Abstract
Purpose
This study aimed to evaluate the increase in tear meniscus height (TMH) induced by 3% diquafosol ophthalmic solution in eyes with contact lens (CL).
Methods
Ten healthy subjects wearing high-water-content CLs received topical instillation of two ophthalmic solutions – 3% diquafosol ophthalmic solution in one eye and artificial tears in the other eye. Lower TMH was measured at 5 minutes, 10 minutes, 15 minutes, 30 minutes, and 60 minutes after instillation by anterior segment optical coherence tomography.
Results
TMH increased significantly (P<0.001) at 5 minutes and 15 minutes after instillation of saline compared with the baseline values. After instillation of 3% diquafosol ophthalmic solution, TMH significantly increased (P<0.05) at 5 minutes, 15 minutes, 30 minutes, and 60 minutes compared with the baseline values. Increases in TMH after diquafosol instillation were significantly greater (P<0.05) at 15 minutes, 30 minutes, and 60 minutes than increases in TMH after saline instillation.
Conclusion
Topical instillation of 3% diquafosol ophthalmic solution increases TMH for up to 60 minutes in eyes with high-water-content CLs.
Introduction
As reported in the “Contact Lens Discomfort” report by the Tear Film and Ocular Surface Society, several biophysical tear film changes have been associated with contact lens (CL) wear. Decrease in tear volume is one of the changes related to CL discomfort.Citation1 Previously, we reported a remarkable decrease of tear meniscus height (TMH) with CL wear,Citation2 especially with high-water-content CL wear by quantitative TMH measurement using anterior segment optical coherence tomography (AS-OCT). Because 75%–90% of tear volume exists in the upper and lower tear menisci,Citation3 reduction of TMH during CL wear may be a major factor that induces CL-related dry eye symptoms. Artificial tears and tear retention agents have been widely used as possible options for tear supplementation during CL wear. Our previous preliminary investigation in a limited number of subjects showed a significant increase in TMH at 5 minutes after instillation of 3% diquafosol ophthalmic solution (Diquas, ophthalmic solution 3%; Santen Pharmaceutical, Osaka, Japan) during high-water-content CL wear.Citation2 Diquafosol ophthalmic solution stimulates secretion of tear fluid and mucin. These actions have been reported to improve tear film stability and provide therapeutic relief for dry eye symptoms in various types of dry eyes.Citation4–Citation9 Therefore, we speculate that eyes with a remarkable reduction in TMH due to high-water-content CL wear might benefit from an increase in wetness by tear fluid secretion caused by diquafosol instillation.
In our current study, we evaluated whether diquafosol had the capacity to prolong increases of TMH in eyes with high-water-content CL wear in a greater number of subjects.
Materials and methods
This study was approved by the Institutional Review Board of Kansai Rosai Hospital. This research adhered to the tenets of the Declaration of Helsinki, and we obtained informed consent from all participants after the nature and possible consequences of the study were explained to them.
Twenty eyes of ten normal subjects (eight men, two women; average age: 38.1±5.8 years) with no ocular disease, except refractive errors, were analyzed. No subject had worn CLs, had undergone ocular surgery, used eye drops, or taken systemic medication. Subjects were asked to wear commercially available CLs, specifically, etafilcon A lenses (1-day ACUVUE; Johnson & Johnson, Tokyo, Japan). This lens has a high water content of 58.0% and a diameter of 14.2 mm, and it was classified by the US Food and Drug Administration as a group IV lens.
In accordance with the procedure we used in our previous study,Citation2 TMH measurements were performed using a commercial AS-OCT (SS-1000; Tomey Corp, Nagoya, Japan). Cross-sectional images of the lower TMH were taken vertically, across the central cornea, in every subject. TMH was defined as the line distance from the fluid surface of the CL–meniscus junction to the lower eyelid–meniscus junction. On the basis of the previously described methods,Citation2,Citation10,Citation11 the lower TMH values were calculated using the cross-sectional AS-OCT images.
At 20 minutes after lens insertion, each subject randomly received a single drop of 3% diquafosol ophthalmic solution in one eye and an isotonic borate-buffered saline solution (Soft Santear; Santen Pharmaceutical, Osaka, Japan) in the other eye. AS-OCT images were taken prior to and at 5 minutes, 15 minutes, 30 minutes, and 60 minutes after eye drop instillation in both eyes of each subject. The change in TMH (ΔTMH) was calculated as the difference between the baseline TMH value and the TMH value after instillation. In addition, the time course of ΔTMH with two eye drops was evaluated, and the ΔTMH values were compared between the two eye drops.
Results
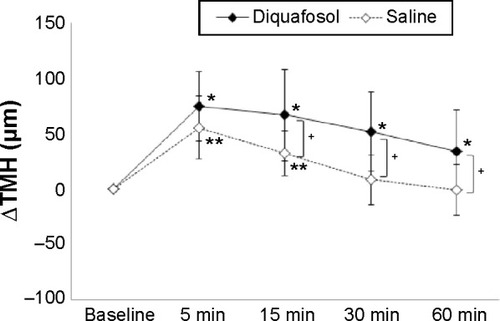
The ΔTMH data obtained before and after instillation of 3% diquafosol ophthalmic solution and saline in eyes with CL wear are shown in . Mean ΔTMHs after saline instillation were 55.8 µm, 32.7 µm, 8.4 µm, and -0.6 µm at 5 minutes, 15 minutes, 30 minutes, and 60 minutes after instillation, respectively. Mean ΔTMHs after diquafosol instillation were 75.1 µm, 66.9 µm, 52.3 µm, and 34.4 µm at 5 minutes, 15 minutes, 30 minutes, and 60 minutes after instillation, respectively. Compared with the baseline TMH values, significant increases of TMH were observed at 5 minutes, 15 minutes, 30 minutes, and 60 minutes after diquafosol instillation and at 5 minutes and 15 minutes after saline instillation. The ΔTMH after diquafosol instillation was significantly greater (P<0.05) at 15 minutes, 30 minutes, and 60 minutes compared with the ΔTMH after saline instillation.
Figure 1 Time course of the lower TMH after eye drop instillation with contact lens wear.
Abbreviations: ΔTMH, change in TMH; TMH, tear meniscus height.
Discussion
In our present study, a significant, prolonged increase in TMH was observed up to 60 minutes after diquafosol instillation in eyes with high-water-content CL wear. Furthermore, increase in TMH after diquafosol instillation was significantly greater at 15 minutes, 30 minutes, and 60 minutes than the increase in TMH after saline instillation. The results of our present study were consistent with those of our previous study,Citation2 and it added convincing evidence of prolonged increase in wetness via use of diquafosol in eyes with CL wear.
Contact lenses divide the tear film into pre- and postlens tear films, and this compartmentalization affects biophysical changes in the tear film.Citation1 According to a previous report,Citation12 after instillation of artificial tears, the thickness of prelens tear film increased temporarily for a few minutes. However, there were no significant changes in the postlens tear film. It has been reported that the postlens tear film plays an important role in interactions with the ocular surface and may influence ocular comfort.Citation1,Citation13–Citation15 Although prolonged increase in prelens TMH of eyes with CL wear after instillation of diquafosol ophthalmic solution was demonstrated in our current study, postlens tear film dynamics remain unknown. Although a study addressing this issue is currently under way, it will be helpful to clarify pre- and postlens tear film dynamics after instillation of artificial tears and tear retention agents in eyes with CL wear, especially symptomatic CL wearers. Using AS-OCT, future quantitative investigations of changes in thickness of pre- and postlens tear films will be possible.
Significant, prolonged increases of prelens TMH were observed after diquafosol instillation. It is expected that diquafosol ophthalmic solution, which has the potential to increase tear volume over the ocular surface beneath CLs, will become a treatment option for CL-related dryness.
Disclosure
The authors report no conflicts of interest in this work.
References
- CraigJPWillcoxMDArgüesoPmembers of TFOS International Workshop on Contact Lens DiscomfortThe TFOS International Workshop on contact lens discomfort: report of the contact lens Interactions with the tear film subcommitteeInvest Ophthalmol Vis Sci20135411123156
- NagaharaYKohSMaedaNNishidaKWatanabeHProminent decrease of tear meniscus height with contact lens wear and efficacy of eye drop instillationEye Contact Lens Epub 201541
- HollyFJPhysical chemistry of the normal and disordered tear filmTrans Ophthalmol Soc U K1985104pt 43743803862270
- MatsumotoYOhashiYWatanabeHTsubotaKDiquafosol Ophthalmic Solution Phase 2 Study GroupEfficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trialOphthalmology2012119101954196022739038
- TakamuraETsubotaKWatanabeHOhashiYDiquafosol Ophthalmic Solution Phase 3 Study GroupA randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patientsBr J Ophthalmol201296101310131522914501
- Shimazaki-DenSIsedaHDogruMShimazakiJEffects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eyeCornea20133281120112523635860
- KohSIkedaCTakaiYWatanabeHMaedaNNishidaKLong-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eyeJpn J Ophthalmol201357544044623740285
- MoriYNejimaRMasudaAEffect of diquafosol tetrasodium eye drop for persistent dry eye after laser in situ keratomileusisCornea201433765966224858017
- YamaguchiMNishijimaTShimazakiJClinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational studyAdv Ther201431111169118125376447
- FukudaRUsuiTMiyaiTYamagamiSAmanoSTear meniscus evaluation by anterior segment swept-source optical coherence tomographyAm J Ophthalmol2013155462062423317654
- OhtomoKUetaTFukudaRTear meniscus volume changes in dacryocystorhinostomy evaluated with quantitative measurement using anterior segment optical coherence tomographyInvest Ophthalmol Vis Sci20145542057206124474266
- ChenQWangJTaoAShenMJiaoSLuFUltrahigh resolution measurement by optical coherence tomography of dynamic tear film changes on contact lensesInvest Ophthalmol Vis Sci20105141988199319933178
- BruceASMainstoneJCLens adherence and postlens tear film changes in closed-eye wear of hydrogel lensesOptom Vis Sci199673128348867679
- LittleSABruceASPostlens tear film morphology, lens movement and symptoms in hydrogel lens wearersOphthalmic Physiol Opt199414165698152823
- LittleSABruceASRole of the post-lens tear film in the mechanism of inferior arcuate staining with ultrathin hydrogel lensesCLAO J19952131751817586476
