschDCSteinJDRacial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care networkAm J Ophthalmol20111522273282.e321696700
- KanskiJJClinical Ophthalmology: A Systematic Approach6th edPhiladelphia, PAElsevier2007