Abstract
Purpose
To produce a two-dimensional reconstruction map of dendritic lesions in patients with herpes simplex keratitis (HSK) using in vivo confocal microscopy.
Methods
Four eyes of four patients (mean 65.8 years) with HSK presenting with a dendritic lesion were enrolled. Slit-lamp biomicroscopy and in vivo laser confocal microscopy were performed. Acquired confocal images at the level of the epithelium were arranged and mapped into subconfluent montages. Changes in the shape and degree of light reflection of abnormal cells and deposits around dendritic lesions as well as other corneal layers were qualitatively evaluated.
Results
Mapping of dendritic lesion was successful in all cases, and the subconfluent montages clearly showed the larger image of dendritic lesion. In all cases, the dendritic lesion consisted of hyperreflective irregular epithelial cells, and was surrounded by distorted and elongated epithelial cells. In three cases, hyperreflective deposits were noted at the midline of the lesion. The corneal stroma showed a hyperreflective honeycomb pattern. In two cases, inflammatory cells were observed at the level of endothelial cell layer.
Conclusion
Mapping of dendritic lesions in patients with HSK was successful in all patients using in vivo confocal microscopy. Cellular level observation of dendritic lesion at a relatively larger magnification may help understand the in vivo morphological change of HSK. Further study in more patients with HSK and nonherpetic dendritic lesion is needed to utilize confocal microscopy images in differential diagnosis and follow-up of the epithelial lesions with dendrite.
Introduction
Herpes simplex keratitis (HSK) is still a major cause of corneal blindness in many countries.Citation1,Citation2 Clinical phenotypes of HSK include infectious epithelial keratitis, neurotrophic keratopathy, immune stromal keratitis, necrotizing stromal keratitis, and endotheliitis. Among these, the most common presentation of HSK is the dendritic lesion of infectious epithelial keratitis. Dendritic lesions may occur with a primary herpes simplex virus infection; however, they occur more commonly due to the reactivation of latent virus. Dendritic lesions are characterized by a branching, linear lesion with a terminal bulb and swollen epithelial borders, all of which are visualized well with slit-lamp biomicroscopy with fluorescein staining.
Recently, in vivo confocal microscopy has become an increasingly popular noninvasive method to facilitate the understanding of the corneal microstructure in the healthy as well as the diseased cornea.Citation3 Prior studies have evaluated the corneal involvement of HSK with different types of confocal microscopes, each investigating the subbasal nerve plexus, the epithelium, the endothelium, or the inflammatory response.Citation4–Citation8 So far, only a single case report was published that described in vivo confocal microscopic features of the dendritic lesion.Citation9 In the current study, to further elucidate the cellular level morphological change of dendritic lesion at a wider angle, we produced larger two-dimensional reconstruction confocal map of the dendritic lesion.
Materials and methods
This clinical study was approved by the Ethical Committee of Kanazawa University Graduate School of Medical Science and followed the tenets of the Helsinki Declaration. Four eyes of four patients (65.8±21.5 years of age) with HSK presenting with a dendritic lesion were evaluated in this study (). The diagnosis of HSK was made according to characteristic clinical features, including dendritic lesion with terminal bulb, history of recurrent HSK, and response to acyclovir treatment (). Only in Case 4 was herpes simplex virus detected by immunochromatography of an epithelial sample.
Figure 1 Fluorescein slit-lamp photographs of dendritic ulcers with terminal bulbs in Case 1 (A), Case 2 (B), Case 3 (C), and Case 4 (D).
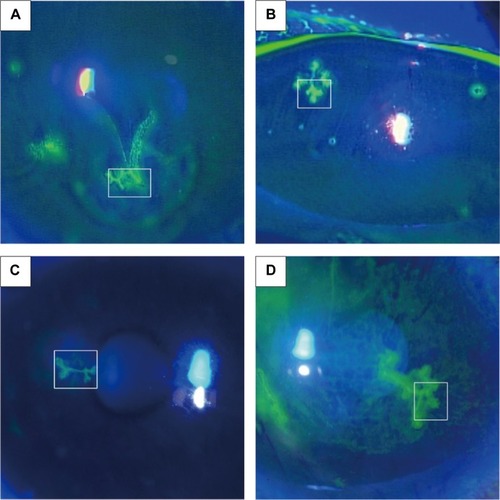
Figure 2 Subconfluent montages of in vivo confocal microscopic images at the area of dendritic lesion in Case 1 (A), Case 2 (B), Case 3 (C), and Case 4 (D). (Bar =200 μm).
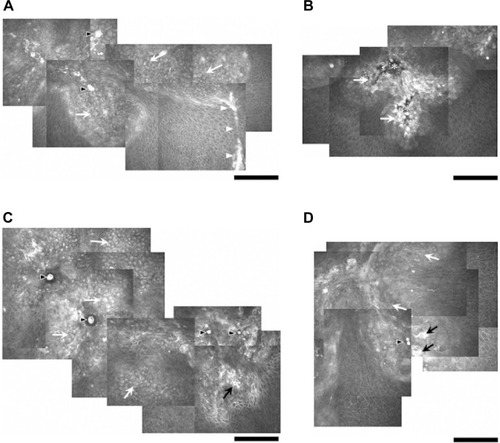
Table 1 Clinical data of four patients with herpes simplex keratitis
In vivo laser confocal microscopy
After the nature and possible consequences of confocal microscopic study (such as superficial punctate keratopathy) were explained to the patients, informed consent was obtained for confocal microscopic examination. For each patient, a fluorescein slit-lamp photograph demonstrating dendritic lesion was obtained. Each dendritic lesion was manually scanned at the level of the epithelium with a Heidelberg Retina Tomograph 2 Rostock Cornea Module (HRT2-RCM, Heidelberg Engineering GmbH, Dossenheim, Germany) with a 60× water-immersion objective lens (Olympus, Tokyo, Japan) (numerical aperture, 0.9). The microscope utilizes a 670-nm red wavelength diode laser as the light source (area of observation, 400×400 μm2). The manufacturer quotes the transverse resolution and optical section thickness as 2 and 4 μm, respectively.
Two-dimensional reconstruction mapping
Acquired confocal images at the level of the epithelium were arranged and mapped into subconfluent montages with a mapping technique and computer software (Adobe Photoshop, Adobe Systems, San Jose, CA, USA) as previously described.Citation10–Citation11 Changes in the shape and degree of light reflection of abnormal cells and deposits around dendritic lesions as well as other corneal layers were qualitatively evaluated.
Results
A total of 530.5±188.0 in vivo confocal microscopic images (400×400 μm2) of all cell layers were obtained for each patient; of them, a mean of 8.5±2.5 images of the epithelial layer at the area of the dendritic lesion were used for sub-confluent montage formation (). The mean dimensions of the mapped areas were 1.0±0.2 mm (horizontal) and 0.8±0.2 mm (vertical).
Mapping of dendritic lesion was successful in all cases, and the subconfluent montages showed clearly the larger image of dendritic lesion. In all cases, the dendritic lesions consisted of hyperreflective irregular epithelial cells, and were surrounded by distorted and elongated epithelial cells. In three cases (75%, Cases 1, 2, and 4), hyperreflective deposits, which may represent necrotic abnormal cells, were noted at the midline of the lesion. In Case 2, the central area of dendritic lesion seemed dark, indicating an epithelial defect. Putative dendritic inflammatory cells were sparsely noted.
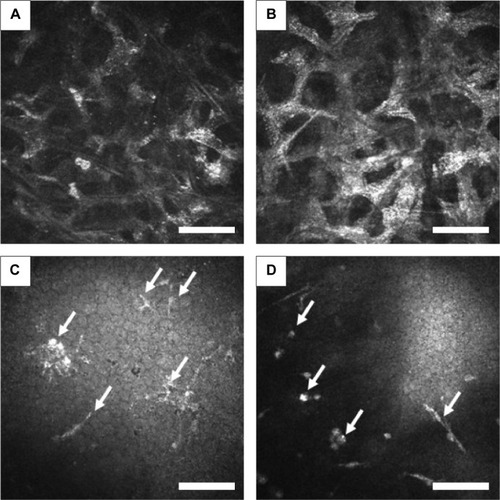
At the level of deep epithelium to subbasal nerve plexus, numerous inflammatory cells including possible Langerhans cells were noted, and were not exclusively located at the dendritic lesion (). At the level of corneal stroma, honeycomb-shaped hyperreflective keratocytes were seen in all cases, suggesting stromal edema (). At the level of endothelium, inflammatory cells were only seen in two cases (Cases 1 and 4) ().
Figure 3 In vivo confocal microscopic images at the level of deep epithelium to subbasal nerve plexus in Case 1 (A), Case 2 (B), Case 3 (C), and Case 4 (D).
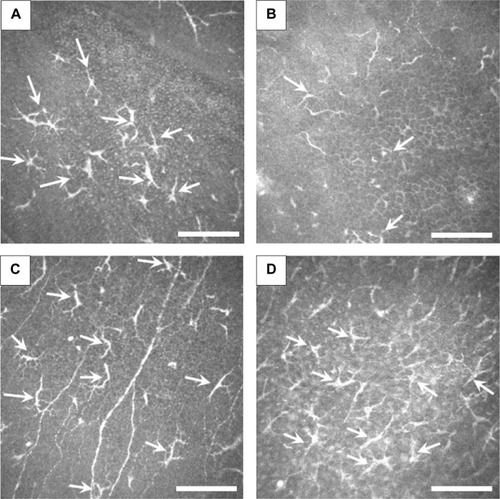
Figure 4 In vivo confocal microscopic images at the level of stroma and endothelium.
Discussion
In the current study, we investigated the confocal microscopic characteristics of in vivo epithelial changes of dendritic lesion as well as other corneal layers using similar mapping technique. As a result, larger images of the dendritic lesion were successfully constructed. In all cases, the dendritic lesion consisted of hyperreflective irregular epithelial cells, and was surrounded by distorted and elongated epithelial cells. Based on previous histopathological examinations,Citation12–Citation17 these cells may represent herpes simplex virus-infected cells, as they were multinucleated giant cells and cells with intranuclear inclusions with koilocytic changes. Hyperreflective deposits were frequently observed (75%) within the dendritic lesion, especially at the branching point. We surmise that they might be infected necrotic abnormal cells. A histopathological study by Maudgal et alCitation12 showed that dendritic ulcers had necrotic areas with fragmented nuclei and ghost cells debris. The usefulness of a mapping technique using numerous in vivo confocal microscopy images was previously reported for visualizing the subbasal nerve plexus,Citation18,Citation19 Kobayashi-structures (K-structures, sub-Bowman’s fibrous structures correlated with anterior mosaic),Citation10 and endothelial cells in cases of cytomegalovirus corneal endotheliitis.Citation11
In addition to the epithelial findings, we found that numerous Langerhans cells were characteristically observed at the level of deep epithelium to subbasal nerve plexus. It should be noted that the Langerhans cells were not exclusively located at the dendritic ulcer lesion. At the level of corneal stroma, honeycomb-shaped hyperreflective keratocytes were seen in all cases. Also, at the level of endothelium, inflammatory cells were only seen in two cases. We surmise that these changes were nonspecific inflammatory response of the cornea caused by HSK.
To date, several in vivo confocal microscopic studies in both epithelial and nonepithelial type HSK have investigated subbasal nerve plexus abnormalities (density, branch, etc) and epithelial/stromal cell abnormalities (density, size, etc) with different types of confocal microscopes.Citation4–Citation8 For example, Rosenberg et alCitation4 reported that dendritic structures, possibly Langerhans cells, were characteristically observed, at the level of the basal epithelium in resolved HSK cases, using a tandem scanning confocal microscope. Their findings were consistent with our observation, in which there were numerous Langerhans cells at the level of deep epithelium to subbasal nerve plexus (). In terms of corneal nerve changes due to HSK, Nagasato et alCitation5 reported decreases in subbasal nerve density parameters in epithelial and stromal-type HSK using HRT2-RCM, the identical device used in this study. Additionally, Hamrah et alCitation6,Citation7 reported that HSK-induced superficial epithelial changes included increases in epithelial cell size and decreases in epithelial cell density with Confoscan 4, and they concluded that these epithelial cell changes correlated with corneal innervation. On the other hand, Mocan et alCitation8 observed inflammatory cell infiltration and keratic precipitates in nonepithelial-type HSK with Confoscan 3.0. Interestingly, Martone et alCitation9 have already published a single case report of in vivo confocal microscopic analysis of dendritic lesion in a patient with bilateral HSK. They used a same confocal device with ours (HRT2-RCM), and obtained quite similar findings with our study: a distortion of the superficial and basal epithelium, the presence of irregular hyperreflective structures, and dendritic particles near the epithelial cells.
One limitation of this study is that the sample size was quite small (n=4). Also, nonexistence of the nonherpetic controls makes the study just an observational one. Nonetheless, we believe that further comparative studies of nonherpetic dendrites (pseudodendrite) frequently seen in early-stage Acanthamoeba keratitis would make the current study more clinically significant. Additionally, it might be important to study the treated healing lesions as a future research.
In conclusion, mapping of dendritic lesions in patients with HSK was successful in all patients using in vivo confocal microscopy. Cellular level observation of dendritic lesion at relatively larger image may help understand the in vivo morphological change of HSK. A further study in more patients with HSK and nonherpetic dendritic lesion is needed to utilize confocal microscopy images in the differential diagnosis and follow-up of the epithelial lesions with dendrite.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research KAKENHI, Japan (No 23890072). The funding organizations had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiesegangTJHerpes simplex virus epidemiology and ocular importanceCornea20012011311188989
- HollandEJSchwartzGSNeffKDHerpes simplex keratitisKrachmerJHMannisMJHollandEJCorneaPhiladelphia, PAElsevier Mosby2011953984
- CavanaghHDPetrollWMAlizadehHHeYGMcCulleyJPJesterJVClinical and diagnostic use of in vivo confocal microscopy in patients with corneal diseaseOphthalmology1993100144414548414403
- RosenbergMETervoTMMüllerLJMoilanenJAVesaluomaMHIn vivo confocal microscopy after herpes keratitisCornea20022126526911917174
- NagasatoDAraki-SasakiKKojimaTIdetaRDogruMMorphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitisJpn J Ophthalmol20115544445021830060
- HamrahPCruzatADastjerdiMHCorneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy studyOphthalmology20101171930193620810171
- HamrahPSahinADastjerdiMHCellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy studyOphthalmology20121191791179722608476
- MocanMCIrkecMMikropoulosDGBozkurtBOrhanMKonstasAGIn vivo confocal microscopic evaluation of the inflammatory response in non-epithelial herpes simplex keratitisCurr Eye Res2012371099110622799438
- MartoneGAlegenteMBalestrazziAIn vivo confocal microscopy in bilateral herpetic keratitis: a case reportEur J Ophthalmol200818699499718988174
- YokogawaHKobayashiASugiyamaKMapping of normal corneal K-structures by in vivo laser confocal microscopyCornea200827887988318724147
- YokogawaHKobayashiASugiyamaKMapping owl’s eye cells of patients with cytomegalovirus corneal endotheliitis using in vivo laser confocal microscopyJpn J Ophthalmol201357808423124832
- MaudgalPCMissottenLHistopathology of human superficial herpes simplex keratitisBr J Ophthalmol1978624652629910
- NakagawaHUchidaYTakamuraEDiagnostic impression cytology for herpes simplex keratitisJpn J Ophthalmol1993375055138145397
- KimJHKoMKShinJCInfectivity of basal epithelial cells in herpetic dendritic epithelial keratitisKorean J Ophthalmol19971184889510649
- AthmanathanSBandlapallySRRaoGNCollection of corneal impression cytology directly on a sterile glass slide for the detection of viral antigen: an inexpensive and simple technique for the diagnosis of HSV epithelial keratitis – a pilot studyBMC Ophthalmol20011311592921
- HolbachLMFontRLWilhelmusKRRecurrent herpes simplex keratitis with concurrent epithelial and stromal involvement. Immunohistochemical and ultrastructural observationsArch Ophthalmol19911096926951850978
- BlaumJLMorphogenesis of the dendritic figure in herpes simplex keratitis. A negative studyAm J Ophthalmol1970707227245477159
- PatelDVMcGheeCNMapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopyInvest Ophthalmol Vis Sci2005464485448816303938
- PatelDVMcGheeCNMapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopyInvest Ophthalmol Vis Sci2006471348135116565367
