Abstract
Purpose
To evaluate the outcomes and complications of patients with diabetic tractional retinal detachment (TRD) treated with pars plana vitrectomy (PPV).
Patients and methods
We retrospectively studied a case series of 24 eyes of 21 patients at a single tertiary, university-affiliated medical center. A review was carried out on patients who underwent PPV for the management of TRD due to proliferative diabetic retinopathy from October 2011 to November 2013. Preoperative and final visual outcomes, intraoperative and postoperative complications, and medical background were evaluated.
Results
A 23 G instrumentation was used in 23 eyes (95.8%), and a 25 G instrumentation in one (4.2%). Mean postoperative follow-up time was 13.3 months (4–30 months). Visual acuity significantly improved from logarithm of the minimum angle of resolution (LogMAR) 1.48 to LogMAR 1.05 (P<0.05). Visual acuity improved by ≥3 lines in 75% of patients. Intraoperative complications included iatrogenic retinal breaks in seven eyes (22.9%) and vitreal hemorrhage in nine eyes (37.5%). In two eyes, one sclerotomy was enlarged to 20 G (8.3%). Postoperative complications included reoperation in five eyes (20.8%) due to persistent subretinal fluid (n=3), vitreous hemorrhage (n=1), and dislocated intraocular lens (n=1). Thirteen patients (54.2%) had postoperative vitreous hemorrhage that cleared spontaneously, five patients (20.8%) required antiglaucoma medications for increased intraocular pressure, seven patients (29.2%) developed an epiretinal membrane, and two patients (8.3%) developed a macular hole.
Conclusion
Patients with diabetic TRD can benefit from PPV surgery. Intraoperative and postoperative complications can be attributed to the complexity of this disease.
Introduction
End-stage diabetic eye disease is an important cause of severe visual impairment in the working-age group.Citation1 One of the feared ocular complications of diabetes mellitus is proliferative diabetic retinopathy (PDR) with tractional retinal detachment (TRD).Citation2,Citation3 When involving or threatening the macula or when accompanied by persistent vitreous hemorrhage (VH), TRD is an indication for surgical intervention by pars plana vitrectomy (PPV).Citation4
The modern era of vitrectomies began with the introduction of 20 G instrumentation in 1975 by O’Malley and Heintz.Citation5 It was a few decades later, a paradigm shift toward small-gauge vitrectomy (SGV) began in 2002 and 2004, with the first- and second-generations of 25 G instrumentations developed by Fujii et alCitation6 and Lesnoni et al,Citation7 respectively. In 2005, EckardtCitation8 developed the 23 G instrumentation.
First-generation 25 G was used only for surgeries that did not require extensive dissection, such as epiretinal membrane peeling, macular pathologies, simple VH, or vitreomacular tractions. Its limited usage was due to several factors including excessive flexibility of the instruments that made some surgical maneuvers more difficult, low flow rates, less efficient cutters, and wound leakage-associated complications (postoperative hypotony and endophthalmitis) that were related to the sutureless closure of the ports.Citation9,Citation10
Second-generation 25 G and 23 G instruments were designed to overcome these limitations through increased rigidity, better fluidics, and a larger variety of instruments. This allowed the management of more complicated surgeries such as TRD, dense VH, and rhegmatogenous retinal detachment.Citation10–Citation12
Diabetic vitrectomy holds significant risk for surgically induced complications, among which are intraoperative bleeding and iatrogenic retinal breaks, early and delayed VHs, recurrent retinal detachment, neovascular glaucoma, and anterior hyaloid fibrovascular proliferation.Citation4,Citation13
In the last 10 years, SGV became the common practice for TRD.Citation14,Citation15 However, doubt has been raised whether diabetic TRD, in which complex intraocular manipulations are needed and complications occur more commonly, can be equally managed by SGV.Citation1,Citation13,Citation15
SGV has several advantages in treatment of diabetic TRD as well as other diseases. The prominent ones are smaller incisions without peritomy, self-sealing sclerotomies that heal faster, diminished conjunctival scarring that allows repeat surgeries, decreased postoperative inflammation, and a decrease in induced astigmatism that may facilitate visual recovery.Citation9,Citation11,Citation13,Citation16 Moreover, because the cutting tip is closer to the edge of the device, the vitrector facilitates segmentation, dissection, and removal of the fibrovascular membranes in PDR.Citation9,Citation13 The smaller lumen diameter also improves fluidics, allowing for better fluid stability and less tissue movement or incarceration. Better fluidics increase the safety of intraoperative maneuvers.Citation17,Citation18
The purpose of this study is to retrospectively evaluate the outcomes and complications of TRD in patients with PDR managed by PPV. Moreover, we tried to find out whether patients with poor visual acuity (VA) had different VA gain than patients with VA better than 20/200.
Patients and methods
Subjects
This retrospective study included a consecutive series of patients who underwent PPV with 23 G and 25 G instrumentation for the management of TRD due to PDR between October 2011 and November 2013 at a single center by three senior surgeons.
Indications for vitrectomy were TRD involving or threatening the macula or unresolved VH combined with TRD. Patients with previous vitrectomy were excluded from the study. Approval to review the patients’ data was obtained from the local ethics committee.
Methods
The medical files were reviewed of all patients who underwent PPV for the management of TRD due to PDR. The following data were retrieved: patient’s age, sex, medical background, past hospitalizations and blood tests, preoperative and postoperative Snellen VA, intraocular pressure (IOP) and results of slit-lamp examination of the anterior segment and fundus, surgical reports, and intraoperative and postoperative complications.
Snellen VAs were converted into the logarithm of the minimum angle of resolution (LogMAR) score for data analysis.
Thirteen (54.2%) of the cases were conducted under regional anesthesia and eleven (45.8%) under general anesthesia. All surgeries were performed with the Stellaris Vitreoretinal Surgical System (Bausch & Lomb Incorporated, Bridgewater, NJ, USA) and a wide-angle viewing system. The surgeon could select either 25 G or 23 G instrument depending on the patient’s preference. Diabetic membrane removal was achieved either with a cutter or with the curved microscissors. In all cases, the vitreous was removed up to the far periphery, involving meticulous removal around the ports and the infusion line. At the completion of the surgery, the peripheral retina was examined using scleral depression. Drainage retinotomy was performed in cases of subretinal fluid. In cases where a retinal tear was noted, fluid air exchange was performed. Endolaser panretinal photocoagulation up to the far periphery and laser around holes and tears were performed in all patients. At the end of surgery, air, gas, or silicone oil tamponade was used. Sclerotomies were checked for leakage, and if needed, a vicryl suture was applied.
All surgeries were performed by experienced vitreoretinal surgeons. Those patients undergoing combined cataract vitrectomy surgery had their crystalline lens removed using phacoemulsification techniques. A clear corneal approach was used in all cases, and a posterior chamber intraocular lens was inserted to the bag when possible.
Statistical analysis
Analyses were performed using SPSS for Windows (SPSS, Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation. Wilcoxon signed-rank test was used to compare preoperative and postoperative LogMAR VA. Furthermore, Pearson’s correlation coefficient was used to search for an existing relationship between the preoperative and postoperative VA, and Fisher’s exact test was used to search for a preoperative VA under which the probability to improve VA would be lower. A P-value <0.05 was used to mark statistical significance.
Results
Twenty-four eyes of 21 PDR patients (58.3% males) who had undergone PPV for the management of TRD during the aforementioned period were included in this study. Patients’ characteristics are summarized in .
Table 1 Characteristics of 21 patients who underwent small-gauge vitrectomy during 2011–2013
Of the 24 surgeries performed, 23 utilized the 23 G instrumentation and one utilized the 25 G instrumentation. Fourteen surgeries (58.3%) were performed on the left eye. Fifteen eyes (62.5%) had VH at the time of surgery. Eleven cases (45.8%) underwent combined cataract and vitrectomy surgery.
The average hemoglobin A1C value as measured in patients’ routine blood tests since 2010 was 11.5% (6.4%–17.7%, normal values 4.0%–5.7%). Each patient’s maximal hemoglobin A1C was taken for average calculation purposes as a marker of the unbalanced diabetes. Seventeen patients were diagnosed with chronic hypertension (81.0%) and 16 were diagnosed with hyperlipidemia (76.2%). The mean number of emergency hospitalizations for nonophthalmologic reasons during the follow-up period was 2.3 (0–7).
Preoprerative examination revealed detached macula in 12 of the cases (50%) and tractions threatening the macula in nine additional cases (37.5%). The remaining three cases (12.5%) involved a thick, long-standing, nonclearing VH with tractional detachment in the posterior pole that could be demonstrated only by an ultrasound examination. Six of the cases (25%) were diagnosed with diabetic macular edema prior to the surgery.
Intraoperative and postoperative complications are described in . Retinal breaks were observed in ten of the cases (41.7%) and seven of them reported as iatrogenic (29.2%). Retinal and vitreal hemorrhages occurred in nine of the operations (37.5%).
Table 2 Intraoperative and postoperative complications in 24 cases that underwent small-gauge vitrectomy during 2011–2013
Intraocular tamponade was perfluoropropane (C3F8) in eight cases (33.3%), sulfur hexafluoride (SF6) in five cases (20.8%), silicone oil in three cases (12.5%), and air in one case (4.2%).
A statistically significant improvement in mean LogMAR VA values was found between preoperative and final follow-up examination (1.48±0.65 and 1.05±0.65, respectively, P=0.04; ). Moreover, 62.5% of the cases had a final VA ≥20/200, and 75% showed an improvement of ≥0.3 LogMAR with 95% confidence interval of 53%–90% exact by nominal confidence interval. Only 12.5% of the cases had decreased VA at the end of the follow-up period when compared with the preoperative VA. One case regressed to no light perception due to uncontrolled glaucoma, which was known prior to the surgery. A correlation between preoperative and postoperative VA was not found (r=−0.1, P=0.7; ).
Figure 1 Postoperative versus preoperative visual acuity (LogMAR).
Abbreviations: LogMAR, logarithm of the minimum angle of resolution; OP, operative.
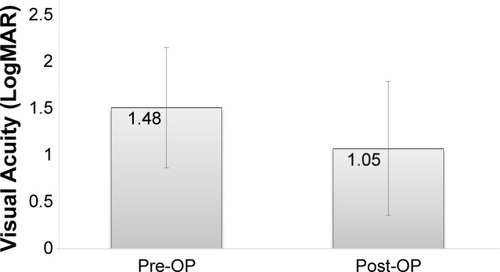
Figure 2 Linear regression analysis of the relationship between preoperative and postoperative visual acuity (VA).
Abbreviation: LogMAR, logarithm of the minimum angle of resolution.
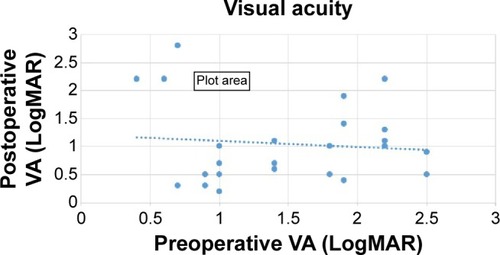
On an attempt to find a cutoff point for the feasibility of the operation, the cases were divided into two subgroups. Those with preoperative VA of LogMAR ≤1 (ten patients) and those with LogMAR >1 (14 patients). Sixty percent of those in the first group showed improvement in their final postoperative VA comparing with 86% in the second group. Fisher’s exact test did not show a significant difference between the groups; however, there is a trend toward a greater probability to improve the postoperative VA with a decreased preoperative VA (P=0.19).
Postoperative complications () of various degrees were documented in 21 cases (87.5%). Although VH occurred in 13 cases (54.2%), an additional surgery to clear the vitreous was performed only in one case, in which a very thick hemorrhage developed several days after the first surgery. In the other cases, the hemorrhage was absorbed spontaneously or with the aid of antivascular endothelial growth factor (anti-VEGF) injection. A second operation was performed in five cases (20.8%), of which three were due to nonabsorbing subretinal fluids, one due to the aforementioned VH, and one owing to a dislocated intraocular lens. Newly diagnosed postoperative glaucoma occurred in five cases (20.8%). None of the cases had new neovascularization of the iris or the angle. Additional postoperative treatment with anti-VEGF intraocular injections due to VH or macular edema was given in six of the cases (25.0%). There were no cases of postoperative hypotony or endophthalmithis.
Discussion
Although SGV became the mainstay of the management of diabetic TRD in the last decade, questions still remain regarding its ability to overcome the complexity of this procedure.Citation10,Citation13 The current study is focusing on the subgroup of diabetic TRD patients, trying to accentuate the advantages of surgery along with the accompanying complications and risks.
The results of the present study showed that VA significantly improved at the end of the follow-up period and thus suggested that SGV is indeed an effective treatment for this subgroup.
A total of 62.5% of the cases had a final VA ≥20/200 and 75% showed an improvement of ≥0.3 LogMAR. Only 12.5% of cases experienced a deterioration in VA. These results are supported by other studies.Citation13,Citation15,Citation19
Moreover, high rates (87.5%) of primary retinal reattachment were found in the present study. The ultimate reattachment rate after repeated surgeries, as evaluated in the final examination, was 100%. These findings are in accordance with previous studies.Citation2,Citation3,Citation13
There was no statistically significant correlation between the preoperative and the postoperative VA. However, a trend was found toward greater probability to have a postoperative VA improvement with a preoperative VA of LogMAR >1 than with LogMAR ≤1. This might be a subject for further research in larger future studies trying to elaborate whether there are cases in which surgery should be recommended.
On the other hand, a considerable amount of intraoperative and postoperative complications occurred in this study. Retinal breaks were found in higher rates (41.7%) compared with other studies. Kumar et alCitation15 reported an incidence of 16% when using the 23 G and 20% when using the 25 G. Similar rates of 21% were noted by Oshima et al.Citation13 Such differences may be attributed to the higher percentage of patients with preoperative VH found in the present study (62.5%), which may have prevented the diagnosis of some existing retinal breaks. Kumer et alCitation15 reported an incidence of 24% in the 23 G group and 20% in the 25 G group, whereas Oshina et alCitation13 reported an incidence of 45%.
In one prospective study on retinal breaks in SGV, a rate of 41.1% of retinal breaks was reported in diabetic patients with traction retinal detachment.Citation20 Latrogenic retinal breaks were previously reported to be related to the complexity of the case with rates of up to 78% in complex fibrovascular proliferation.Citation21
When comparing rates of retinal breaks in PDR in 20 G versus 23 G vitrectomy, lower rates of retinal breaks were reported in the latter.Citation13,Citation22,Citation23
Postoperative VH was a major complication in this study (54.3%). However, only one single case justified a second surgical intervention due to its thickness and occurrence several days postoperatively. In all other cases, postoperative VHs were reabsorbed spontaneously or with the assistance of intraocular anti-VEGF injection. Ozone et alCitation10 reported a 22% incidence of postoperative VH in their study following 25 G vitrectomies in diabetic patients with VH, TRD, or fibrovascular proliferation. A similar rate of 21.4% was noted by Altan et al.Citation19 The higher incidence found in the present study may be attributed to the patients’ poorly controlled underlying diabetes. Additionally, our study included all vitreous bleeding regardless of volume or thickness, while other studies may have disregarded negligible bleedings.
Another important postoperative complication in our study was elevated IOP in five cases (20.8%), which was unrelated to neovascularization of the iris or the anterior chamber angle. Both Wu et alCitation24 and ChangCitation25 described similar findings in their retrospective studies of vitrectomized eyes; however, they excluded cases with severe PDR. Other studies reported lower incidence of this complication.Citation26,Citation27 The mechanism suggested for this complication was increased oxidative stress in the anterior chamber after vitrectomy that damaged the trabecular meshwork.Citation24,Citation25 Elevated oxidative stress is considered an important factor in the development of diabetic retinopathyCitation28 and may therefore also amplify the risk of developing high IOP after vitrectomy by the aforementioned mechanism.
In the present study, no case of endophthalmitis was documented. Increased experience with SGV, as demonstrated by more complete vitrectomy, careful checking for wound leakage, closure of the sclerotomies by massage, and the use of angled sclerotomies dramatically decreased the incidence of endophthalmitis.Citation29
Hypotony is another common immediate postoperative complication following SGV, with incidence of up to 16%.Citation30,Citation31 However, none of the patients included in this study developed hypotony postoperatively. This could be attributed to the common use of intraocular tamponade, the use of biplanar oblique sclerotomies, and the liberal use of sutures whenever suspected leakage or incomplete closure occurred at the end of the surgery.
In most cases, inadequate treatment of diabetes over the years may have been the cause of severe retinopathy and may explain the high number of associated cardiovascular diseases and hospitalizations, and low compliance of patients to treatment.
Conclusion
In conclusion, the current study demonstrates that patients with diabetic TRD benefit from surgical intervention. Moreover, SGV is an effective and safe procedure for the management of this complex disease owing to the experience and improved instruments and techniques gained over the years. The complications associated with SGV seem to be less pronounced than thought previously.
The limitations of the current study include its small size, its retrospective nature, and lack of control group. A prospective randomized controlled trial would be beneficial and strengthen the results shown in the current study.
Disclosure
The authors report no conflicts of interest in this work.
References
- GuptaBSivaprasadSWongRVisual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK studyEye201226451051622222268
- TaoYJiangYRLiXXGaoLJonasJBLong-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachmentRetina201030344745120216292
- QamarRMSaleemMISaleemMFThe outcomes of pars plana vitrectomy without endotamponade for tractional retinal detachment secondary to proliferative diabetic retinopathyInt J Ophthalmol20136567167424195047
- NewmanDKSurgical management of the late complications of proliferative diabetic retinopathyEye201024344144920139916
- O’MalleyCHeintzRMSrVitrectomy with an alternative instrument systemAnn Ophthalmol1975745855885915941147502
- FujiiGYDe JuanEJrHumayunMSA new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgeryOphthalmology20021091018071812 discussion 181312359598
- LesnoniGRossiTGelsoA25 Gauge vitrectomy instrumentation: a different approachSemin Ophthalmol2004191–2495415590534
- EckardtCTransconjunctival sutureless 23-gauge vitrectomyRetina200525220821115689813
- ArumiJGBoixaderaAMartinez-CastilloVCorcosteguiBTransconjunctival sutureless 23-gauge vitrectomy for diabetic retinopathy. ReviewCurr Diabetes Rev200951636619199901
- OzoneDHiranoYUedaJYasukawaTYoshidaMOguraYOutcomes and complications of 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathyOphthalmologica20112262768021613797
- FineHFIranmaneshRIturraldeDSpaideRFOutcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment diseaseOphthalmology200711461197120017544779
- BourlaDHBorEAxer-SiegelRMimouniKWeinbergerDOutcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomyAm J Ophthalmol20101494630634.e120138604
- OshimaYShimaCWakabayashiTMicroincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachmentOphthalmology2009116592793819269033
- FaroukMMNaitoTSayedKMOutcomes of 25-gauge vitrectomy for proliferative diabetic retinopathyGraefes Arch Clin Exp Ophthalmol2011249336937620848125
- KumarADuraipandiKGogiaVSehraSVGuptaSMidhaNComparative evaluation of 23- and 25-gauge microincision vitrectomy surgery in management of diabetic macular traction retinal detachmentEur J Ophthalmol201424110711323709329
- ThompsonJTAdvantages and limitations of small gauge vitrectomySurv Ophthalmol201156216217221236459
- SteelDHCharlesSVitrectomy fluidicsOphthalmologica2011226suppl 1273521778777
- DugelPUZhouJAbulonDJBuboltzDCTissue attraction associated with 20-gauge, 23-gauge, and enhanced 25-gauge dual-pneumatic vitrectomy probesRetina20123291761176622466488
- AltanTAcarNKapranZUnverYBOzdoganSTransconjunctival 25-gauge sutureless vitrectomy and silicone oil injection in diabetic tractional retinal detachmentRetina20082891201120618728620
- EhrlichRGohYWAhmadNPolkinghornePRetinal breaks in small-gauge pars plana vitrectomyAm J Ophthalmol2012153586887222306371
- CarterJBMichelsRGGlaserBMDe BustrosSIatrogenic retinal breaks complicating pars plana vitrectomyOphthalmology1990977848853 discussion 8542381696
- IssaSAConnorAHabibMSteelDHComparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathyClin Ophthalmol2011510911421339803
- ParkDHShinJPKimSYComparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathyRetina201030101662167020661174
- WuLBerrocalMHRodriguezFJIntraocular pressure elevation after uncomplicated pars plana vitrectomy: results of the Pan American Collaborative Retina Study GroupRetina201434101985198924736465
- ChangSLXII Edward Jackson lecture: open angle glaucoma after vitrectomyAm J Ophthalmol200614161033104316765671
- KoreenLYoshidaNEscariaoPIncidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomyRetina201232116016721765372
- ToyokawaNKimuraHMatsumuraMKurodaSIncidence of late-onset ocular hypertension following uncomplicated pars plana vitrectomy in pseudophakic eyesAm J Ophthalmol2015159472773225597837
- BehlTKaurIKotwaniAImplication of oxidative stress in progression of diabetic retinopathySurv Ophthalmol Epub2015
- ScottIUFlynnHWJrAcarNIncidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomyGraefes Arch Clin Exp Ophthalmol2011249337738020853005
- HsuJChenEGuptaOFinemanMSGargSJRegilloCDHypotony after 25-gauge vitrectomy using oblique versus direct cannula insertions in fluid-filled eyesRetina200828793794018698294
- KellnerLWimpissingerBStolbaUBrannathWBinderS25-Gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trialBr J Ophthalmol200791794594817202200
