Abstract
Purpose
Many patients with glaucoma require combination therapies to achieve target intraocular pressure (IOP) and preserve visual function. Ocular hypotensives often contain a preservative (eg, benzalkonium chloride [BAK]), but preservative-free (PF) formulations have been developed for patients with sensitivity. A Phase III study found the efficacy of bimatoprost 0.03%/timolol 0.5% (bim/tim, Ganfort®) PF to be equivalent to that of preserved bim/tim, although a trend favoring bim/tim PF was observed. As BAK is a corneal penetration enhancer, this literature review aims to explain these findings by exploring the relationship between timolol concentration and its IOP-lowering effect.
Methods
Systematic searches were performed in Scopus and PubMed for clinical trials published in English between 1960 and July 2014 using the keywords “timolol”, “intraocular pressure”, and the concentrations “1%, 0.5%, OR 0.25%”. Articles that directly compared IOP-lowering effects of ≥2 concentrations of timolol were identified by manual screening, and cross-checked for duplication.
Results
Seventeen studies that included 10–371 patients were evaluated; the majority were randomized (16/17), double-masked (14/17), and enrolled patients with open-angle glaucoma or ocular hypertension (12/17). All studies investigated timolol in preserved formulations. Timolol concentrations tested ranged from 0.008% to 1.5%. Of 13 studies comparing timolol 0.25% versus 0.5%, two found the 0.25% dose to have greater IOP-lowering effects, and three reported the opposite; eight reported similar IOP lowering. Results also indicate that timolol 0.5% may be more effective than higher concentrations.
Conclusion
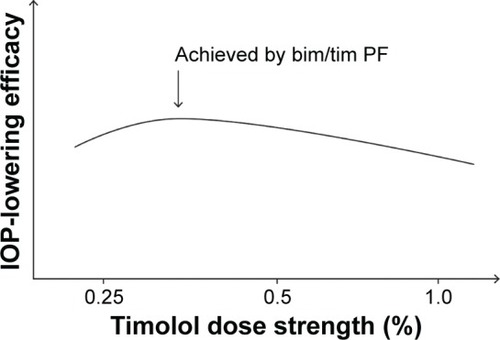
The evidence suggests that timolol may have an inverted U-shaped dose–response curve, and that its optimal IOP-lowering concentration is between 0.25% and 0.5%. Compared with bim/tim, removal of the permeability enhancer BAK in bim/tim PF could have resulted in a lower timolol concentration at the target site, bringing the effective concentration within the 0.25%–0.5% range and enhancing the efficacy of bim/tim PF.
Introduction
Worldwide, open-angle glaucoma (OAG) is estimated to affect almost 45 million adults over the age of 40 years, and the number is expected to reach 59 million by 2020.Citation1 Since the loss of vision associated with glaucoma is irreversible,Citation2 early diagnosis and treatment are key to preserving visual function. Because intraocular pressure (IOP) is a major risk factor in glaucoma and glaucoma suspects, all currently marketed treatments for glaucoma aim to lower IOP. Accordingly, the administration of topical agents that reduce the production of aqueous humor and/or increase outflow is the mainstay of therapy.Citation2–Citation6
Multidose formulations of topical antiglaucoma medications contain preservatives, of which the most commonly used is benzalkonium chloride (BAK), a potent bactericidal and fungicidal agent.Citation7,Citation8 The majority of patients tolerate BAK, but it has been associated with ocular sensitivity in some cases.Citation9–Citation12 Patients who require multiple medications to reach/maintain target IOP may be at higher risk of BAK sensitivity, as are those with severe dry eye disease because of reduced dilution in the tear film.Citation9 Consequently, preservatives causing less irritation, as well as many single-dose, preservative-free (PF) formulations, have been developed. In clinical trials and clinical practice, PF formulations have demonstrated noninferior or equivalent efficacy to their respective preserved formulations, with potential for a reduced incidence of ocular adverse events.Citation13–Citation22
Bimatoprost/timolol (bim/tim) ophthalmic solution (Ganfort®; Allergan plc, Irvine, CA, USA) is a fixed-combination formulation of bimatoprost 0.03% (a synthetic prostamide) and timolol 0.5% (a nonselective β-adrenergic receptor antagonist) preserved with BAK.Citation23 Clinical trials have demonstrated that when dosed once daily, the fixed combination produces greater IOP reduction than each of the active components dosed as monotherapy.Citation24,Citation25 Accordingly, bim/tim is used in a number of countries worldwide to treat patients with primary OAG (POAG) or ocular hypertension (OHT) who do not reach target IOP with monotherapy. However, because of the increasing awareness of patient sensitivity to preservatives, bim/tim PF (Allergan plc, Irvine, CA, USA) was developed; this PF formulation is identical to the original bim/tim formulation, except for the removal of 50 ppm of BAK.
In a 12-week, multicenter, randomized, Phase III study of 561 patients with OAG or OHT, bim/tim PF demonstrated noninferiority and equivalence to bim/tim in terms of IOP-lowering efficacy (without significant differences in safety/tolerability),Citation26 which led to its marketing approval in the European Union in September 2013.Citation27 Despite these findings, when the between-treatment difference in average eye IOP was compared, a consistent trend toward lower IOP favoring bim/tim PF over bim/tim was observed in seven of the nine time points measured.Citation26 This observation suggested that BAK removal might have enhanced the efficacy of bim/tim. Given that BAK is a well-known permeability enhancer that has been reported to increase drug penetration into ocular tissues,Citation28–Citation31 it was surprising that bim/tim PF produced a greater IOP-lowering effect than the bim/tim formulation containing BAK.Citation26
A preliminary literature search exploring possible explanations for this clinical observation found data suggesting that timolol in preserved ophthalmic solutions may display an inverted, U-shaped dose–response curve for IOP lowering, with an optimal concentration between 0.25% and 0.5%.Citation32 Removal of BAK from 0.5% timolol ophthalmic solution formulations could reduce timolol bioavailability enough to achieve a concentration that optimizes intraocular efficacy.Citation32 This literature review aims to explain the findings of the Phase III study demonstrating enhanced efficacy of bim/tim PF over preserved bim/tim by exploring the relationship between the concentration of timolol in preserved ophthalmic solutions and its IOP-lowering efficacy.
Methods
Study selection
An initial search of the literature for timolol dose–response in humans was performed in Scopus (Elsevier, Philadelphia, PA, USA). Articles that were published in English between 1960 and July 2014 were identified using the keywords “timolol AND (intraocular pressure OR IOP) AND human (or derivatives) AND (1% OR 0.5% OR 0.25%)”, and screened for relevance to IOP lowering after ocular instillation of topical timolol 0.25%, 0.5%, or 1.0%. Similarly, a literature search spanning the years 1960 to July 2014 was conducted in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using the keywords “timolol AND (intraocular pressure OR IOP) AND (1% OR 0.5% OR 0.25%)”, with filters set to display articles involving human patients in clinical trials published in English. Articles that reported direct comparisons of the effect of at least two concentrations of timolol on IOP in adult patients were included. Studies in which patient subgroups received a higher concentration of timolol after responding poorly to an initial lower concentration were excluded.
Results and discussion
Selected studies
Given its long history in the management of glaucoma, many reports of the IOP-lowering effects of timolol have been published, but only a few provide data related to dose response. The Scopus search identified 548 citations, eleven of which contained relevant studies. A comparable search in PubMed yielded 607 references, including ten relevant clinical trials. Accounting for overlap, the Scopus and PubMed searches together found a total of 17 relevant studies ().
A summary of each of the 17 publications identified is presented in . All trials were randomized except one,Citation33 and 14 were double-masked.Citation34–Citation47 Eight studies included parallel groups,Citation34,Citation37,Citation41,Citation43,Citation44,Citation46–Citation48 six involved dose escalation,Citation33,Citation35,Citation38,Citation42,Citation45,Citation49 and three had one or two crossover(s).Citation36,Citation39,Citation40 Five studies enrolled healthy volunteers,Citation34,Citation38,Citation42,Citation43,Citation49 whereas the other 12 enrolled patients with OAG or OHT. Sample size ranged from ten to 371 patients.
Table 1 Summary of selected clinical trials
Overall IOP response to topical timolol
Timolol is commonly prescribed as a 0.25% or 0.5% preservative-containing (usually BAK) ophthalmic solution. However, the selected studies covered a broader range of concentrations: 0.008%, 0.025%, 0.0625%, 0.08%, 0.1%, 0.125%, 0.25%, 0.5%, 1.0%, and 1.5% (). Nine studies measured IOP after weeks to months of timolol administration onceCitation40,Citation48 or twiceCitation33,Citation35–Citation37,Citation41,Citation44,Citation45 daily. The remaining eight studies assessed the short-term (ie, within ≤28 hours) IOP-lowering effect following a single application.Citation34,Citation38,Citation39,Citation42,Citation43,Citation46,Citation47,Citation49
All studies that evaluated the efficacy of timolol over weeks or months found that the concentrations tested (ie, 0.1%, 0.25%, 0.5%, and 1%) provided statistically significant IOP reductions compared with baseline and/or controls (untreated or placebo-treated eyes), regardless of the instillation schedule (ie, once versus twice daily).Citation33,Citation35–Citation37,Citation40,Citation41,Citation44,Citation45,Citation48 However, results of short-term studies varied, depending on the study population. In patients with elevated IOP, concentrations of 0.1%, 0.25%, 0.5%, 1.0%, and 1.5% provided statistically significant IOP reductions compared with baseline and/or controls (untreated or placebo-treated eyes).Citation34,Citation38,Citation43,Citation46,Citation47 Even a concentration as low as 0.008% (administered as gel or solution) was effective in lowering IOP in patients with OHT and a baseline IOP >22 mmHg, compared with placebo controls.Citation39 In contrast, studies of healthy volunteers with baseline IOP of approximately 13–14 mmHg reported no significant IOP-lowering effect when treated with timolol 0.008% or 0.025%,Citation42 or 0.0625%, 0.125%, or 0.25%.Citation49
Determining the optimal dose – timolol 0.5% versus higher concentrations
Four studies compared the IOP-lowering effect of timolol ophthalmic solution at concentrations of 0.5% or greater and found that timolol 0.5% was as effective as or more effective than higher concentrations. Three of these studies involved a single application of drug.Citation38,Citation46,Citation47 In a parallel-group, single-dose study in 30 patients with OAG, timolol 0.5% was as effective as or numerically more effective than timolol 1.5% at six of eight time points measured ().Citation46 In a similarly designed study in 20 patients with OAG, timolol 0.5% was as effective as or numerically more effective than timolol 1.0% at the 4-, 12-, and 24-hour time points ().Citation47 Also, in a dose escalation study in 30 healthy volunteers, Katz et alCitation38 found that IOP reduction from baseline was numerically greater at 3, 5, and 7 hours after a single application of timolol 0.5% (25%, 26%, and 23%), compared with timolol 1.0% (23%, 19%, and 22%) or 1.5% (24%, 17%, and 17%), respectively. Timolol 0.5% was also numerically more effective than timolol 1.0% at 2 hours postinstillation (). The fourth and most clinically relevant study involved administration of timolol 0.5% and 1.0% twice daily for 1 week in a dose escalation design,Citation45 and found that patients with OHT or OAG had a numerically lower mean IOP at four of six postinstillation time points during treatment with timolol 0.5% than during treatment with timolol 1.0% ().Citation45
Figure 2 Timolol 0.5% is at least as effective as timolol 1.0% and 1.5% at lowering IOP.
Abbreviations: IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension.
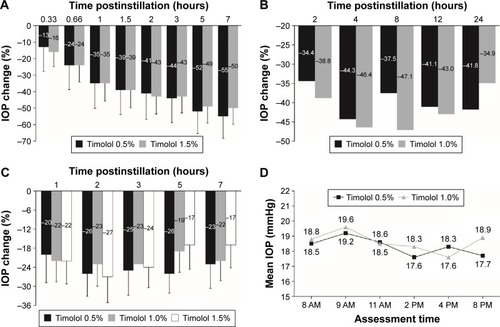
The consistent findings suggest that on a concentration basis, timolol 0.5% is more effective than higher concentrations. Such an inverted U-shaped dose–response relationship, in conjunction with the systemic cardiovascular risk associated with timolol as a nonselective β-adrenoceptor antagonist,Citation50–Citation56 explains why timolol is not prescribed at a dose strength higher than 0.5%.
Determining the optimal dose – timolol 0.25% versus 0.5%
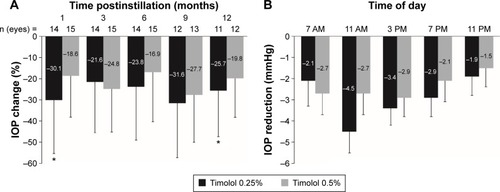
Of the 17 studies, 13 compared timolol 0.25% and 0.5%, currently the most commonly used concentrations. Among these, two studies showed that timolol 0.25% was either significantly or numerically more effective than timolol 0.5% in IOP lowering. The first, a parallel-group study of timolol administered twice daily over 12 months, indicated that when statistically significant differences were found between doses, they always favored timolol 0.25% ().Citation41 The second, a double-masked, three-phase study of the effects of timolol 0.25% and 0.5% administered once daily for 10 days, reported that timolol 0.25% was numerically (but not statistically significantly) better than the 0.5% concentration in lowering IOP over the majority of time points measured ().Citation40
Figure 3 IOP lowering with timolol 0.25% is greater than that with timolol 0.5%.
Abbreviations: IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension.
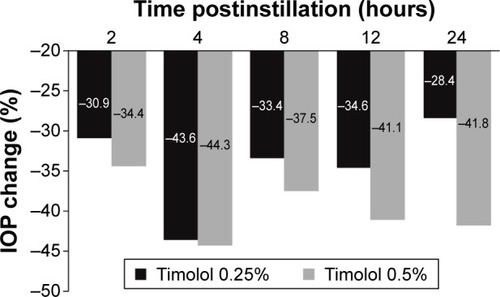
Three other studies provided data supporting a dose-dependent difference in IOP-lowering favoring the 0.5% concentration. Results from a 1-week, dose escalation study of twice-daily administration indicated that IOP lowering from baseline was numerically greater with timolol 0.5% (21% and 23%) than with timolol 0.25% (17% and 20%) at 1 and 6 hours postinstillation, respectively, but similar or greater with timolol 0.25% (21% and 24%) than with timolol 0.5% (21% and 15%) at 3 and 8 hours postinstillation, respectively.Citation45 However, the IOP-lowering effect appeared to be better sustained at 12 hours postinstillation (trough) with timolol 0.5% (19%) than timolol 0.25% (11%).Citation45 Similarly, two short-term studies with assessments that spanned 90 minutesCitation43 and 28 hoursCitation47 postinstillation of timolol 0.25% or 0.5% found a dose-dependent, numerical difference in IOP lowering at later time points that favored the 0.5% concentration (), although statistical analysis of the difference between concentrations was not provided.
Figure 4 IOP lowering with timolol 0.5% is greater than that with timolol 0.25%.
Abbreviation: IOP, intraocular pressure.
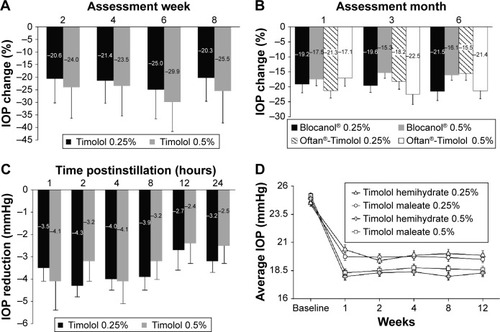
The remaining eight studies concluded that there was no difference in IOP lowering between timolol 0.25% and 0.5%. In a double-masked, three-period crossover study of timolol administered twice daily for 4 weeks, the mean IOP reduction at study end was 11.31±3.18 mmHg (34.67%) with timolol 0.25% versus 12.03±3.72 mmHg with timolol 0.5% (35.95%; P>0.5).Citation36 Findings were comparable when timolol 0.25% and 0.5% were dosed once daily for 8 weeks (),Citation48 or twice daily for 6 months ().Citation44 In a randomized, double-masked, dose escalation study, timolol 0.25% induced a 26% reduction in IOP from baseline when administered twice daily for 1 week. An increase in concentration to timolol 0.5% only produced a modest, additional IOP reduction of approximately 4%, but a statistical analysis of the between-concentration difference in IOP lowering was not provided.Citation35 In a similar dose escalation study of timolol 0.25% and 0.5% twice daily, increasing the concentration from 0.25% to 0.5% did not result in significant additional IOP lowering from baseline; IOP reduction reached 25% and 27% after 3–4 weeks of treatment with timolol 0.25% and 0.5%, respectively.Citation33 A short-term study that assessed IOP levels at 1, 2, 4, 6, 8, 12, and 24 hours postinstillation and included statistical analysis of the between-concentration difference in IOP lowering from baseline also concluded that the effect of timolol 0.5% was not superior to that of timolol 0.25% ().Citation34 An area under the curve analysis of IOP versus time (ie, 12 and 24 hours) yielded similar values for both concentrations: 43.6±6.2 and 74.9±11.0 for timolol 0.25% versus 38.1±9.4 and 69.1±16.4 for timolol 0.5%, respectively.Citation34 Importantly, the largest study identified in this systematic review compared the IOP-lowering efficacy of timolol 0.25% and 0.5% administered twice daily as a maleate or hemihydrate solution over 8 weeks in 371 patients with POAG or OHT.Citation37 The authors concluded that when either formulation was administered, the 0.25% and 0.5% dose strengths were equally effective from week 1 to week 12 (); equivalence between the two concentrations at the final visit was established by statistical analysis.
Figure 5 IOP lowering is similar with timolol 0.25% and 0.5%.
Abbreviations: IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension.
Taken together, the aforementioned results suggest that an inverted U-shaped dose–response curve may exist for timolol in terms of IOP lowering, and that the concentration that elicits maximum IOP lowering likely lies between 0.25% and 0.5%, as illustrated in . A possible explanation for the inverted U-shaped dose–response curve might involve receptor upregulation and tachyphylaxis. The association between repeated ocular timolol use and tachyphylaxis has long been described,Citation57–Citation65 and although some patients exhibit relatively stable IOP reductions for years in response to timolol ophthalmic solution, some demonstrate an upward IOP drift after days or months, reflecting a partial or complete loss of response to timolol.Citation59,Citation62 Timolol concentrations of 0.5% and higher may be associated with an increased occurrence of receptor upregulation,Citation62,Citation66,Citation67 which leads to tachyphylaxis. In contrast, there has been no report of an association between bimatoprost and tachyphylaxis in the literature, and preclinical studies of bimatoprost in dogs and monkeys have revealed a flat dose–response curve between 0.001% and 0.1%, suggesting that preservative removal is unlikely to affect the effective concentration of bimatoprost.Citation68
Conclusion
The evidence gathered appears to support our hypothesis that the optimal IOP-lowering concentration of timolol lies between 0.25% and 0.5% when administered as a BAK-preserved formulation. The majority of studies found no significant differences in IOP lowering between the 0.25% and 0.5% concentrations, and some studies showed statistically significantly increased efficacy with the 0.25% concentration. Therefore, if the removal of BAK would result in lower ocular concentrations of timolol in patients treated with bim/tim PF (containing 0.5% timolol) than in those treated with preserved bim/tim (also containing 0.5% timolol), it is reasonable to believe that the reduced exposure to timolol 0.5% in the PF formulation at the target site may bring the effective concentration within the 0.25%–0.5% range, thus maximizing the IOP-lowering effect of timolol. This supposition, however, should be considered in light of the limitations of the available data: the variability of the study designs, the small sample sizes of some studies, and the lack of studies that compared the efficacy of various concentrations of timolol in preserved versus PF formulations.
Timolol ophthalmic solution was a much needed breakthrough for the treatment of OAG. Early pharmacokinetic and pharmacodynamic studies showed that timolol is well absorbed through the cornea and rapidly distributes into ocular tissues following topical ocular administration; it can be measured in the human aqueous humor for up to 12 hours. Timolol significantly lowered IOP in healthy volunteers and in patients with OAG, and in dose ranging studies, the maximum effect appeared to occur with the 0.5% concentration.Citation69 The current knowledge of the dose–response relationship between IOP lowering and timolol concentration in preserved topical ophthalmic solutions is highlighted in our systematic literature review. Studies that evaluated the efficacy of timolol over weeks or months in patients with OAG or OHT are likely more relevant to our hypothesis as they are indicative of the relationship between efficacy and steady-state concentrations of timolol in the eye (compared with studies assessing the IOP-lowering effects within minutes or hours of treatment with a single dose). These studies concluded that timolol ophthalmic solutions at 0.1%, 0.25%, 0.5%, and/or 1% provide statistically significant IOP reductions from baseline and/or compared with untreated or placebo-treated eyes, regardless of the instillation schedule. Nevertheless, all but two short-term studies (including one study in eleven patients that could not detect significant IOP reduction at every time point with timolol 0.5%), reached the same conclusion, even for timolol concentrations ≤0.1%.
Overall, these data suggest that on a concentration basis, timolol 0.5% is more effective at lowering IOP than higher concentrations, and that the optimal IOP-lowering concentration of timolol is likely between 0.25% and 0.5%. Furthermore, these findings support the clinical observation that removal of BAK from the bim/tim formulation provided more optimal IOP reduction.
Acknowledgments
This article was sponsored by Allergan plc. Medical writing and editorial assistance was provided to the authors by Michele Jacob, PhD, and Jennifer Bodkin, PhD, of Evidence Scientific Solutions (Philadelphia, PA, USA), and funded by Allergan plc.
Disclosure
Jie Shen and Marina Bejanian are employees of Allergan plc. The authors report no other conflicts of interest in this work.
References
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- BagnisAPapadiaMScottoRTraversoCECurrent and emerging medical therapies in the treatment of glaucomaExpert Opin Emerg Drugs201116229330721406029
- American Academy of OphthalmologyPreferred Practice Pattern® Guidelines. Primary Open-Angle Glaucoma [homepage on the Internet]San Francisco, CAAmerican Academy of Ophthalmology (AAO) Glaucoma Panel2010 Available from: http://www.aaojournal.org/article/S0161-6420(15)01276-2/pdfAccessed January 29, 2016
- MarquisREWhitsonJTManagement of glaucoma: focus on pharmacological therapyDrugs Aging200522112115663346
- McKinnonSJGoldbergLDPeeplesPWaltJGBramleyTJCurrent management of glaucoma and the need for complete therapyAm J Manag Care200814Suppl 1S20S2718284312
- StamperRLIntroduction and classification of the glaucomasStamperRLLiebermanMFDrakeMVBecker-Shaffer’s Diagnosis and Therapy of the Glaucomas8th edAmsterdam, the NetherlandsMosby Elsevier200917
- LouatiYShaarawyTControversy: is benzalkonium chloride necessary in antiglaucoma drops?J Curr Glaucoma Pract201263104107
- NoeckerRMillerKVBenzalkonium chloride in glaucoma medicationsOcul Surf20119315916221791190
- BaudouinCLabbéALiangHPaulyABrignole-BaudouinFPreservatives in eyedrops: the good, the bad and the uglyProg Retin Eye Res201029431233420302969
- TrocmeSHwangLJBeanGWSultanMBThe role of benzalkonium chloride in the occurrence of punctate keratitis: a meta-analysis of randomized, controlled clinical trialsAnn Pharmacother201044121914192121119099
- LeungEWMedeirosFAWeinrebRNPrevalence of ocular surface disease in glaucoma patientsJ Glaucoma200817535035518703943
- StewartWCStewartJANelsonLAOcular surface disease in patients with ocular hypertension and glaucomaCurr Eye Res201136539139821501071
- de JongCStolwijkTKuppensEde KeizerRvan BestJTopical timolol with and without benzalkonium chloride: epithelial permeability and autofluorescence of the cornea in glaucomaGraefes Arch Clin Exp Ophthalmol199423242212248034210
- BronAVelasqueLRebicaHPouliquenPElenaPPRoulandJFComparaison du timolol sans conservateur et du timolol à délivrance prolongée donnés une fois par jour en association à du latanoprost. [Comparison of once-daily nonpreserved timolol and timolol maleate gel-forming solution associated with latanoprost]J Fr Ophthalmol2004279 Pt 1971977 French
- FrezzottiPFogagnoloPHakaGIn vivo confocal microscopy of conjunctiva in preservative-free timolol 0.1% gel formulation therapy for glaucomaActa Ophthalmol2014922e133e14024020826
- ManniGCentofantiMOddoneFParravanoMBucciMGInterleukin-1β tear concentration in glaucomatous and ocular hypertensive patients treated with preservative-free nonselective beta-blockersAm J Ophthalmol20051391727715652830
- MastropasquaLAgnifiliLFasanellaVConjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology studyActa Ophthalmol2013915e397e40523601909
- RoulandJFTraversoCEStalmansIfor the T2345 Study GroupEfficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucomaBr J Opthalmol2013972196200
- UusitaloHKaarnirantaKRopoAPharmacokinetics, efficacy and safety profiles of preserved and preservative-free tafluprost in healthy volunteersActa Ophthalmol Suppl (Oxf)200824271318752509
- JaenenNBaudouinCPouliquenPManniGFigueiredoAZeyenTOcular symptoms and signs with preserved and preservative-free glaucoma medicationsEur J Ophthalmol200717334134917534814
- PisellaPJPouliquenPBaudouinCPrevalence of ocular symptoms and signs with preserved and preservative free glaucoma medicationBr J Ophthalmol200286441842311914211
- BronAChiambarettaFPouliquenPRigalDRoulandJFIntérêt de la substitution d’un traitement journalier de 2 instillations de timolol par 1 instillation quotidienne de bêtabloquant non conservé chez des patients présentant un glaucome chronique ou une hypertonie oculaire [Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension]J Fr Ophtalmol2003267668674 French13130253
- European Medicines AgencyGanfort® – Summary of Product Characteristics [homepage on the Internet]Irvine, CAAllergan, plc2006 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000668/WC500020625.pdfAccessed April 2, 2015
- BrandtJDCantorLBKatzLJBatoosinghALChouCBossowskaIfor the Ganfort Investigators Group IIBimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertensionJ Glaucoma200817321121618414107
- LewisRAGrossRLSallKNSchiffmanRMLiuCCBatoosinghALfor the Ganfort Investigators Group IIThe safety and efficacy of bimatoprost/timolol fixed combination: a 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertensionJ Glaucoma201019642442619855289
- GoldbergIGil PinaRLanzagorta-ArestiASchiffmanRMLiuCBejanianMBimatoprost 0.03%/timolol 0.5% preservative-free ophthalmic solution versus bimatoprost 0.03%/timolol 0.5% ophthalmic solution (Ganfort) for glaucoma or ocular hypertension: a 12-week randomised controlled trialBr J Ophthalmol201498792693124667994
- European Medicines AgencySummary of the European Public Assessment Report (EPAR) for Ganfort (Bimatoprost/Timolol) [homepage on the Internet]London, UKEMA Committee for Medicinal Products for Human Use (CHMP)2013 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000668/human_med_000804.jsp&mid=WC0b01ac058001d124Accessed April 1, 2015
- OkabeKKimuraHOkabeJEffect of benzalkonium chloride on transscleral drug deliveryInvest Ophthalmol Vis Sci200546270370815671302
- DesbenoitNSchmitz-AfonsoIBaudouinCLocalisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometryAnal Bioanal Chem2013405124039404923430186
- van der BijlPvan EykADMeyerDEffects of three penetration enhancers on transcorneal permeation of cyclosporineCornea200120550550811413407
- PellinenPHuhtalaATolonenALokkilaJMäenpääJUusitaloHThe cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivoCurr Eye Res201237214515422049909
- ShenJBejanianMSchiffmanRRemoval of preservative from Ganfort improves intraocular pressure (IOP) lowering in patients – a timolol dose-response phenomenonActa Ophthalmol (Copenh)201391s252 Abstract 4226. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1755-3768.2013.4226.x/abstractAccessed January 8, 2016
- KrupinTSingerPRPerlmutterJKolkerAEBeckerBOne-hour intraocular pressure response to timolol. Lack of correlation with long-term responseArch Ophthalmol19819958408417236087
- AlmAKoskelaTTaarnhøjJEffects of D-timolol and L-timolol eye drops on intraocular pressure and aqueous flow. A dose-response study in normal eyesActa Ophthalmol (Copenh)199068119222336928
- BatchelorEDO’DayDMShandDGWoodAJInteraction of topical and oral timolol in glaucomaOphthalmology19798616065394062
- CampbellSHHickey-DwyerMHardingSPDouble-masked three-period crossover investigation of timolol in control of raised intraocular pressureEye (Lond)1993711051088325398
- DuBinerHBHillRKaufmanHTimolol hemihydrate vs timolol maleate to treat ocular hypertension and open-angle glaucomaAm J Ophthalmol199612155225288610795
- KatzIMHubbardWAGetsonAJGouldALIntraocular pressure decrease in normal volunteers following timolol ophthalmic solutionInvest Ophthalmol19761564894926402
- LaurenceJHolderDVogelRA double-masked, placebo-controlled evaluation of timolol in a gel vehicleJ Glaucoma19932317718219920515
- LetchingerSLFrohlichsteinDGlieserDKCan the concentration of timolol or the frequency of its administration be reduced?Ophthalmology19931008125912628341511
- MillsKBBlind randomised non-crossover long-term trial comparing topical timolol 0.25% with timolol 0.5% in the treatment of simple chronic glaucomaBr J Ophthalmol19836742162196338905
- Mottow-LippaLSLippaEANaidoffMAClementiRBjornssonTJonesK008% timolol ophthalmic solution. A minimal-effect dose in a normal volunteer modelArch Ophthalmol1990108161642404487
- RowleySStauntonJEToschAStewart-JonesJHEdgarDFTurnerPA noninvasive tonometer in the measurement of the effects of pindolol and timolol on intraocular pressure in normal subjectsBr J Ophthalmol19816585365387295615
- UusitaloRJPalkamaAStjernschantzJA study of the efficacy of two commercial preparations of timolol maleate with special reference to side effectsActa Ophthalmol (Copenh)19856366346413913273
- ZimmermanTJKassMAYablonskiMEBeckerBTimolol maleate: efficacy and safetyArch Ophthalmol1979974656658371597
- ZimmermanTJKaufmanHETimolol: a β-adrenergic blocking agent for the treatment of glaucomaArch Ophthalmol1977954601604322648
- ZimmermanTJKaufmanHETimolol: dose response and duration of actionArch Ophthalmol1977954605607322649
- YamamotoTKitazawaYAzumaITsukaharaSNakashimaMfor the WP-934 Study GroupClinical evaluation of a new formula of timolol maleate (WP-934 ophthalmic solution)Jpn J Opthalmol1997414244250
- OlatejuSOAjayiAAThe lack of efficacy of topical beta-blockers, timolol and betaxolol on intraocular pressure in Nigerian healthy volunteersEye (Lond)199913Pt 675876310707140
- Le JeunneCLHuguesFCDufierJLMuneraYBringerLBronchial and cardiovascular effects of ocular topical B-antagonists in asthmatic subjects: comparison of timolol, carteolol, and metipranololJ Clin Pharmacol1989292971012565918
- LeierCVBakerNDWeberPACardiovascular effects of ophthalmic timololAnn Intern Med198610421971993946944
- NelsonWLFraunfelderFTSillsJMArrowsmithJBKuritskyJNAdverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978–1985Am J Ophthalmol198610256066113777080
- NetlandPAWeissHSStewartWCCohenJSNussbaumLLfor the Night Study GroupCardiovascular effects of topical carteolol hydrochloride and timolol maleate in patients with ocular hypertension and primary open-angle glaucomaAm J Opthalmol19971234465477
- StewartWCStewartJAJacksonALCardiovascular effects of timolol maleate, brimonidine or brimonidine/timolol maleate in concomitant therapyActa Ophthalmol Scand200280327728112059866
- UmetsukiMHKotegawaTNakamuraKNakanoSNakatsukaKTemporal variation in the effects of ophthalmic timolol on cardiovascular and respiratory functions in healthy menJ Clin Pharmacol199737158639048274
- SicaDAPharmacotherapy in congestive heart failure: cardiopulmonary effects of ophthalmically administered beta-blockersCongest Heart Fail199952818512189325
- ZimmermanTJCanalePTimolol – further observationsOphthalmology1979861166169530562
- KosmanMETimolol in the treatment of open angle glaucomaJAMA19792412123012303439303
- RosFEDakeCLTimolol eye drops: bradycardia or tachycardia?Doc Ophthalmol19804822832897398530
- CherITransfer to timolol: selective use of a new mode of therapyAust J Ophthalmol1980821651727447802
- SteinertRFThomasJVBogerWP3rdLong-term drift and continued efficacy after multiyear timolol therapyArch Ophthalmol19819911001037006575
- BogerWP3rdShortterm “escape” and longterm “drift.” The dissipation effects of the beta adrenergic blocking agentsSurv Ophthalmol198328Suppl2352426141645
- MaclureGMChronic open angle glaucoma treated with Timolol. A four year studyTrans Ophthalmol Soc U K1983103Pt 178836581640
- GandolfiSARestoring sensitivity to timolol after long-term drift in primary open-angle glaucomaInvest Ophthalmol Vis Sci19903123543582406218
- GandolfiSAVecchiMSerial administration of adrenergic antagonist and agonist (“pulsatile therapy”) reduces the incidence of long-term drift to timolol in humansInvest Ophthalmol Vis Sci19963746846888595970
- NeufeldAHZawistowskiKAPageEDBrombergBBInfluences on the density of beta-adrenergic receptors in the cornea and iris – ciliary body of the rabbitInvest Ophthalmol Vis Sci1978171110691075212382
- KahleGKaulenPSchererVWollensakJZunahme der beta-adrenergen rezeptoren in kaninchen in langfristige lokale verabreichung von betablockern. [Increase in beta-adrenergic receptors in rabbits in long-term local administration of beta-blockers]Ophthalmologe1993906626630 German7907242
- WoodwardDFPhelpsRLKraussAHBimatoprost: a novel antiglaucoma agentCardiovasc Drug Rev200422210312015179448
- HeelRCBrogdenRNSpeightTMAveryGSTimolol: a review of its therapeutic efficacy in the topical treatment of glaucomaDrugs19791713855369807