Abstract
Purpose
To describe the clinical features, management, and outcomes of patients with diffuse unilateral subacute neuroretinitis (DUSN).
Methods
A noncomparative, consecutive analysis of case series from two tertiary care campuses of LV Prasad Eye Institute, India, between January 2011 and April 2014 was performed. Medical records of the patients presenting with DUSN (early or late stage) were reviewed.
Results
The current study included 13 patients. The majority (10/13, 76.92%) of the patients were aged 20 years or less. All patients had unilateral eye involvement. Visual acuity at presentation was 20/200 or worse in 9/13 (69.23%) patients. A delay in diagnosis occurred in 6/13 patients, and initial diagnosis in these patients included retinitis pigmentosa (4 patients) and posterior uveitis (2 patients). Clinical features included early presentation (prominent vitritis, localized retinitis, and vasculitis) in 7/13 (53.85%) patients and late presentation (attenuation of vessels, retinal pigment epithelium atrophic changes, and optic atrophy) in 6/13 (46.15%) patients. Worm could not be identified in any of the cases. All the patients received laser photocoagulation of retina and oral albendazole treatment for a period of 30 days. With treatment, visual acuity improved in seven patients (six early stage, one late stage) and remained unchanged in six patients. Mean follow-up period was 8.69 months (range, 1–21 months). The mean central foveal thickness in the affected eye, done by optical coherence tomography, during the late stage of the disease was 188.20±40 µm (range, 111–242 µm), which was significantly thinner than the fellow eye, 238.70±36.90 µm (range, 186–319 µm), P=0.008.
Conclusion
DUSN is a serious vision threatening disease, which may progress to profound vision loss in the later stage of the disease. Visualization of subretinal worm is usually not possible. Treatment with high-dose albendazole therapy and laser photocoagulation may alter the blood–retinal barrier and may be useful in achieving visual recovery.
Introduction
Diffuse unilateral subacute neuroretinitis (DUSN), also known as “unilateral wipe out syndrome”, is a progressive ocular infectious disease caused by various species of nematodes, leading to inflammation and degeneration of the outer retina and the retinal pigment epithelium (RPE).Citation1 Nematode infection of the eye was first reported in the year 1950 by WilderCitation2 in an enucleated eye specimen. In 1978, Gass et alCitation1 was the first to describe the clinical entity of DUSN after finding a worm in 2 out of 36 cases with such clinical findings. DUSN is a progressive inflammatory ocular pathology which is characterized by unilateral visual loss, vitreous cells, degenerative retinal pigmentary changes, optic disc and retinal vessel inflammation.Citation1,Citation3–Citation5
Clinical presentation of DUSN is divided into two stages – early and late.Citation1 In early stage, the patient is either asymptomatic or presents with acute onset of multiple and rapidly changing, central/paracentral scotomata, photopsias, or unilateral vision loss.Citation1,Citation3–Citation7 Anterior segment inflammation is uncommon in early stage, with a few exceptional case reports where keratic precipitates and a hypopyon have been reported.Citation1,Citation8 Cystoid macular edema, intraretinal/subretinal hemorrhages, retinal exudation, and neovascularization may be less commonly seen during the early stage of the disease. In late stage, the patients may present with severe vision loss. Clinically, the patients may have vitritis, narrowing of the retinal vessels, optic disc swelling/atrophy, diffuse/focal depigmentation of RPE, multiple RPE track lesions leading to gray–white lesions. These evanescent, multifocal subretinal lesions are present at the level of the outer retinaCitation4,Citation9 and are believed to be the area where the worm is present. These lesions characteristically migrate and change along with the worm and may or may not leave a residual retinal lesion depending on degree of host immune response against the toxic products of the worm.Citation4
DUSN often poses a diagnostic challenge. A retrospective analysis of case series from two tertiary care centers of LV Prasad Eye Institute, India was conducted. The spectrum of clinical features, management, and outcomes were reviewed.
Methods
This was a retrospective analysis of medical records of patients diagnosed with DUSN between January 2011 and April 2014 in the two campuses of LV Prasad Eye Institute (Vijayawada and Visakhapatnam), India. The study was approved by the Institutional Review Board of LV Prasad Eye Institute, India. After informed and signed consent was obtained from the patients or legal guardians (in the case of minors), all subjects underwent detailed comprehensive eye examination. The study was performed in accordance with ethical principles as per the Declaration of Helsinki. Records of the patients presenting with anterior segment inflammation, vitritis, vasculitis, multifocal-migrating yellow–white subretinal lesions, diffuse and focal RPE damage, optic nerve atrophy, retinal vessel narrowing, and white subretinal track lesions suggestive of DUSN were included in the study. In the absence of identification of worm, the diagnosis of presumed DUSN was made on the bases of clinical features and exclusion of any other ocular disease. Patient data including age at presentation, sex, best-corrected Snellen visual acuity at presentation and last visit, presence of relative afferent pupillary defect, anterior segment inflammation, posterior segment findings (using 3-mirror Goldmann lens or 78 D lens), and central macular thickness (CMT) on optical coherence tomography (OCT) were recorded. Mean follow-up was 8.69 months (range, 1–21 months).
Results
A total of 13 patients (5 from Vijayawada and 8 from Visakhapatnam) were included in the study. provides the detailed summary of these patients. Mean age at presentation was 22.5 years (range, 8–62 years). Most patients (10/13, 76.92%) were ≤20 years at the time of presentation (). No predominant predilection for either sex was observed (male to female, 8:5). The patients were symptomatic before presentation for a period ranging from 1 day to 1 year. All patients presented with blurred vision in the affected eye. Visual acuity at presentation was 20/200 or worse in 9/13 (69.23%) patients (). All patients had unilateral presentation, and relative afferent papillary defect was observed in 10/13 cases. Mild anterior segment inflammation was seen in only two cases (patient 2 and 4). Vitreous cells were present in all of the patients (vitreous cell in nine patients were 1+ and in the remaining four patients 2+ as per Standardization Uveitis Nomenclature – SUN classification). Seven patients (53.85%) presented in the early stage, while six patients (46.15%) presented in the late stage of the disease. The posterior segment findings included vitritis, vasculitis, multifocal-migrating yellow–white subretinal lesions (), diffuse and focal RPE damage, optic nerve atrophy, retinal vessel narrowing, and white subretinal track lesions. shows the frequency of the various clinical features at the time of presentation in this case series. RPE changes (13/13, 100%), focal narrowing of vessels (13/13, 100%), and optic disc pallor (7/13, 57.14%) were the most frequent findings. A worm could not be identified in any of these patients. All the patients received oral, high-dose antihelminthic (albendazole 400 mg or 15 mg/kg body weight, once daily) treatment for a period of 30 days. Laser photocoagulation was done either in the vicinity of the subretinal lesion or at the inferior peripheral retina in order to alter the blood–retina barrier and possibly allow increasing ocular penetration of albendazole. Improvement in visual acuity was noted as early as 1 week and stabilized by 1 month in all patients. Visual acuity improved in seven patients (six patients who presented during early stage and one patient who presented during late stage) and remained stable in six patients. During the late stage, the mean central foveal thickness in the affected eye was 188.20±40 µm (range, 111–242 µm), while in the other eye it was 238.70±36.90 µm (range, 186–319 µm).
Figure 1 Fundus pictures of the left eye of an 11-year-old child who presented with blurred vision.
Abbreviations: 20/20, Snellen visual acuity equivalent to 20/20; CFCF, visual acuity counting fingers close to face; 20/40, Snellen visual acuity equivalent to 20/40.
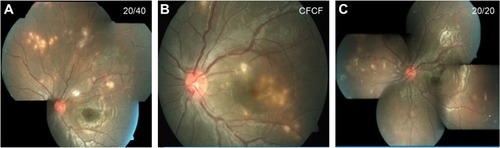
Table 1 Clinical features of patients presenting with presumed diffuse unilateral subacute neuroretinitis (DUSN)
Table 2 Age distribution of patients with DUSN
Table 3 Visual acuity at presentation
Table 4 Posterior segment findings in patients with presumed DUSN
Discussion
The current study included patients with presumed DUSN, where worm could not be identified and the diagnosis was based on the clinical findings. As per literature, the worm is visible in 33.3%–40.2%Citation10,Citation11 of cases. The light sensitivity of the worm (on light exposure, the worm moves deeper and hides subretinally) and difficult peripheral retinal examination are the main hurdles in identifying subretinal worm.Citation9,Citation12 Entry of the worm inside the eye occurs when nematode eggs, after being ingested, find their way to eye hematogenously,Citation13 and then, for months to years the worm can wander in the subretinal space. DUSN was first thought to be caused by Toxocara, but later on, it was found to be caused by other species of nematodes as well, like Ancylostoma caninum, Baylisascaris procyonis, Strongyloides sp, Alaria mesocercaria, and filarial worms.Citation14 The causative nematodes are of two types based on their length – small and large. The smaller nematodes (length range, 400–1,000 µm in length, with diameter being approximately 1/20th of its length; more common) include Ancylostoma caninum and Toxocara canis; endemic in Southeastern United States, the Caribbean, Venezuela, Brazil, and northern parts of South America.Citation3,Citation15,Citation16 The larger nematodes – Baylisascaris procyonis (length range, 1,000–2,000 µm) – are found in Northern midwestern United States, Europe, and Brazil.Citation3,Citation16,Citation17 DUSN is usually unilateral, though a few bilateral cases have been reported in literature.Citation2,Citation11,Citation18 In the current study, all patients had unilateral presentation. Majority (69.42%) of the patients in a retrospective case series of 121 Brazilian patients with DUSNCitation12 were younger than 20 years, which is similar to the number of young patients in this case series (76.92% – age 20 or less). Approximately 92% patients in Brazil studyCitation12 presented at late stage of the disease; however, in this case series, 53.85% patients (seven patients) presented in early stage. The exact pathogenesis of clinical findings seen in DUSN remain unclear, although the toxic inflammation due to larval products and immune response of host to the toxin are believed to damage the inner as well as the outer retina, hence affecting retinal nerve fiber layer (RNFL), optic nerve, and RPE, anatomically and functionally.Citation1,Citation4,Citation5,Citation19,Citation20
Patients, who present during the early stage of the disease, are more likely to get improvement in visual acuity with treatmentCitation12,Citation21,Citation22 as compared to patients presenting during later stage of the disease. This pattern of response was observed in this study as well. Few uncommon, unusual presentations have been reported like the presence of multiple worms or macular cyst or worm at macula.Citation23–Citation25 Treatment of choice is the destruction of worm with laser photocoagulation (using 500 µm, 0.5-second argon laser applications) whenever it was visible.Citation4,Citation16 The worm is usually found in the vicinity of yellow–white retinal lesions.Citation16 Less than 1% of the lesions (which are supposed to be the clue to the presence of worm) are of sufficient clarity to be identified clinically, and therefore it may not be possible to find a worm in all patients. In cases where worm cannot be identified despite careful and intensive time consuming eye examination, high-dose oral antihelminthic therapy (albendazole 400 mg/d or 15 mg/kg body weight, once daily for 30 days) is recommended (similar to the treatment of neurocysticercosis) along with application of laser photocoagulation adjacent to the yellow–white retinal lesion (or in periphery where no focal lesions are present).Citation3,Citation26 Albendazole is a broad-spectrum benzimidazole antihelminthic developed approximately 35 years ago.Citation27 It binds to β-tubulin of parasites and inhibits its polymerization and impairs glucose uptake. High-dose oral albendazole therapy has been reported to be effective.Citation10 Ocular penetration of albendazole is supposed to increase by disrupting blood–retina barrier by laser photocoagulation,Citation28,Citation29 as was done for all patients in this case series. Posttreatment recovery of visual fields has been reported,Citation10,Citation21,Citation22 and in the current study, visual acuity improved in 7/13 (53.85%) cases.
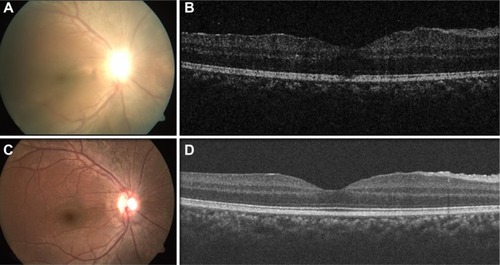
DUSN resembles few other ocular diseases that are difficult to diagnose. In early stage of the disease, it may resemble conditions such as retinal sarcoidosis, acute multifocal posterior placoid pigment epitheliopathy, serpiginous choroiditis, multiple evanescent white dot syndrome, Behçet’s disease, multifocal toxoplasmosis, and presumed ocular histoplasmosis syndrome. In the later stage, the disease may mimic unilateral optic atrophy caused by retrobulbar or intracranial lesions, the presumed ocular histoplasmosis syndrome, unilateral retinitis pigmentosa, posttraumatic chorioretinopathy, and chorioretinal atrophy after central retinal artery occlusion, syphilis, sarcoidosis etc.Citation3,Citation9 In this case series, 6/13 patients were initially diagnosed as having retinitis pigmentosa (4) and posterior uveitis (2), which delayed treatment. A detailed workup, including history (profound vision loss in one eye, photopsia) and ocular examination (optic atrophy, vascular attenuation, presence of normal looking RPE in between punched out lesions), are the initial steps toward making clinical diagnosis. The ancillary tests include fluorescein angiography (in active stage of DUSN, gray–white lesions are hypofluorescent in early phase of angiogram and stain in the later phase of angiogram along with disc leak, while in late stage of DUSN, irregular increase in background choroidal fluorescence is noted),Citation3,Citation16 electroretinography (subnormal waveforms with b waves affected more than a waves),Citation3,Citation30,Citation31 multifocal electroretinography (decreased foveal response and increased parafoveal and perifoveal waveform amplitudes),Citation31 indocyanine green angiography (dark spot in early phase of angiogram which disappears or persists in later phase of angiogram),Citation32 and OCT (decreased RNFL and CMT)Citation19 that may help in assessing the stage of the disease. In this case series, the CMT of patients in late stage showed thinning compared to the other eye. In a case series of eight patients from Brazil, Garcia Filho et alCitation19 found that RNFL and CMT were thinner in DUSN eyes compared to the normal eyes, and this thinning was more prominent in the late stage of the disease. shows increased CMT during active stage (=315 µm) and gradual decrease in CMT during late stage of the disease (257 µm), which was seen during follow-up of a patient from this case series. Other OCT abnormalities such as loss of foveal depression and focal/diffuse defects at the ellipsoid zone (inner segment/outer segment junction) have also been mentioned in DUSN patients.Citation19 Other tests such as visual field, serological studies, stool examination, and peripheral blood smears are of limited value in making the diagnosis of DUSN.Citation1 Gass et alCitation1 mentions that patients may have choroidal neovascular membrane (CNVM) or disciform scar in later stage of the disease. A poster presentation at ARVO (Association for Research in Vision and Ophthalmology) in 2011 by Shah et alCitation33 from Bascom Palmer Eye Institute, (three cases) mentioned that atrophic changes of macula precede the onset of type 2 CNVM; however, in the current study, CNVM was not noted in any of the patients. A longer follow-up to look for delayed changes is recommended.
Figure 2 Fundus pictures and OCT images of early stage and chronic stage of the disease.
Abbreviation: OCT, optical coherence tomography.
In conclusion, DUSN is a vision threatening ocular infectious disease, which may progress to profound vision loss in the later stages of the disease. The presence of focal RPE alterations, vitreous inflammation, narrowing of retinal vessels, optic disc pallor, and yellow–white subretinal lesions are highly suggestive of DUSN. Detailed ophthalmoscopic examination and fundus photography are helpful in making a diagnosis. However, it is not possible to identify the subretinal worm in most cases. In such patients, high-dose albendazole therapy and laser photocoagulation may help in controlling the disease activity and may reduce further damage by the subretinal worm. The disease process may be reversible in the early stages as improvement in visual acuity in some patients has been observed with treatment.
Acknowledgments
We gratefully acknowledge Rajawardhan Mallipudi and Podili Srinivas Rao for collection of data and photographic support. Both are working as faculty optometrists at LV Prasad Eye Institute, Vijayawada, India.
Disclosure
The authors declare that they have no competing interests (financial or nonfinancial).
References
- GassJDGilbertWRJrGuerryRKScelfoRDiffuse unilateral sub-acute neuroretinitisOphthalmology1978855521545673332
- WilderHCNematode endophthalmitisTrans Am Acad Ophthalmol Otolaryngol1950559910914798648
- GassJDMStereoscopic atlas of macular diseases: diagnosis and treatment4th edSt Louis, MOMosby1997
- GassJDBraunsteinRAFurther observations concerning the diffuse unilateral subacute neuroretinitis syndromeArch Ophthalmol198310111168916976639421
- GassJDScelfoRDiffuse unilateral subacute neuroretinitisJ R Soc Med197871295111564964
- CarneyMDCombsJLDiffuse unilateral subacute neuroretinitisBr J Ophthalmol199175106336351954217
- AudoIWebsterARBirdACHolderGEKiddMNProgressive retinal dysfunction in diffuse unilateral subacute neuroretinitisBr J Ophthalmol200690679379416714269
- MuccioliCBelfortRJrHypopyon in a patient with presumptive diffuse unilateral subacute neuroretinitisOcul Immunol Inflamm20008211912110980685
- CortezRTRamirezGColletLGiuliariGPOcular parasitic diseases: a review on toxocariasis and diffuse unilateral subacute neuroretinitisJ Pediatr Ophthalmol Strabismus201148420421220669882
- SouzaECCasellaAMNakashimaYMonteiroMLClinical features and outcomes of patients with diffuse unilateral subacute neuroretinitis treated with oral albendazoleAm J Ophthalmol2005140343744516026752
- CortezRDennyJPMuci-MendozaRRamirezGFuenmayorDJaffeGJDiffuse unilateral subacute neuroretinitis in VenezuelaOphthalmology2005112122110211416242189
- de Amorim Garcia FilhoCAGomesAHde A Garcia SoaresACde Amorim GarciaCAClinical features of 121 patients with diffuse unilateral subacute neuroretinitisAm J Ophthalmol2012153474374922244523
- HollandGNPeposeJSWilhelmusKROcular infection and immunitySt Louis, MOMosby1996
- AlbertDMJakobiecFAPrinciples and practice of ophthalmology2nd edPhiladelphia, PAW.B. Saunders Co2000
- de SouzaECda CunhaSLGassJDDiffuse unilateral subacute neuroretinitis in South AmericaArch Ophthalmol19921109126112631520112
- RyanSJRetina3rd edSt Louis, MOMosby2001
- GoldbergMAKazacosKRBoyceWMAiEKatzBDiffuse unilateral subacute neuroretinitis. Morphometric, serologic, and epidemiologic support for Baylisascaris as a causative agentOphthalmology199310011169517018233397
- de SouzaECAbujamraSNakashimaYGassJDDiffuse bilateral subacute neuroretinitis: first patient with documented nematodes in both eyesArch Ophthalmol1999117101349135110532442
- Garcia FilhoCASoaresACPenhaFMGarciaCASpectral domain optical coherence tomography in diffuse unilateral subacute neurore-tinitisJ Ophthalmol2011201128529621860780
- JohnTBarskyHJDonnellyJJRockeyJHRetinal pigment epitheliopathy and neuroretinal degeneration in ascarid-infected eyesInvest Ophthalmol Vis Sci1987289158315983623842
- GarciaCAGomesAHGarcia FilhoCAViannaRNEarly-stage diffuse unilateral subacute neuroretinitis: improvement of vision after photocoagulation of the wormEye (Lond)200418662462714716322
- GarciaCAGomesAHViannaRNSouza FilhoJPGarcia FilhoCAOreficeFLate-stage diffuse unilateral subacute neuroretinitis: photocoagulation of the worm does not improve the visual acuity of affected patientsInt Ophthalmol2005261–2394216786179
- HartoMARodriguez-SalvadorVAvinoJADuch-SamperAMMenezoJLDiffuse unilateral subacute neuroretinitis in EuropeEur J Ophthalmol199991586210230594
- VedanthamVVatsMMKakadeSJRamasamyKDiffuse unilateral subacute neuroretinitis with unusual findingsAm J Ophthalmol2006142588088317056380
- YusoffMAlwiAASaidMMZakariahSGhaniZAZunainaEIntraocular nematode with diffuse unilateral subacute neuroretinitis: case reportBMC Ophthalmol2011111521679403
- GassJDCallananDGBowmanCBOral therapy in diffuse unilateral subacute neuroretinitisArch Ophthalmol199211056756801580843
- VenkatesanPAlbendazoleJ Antimicrob Chemother19984121451479533454
- MaguireAMZarbinMAConnorTBJustinJOcular penetration of thiabendazoleArch Ophthalmol19901081216752256834
- GassJDCallananDGBowmanCBSuccessful oral therapy for diffuse unilateral subacute neuroretinitisTrans Am Ophthalmol Soc19918997112 discussion 113–1161808823
- KelseyJHDiffuse unilateral subacute neuroretinitisJ R Soc Med1978714303304641935
- SabrosaNAde SouzaECNematode infections of the eye: toxocariasis and diffuse unilateral subacute neuroretinitisCurr Opin Ophthalmol200112645045411734685
- ArevaloJFArevaloFAGarciaRAde AmorimGFCde AmorimGCDiffuse unilateral subacute neuroretinitisJ Pediatr Ophthalmol Strabismus201350420421223244243
- ShahMJacobsDJBerrocalAMSpectral domain OCT findings in Diffuse Acute Unilateral Neuroretinitis (DUSN) with subretinal neovascularizationInvest Ophthalmol Vis Sci201152 E-abstract 2172
