Abstract
To manage ocular redness effectively, health-care practitioners require an understanding of the pathophysiology, clinical features and differential diagnosis of ocular redness, as well as comprehensive knowledge of medical therapies available and their pharmacologic properties. This review aims to provide a clinically relevant summary of the current literature on the mechanism of action, efficacy, and safety of current over-the-counter (OTC) decongestants available for reduction of ocular redness due to minor irritations. Currently marketed OTC products indicated for such use in the United States include topical solutions of tetrahydrozoline 0.05%, naphazoline 0.012% to 0.03%, and brimonidine 0.025%. All 3 agents are adrenergic receptor agonists but vary in their receptor-binding profiles: tetrahydrozoline is a selective α1 receptor agonist; naphazoline is a mixed α1/α2 receptor agonist; and brimonidine is a selective α2 receptor agonist. These OTC decongestants produce vasoconstriction of conjunctival blood vessels, which results in a rapid reduction in ocular redness. In general, ocular adverse events reported in published studies of these OTC decongestants were minimal, mild, and transient, with no significant adverse systemic effects. However, ocular decongestants with α1-adrenergic receptor agonist activity can be associated with loss of effectiveness with continued use (ie, tachyphylaxis) and rebound redness upon treatment discontinuation. In clinical trials of the selective α2-adrenergic receptor agonist brimonidine 0.025%, tachyphylaxis was not observed, and rebound redness was rarely reported.
Introduction
Ocular redness, or hyperemia, is a common ophthalmic sign, occurring with or without symptoms and may be associated with reduced quality of life due to associated discomfort and/or cosmetic concerns. Ocular redness is often associated with conjunctivitis and has a number of potential causes including infectious agents (eg, bacterial or viral conjunctivitis), exposure to allergens (eg, pollen, animal dander) or environmental irritants (eg, smoke, air pollution), eye strain, fatigue, dry eye, and contact lens wear.Citation1–Citation3
For noninfectious and nonallergic ocular redness caused by minor, nonspecific eye irritations with no apparent underlying pathology, topical application of over-the-counter (OTC) ocular decongestants may be considered to reduce ocular redness through the constriction of conjunctival blood vessels.Citation4,Citation5 In this review, we provide a brief overview of the current understanding of the regulation of vascular tone in the eye followed by a summary of clinically relevant peer-reviewed literature on the mechanism of action, efficacy, and safety of currently marketed OTC decongestants available for reduction of ocular redness due to minor irritations in the United States: topical solutions of tetrahydrozoline 0.05%, naphazoline 0.012% to 0.03%, and brimonidine 0.025%. With this information, health-care providers will be better able to recommend an OTC decongestant(s) appropriate for their patients whilst minimizing adverse drug reactions.
Regulation of Vascular Tone
Ocular redness results from vasodilation of conjunctival blood vessels.Citation6 Research on the control of vascular tone in the eye is limited; however, results from in vitro and animal studies on the regulation of other (ie, non-ocular) blood vessels provide pertinent information applicable to understanding the mechanistic basis of ocular redness (). In mammalian species, vascular tone is primarily mediated by activity at adrenergic receptors found on vascular smooth muscle, vascular endothelium, and nerve cell terminals.Citation7–Citation10 Contractile activity of vascular smooth muscle cells alters the diameter of resistance (ie, arteries, arterioles) and capacitance (ie, veins, venules) vessels.Citation11 In vascular smooth muscle cells, stimulation of α1- and/or α2-adrenergic receptors leads to vasoconstriction, whereas stimulation of β-adrenergic receptors leads to vasodilation; in addition, stimulation of α1-, α2-, and/or β-adrenergic receptors on vascular endothelial cells leads to vasodilation via a process possibly mediated by increased nitric oxide release.Citation8,Citation9,Citation12–Citation15 Presynaptic neuronal α2- and β-adrenergic receptors function as autoreceptors; stimulation of these receptors decreases norepinephrine release from neuron terminals, which may also contribute to vasodilation.Citation9 Thus, basal vascular tone is a consequence of the vasoconstrictive and vasodilatory activity mediated by norepinephrine, and ocular redness occurs when vasodilation predominates.
Figure 1 Schematic illustration of the regulation of ocular vascular tone and potential vasoactive effects of topical ophthalmic vasoconstrictors, mediated by adrenergic receptors found on vascular smooth muscle, vascular endothelium, and nerve cell terminals. Norepinephrine released from the nerve cell terminal diffuses to vascular smooth muscle cells and endothelial cells. Stimulation of α1- and/or α2-adrenergic receptors on vascular smooth muscle cells leads to vasoconstriction; whereas, stimulation of α1-, α2- and/or β-adrenergic receptors on vascular endothelial cells, stimulation of β-adrenergic receptors on vascular smooth muscle cells, and stimulation of α2- and/or β-adrenergic receptors on nerve cell terminals, leads to vasodilation. Adapted with permission from Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol. 2018;10(1):17–28.Citation14
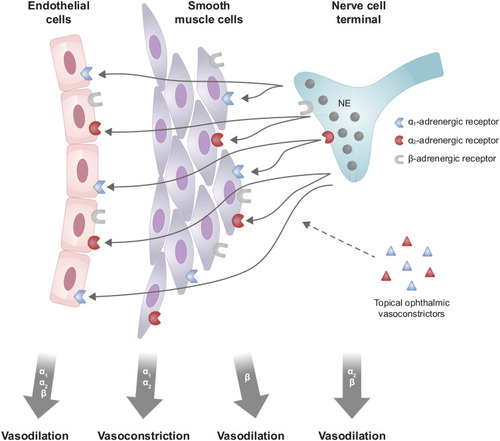
By analogy, the vasoactive effect of a drug with adrenergic receptor agonist activity is also thought to depend on the balance between vasoconstrictive and vasodilatory activity. In addition, anatomic differences in the distribution of α1- and α2-adrenergic receptors are expected and have been noted, with α1-adrenergic receptors observed on both arteries and veins and α2-adrenergic receptors observed predominantly on veins.Citation9,Citation10,Citation12 Consequently, the effects of medications that target ocular α-adrenergic receptors are largely dependent on the receptor type they activate (α1 vs α2), blood vessel type primarily impacted (artery vs vein), and ocular distribution (vascular smooth muscle, vascular endothelium, synapse).
Methods
For data on the mechanisms of action, efficacy, and safety of US OTC decongestant products, a literature search of the Medline database was conducted in June 2020 using the following search terms: (“ocular decongestant” OR “phenylephrine” OR “tetrahydrozoline” OR “naphazoline” OR “oxymetazoline” OR “brimonidine”) AND (“conjunctival hyperemia” OR “ocular hyperemia” OR “ocular redness” OR “ocular erythema” OR “eye redness” OR “eye erythema” OR “eye hyperemia”). Searches were limited to English language publications, with no date restriction. Articles were considered relevant if they included research data or clinical information regarding ocular use for hyperemia in humans or described potential mechanisms of action. Reference lists in relevant publications were reviewed to identify additional articles for inclusion. Also accessed were abstract archives spanning 2013‒2019 from scientific meetings for eye care professionals (American Optometric Association/American Optometric Student Association, American Academy of Optometry, Vision Sciences Society, American Academy of Ophthalmology, Association for Research in Vision and Ophthalmology, and American Ophthalmological Society). Additional articles not identified by the formal searches were included at the discretion of the authors.
Ocular Decongestants
Currently available OTC ocular decongestants in the United States (US) are all α-adrenergic receptor agonists but vary in their receptor-binding profiles (). First-generation ocular decongestants included phenylephrine, tetrahydrozoline, naphazoline, and oxymetazoline. Phenylephrine, a sympathomimetic amine with selective affinity for α1-adrenergic receptors,Citation4,Citation16,Citation17 and oxymetazoline, an imidazole derivative with an affinity of ~5:1 for α2:α1 receptors,Citation18–Citation21 are no longer actively marketed in the US, although oxymetazoline may be available through e-commerce channels. Tetrahydrozoline hydrochloride, an imidazole derivative that has been available since the 1950s, is a selective α1-adrenergic receptor agonist.Citation4,Citation22 Naphazoline hydrochloride, another imidazole derivative, is a mixed α1/α2 receptor agonist with a binding affinity of ~2:1 for α2:α1 receptors.Citation23 Naphazoline (0.1%) received US Food and Drug Administration (FDA) approval in 1974 as a prescription ocular decongestantCitation22 and is now available OTC in lower concentration solutions (0.012–0.03%) for the purpose of redness relief.
Table 1 Representative Over-the-Counter Ocular Decongestants in the United States for the Treatment of Eye Redness*
Brimonidine tartrate, an adrenergic receptor agonist with markedly greater binding affinity for α2 receptors relative to α1 receptors (1000:1),Citation24,Citation25 is the first selective α2-adrenergic receptor agonist for relief of ocular redness.Citation26 Brimonidine was first approved in 1996, in an ophthalmic solution with a concentration of 0.2%, for lowering intraocular pressure in patients with open-angle glaucoma or ocular hypertension and was subsequently approved in 2013, in a topical gel with a concentration of 0.33%, for the treatment of persistent facial erythema of rosacea. Off-label use of brimonidine prior to laser in situ keratomileusis (LASIK) and pterygium surgery demonstrated that brimonidine was effective at reducing subconjunctival hemorrhage and ocular redness at prescription ophthalmic strength and lower doses (0.025%).Citation27–Citation29 Brimonidine, in an ophthalmic solution with the lower concentration of 0.025%, received FDA approval in 2017 to reduce the redness of the eye due to minor irritations.
Mechanisms of Action
There is a wide distribution of α-adrenergic receptors in the eye, including smooth muscle cells and blood vessels (eg, conjunctiva, iris-ciliary structures, aqueous outflow tract).Citation30 Selective α1 receptor agonists (eg, tetrahydrozoline) mixed α1/α2 receptor agonists (eg, naphazoline), and selective α2 receptor agonists (eg, brimonidine) all produce constriction of conjunctival blood vessels through activation of adrenergic receptors, which, in turn, initiates G-protein signaling cascades that culminate in vascular smooth muscle contraction.Citation9,Citation31 More specifically, activation of α1-adrenergic receptors, which are linked to Gq proteins, produces vascular smooth muscle contraction via the inositol triphosphate (IP3) signal transduction pathway, whereas activation of α2-adrenergic receptors, which are linked to Gi proteins, produces vascular smooth muscle contraction as a result of decreased intracellular cyclic adenosine monophosphate (cAMP).Citation9,Citation31
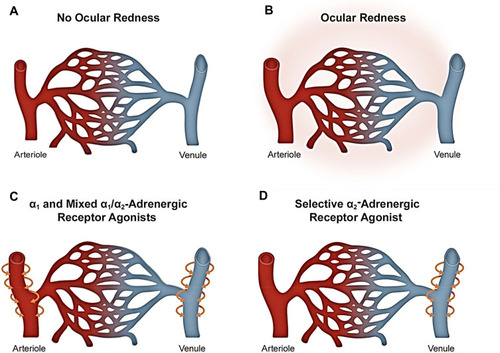
Because of differences in α-adrenergic receptor distribution, ocular decongestants with α1-adrenergic receptor agonist activity are understood to produce constriction of both conjunctival arterioles and venules; whereas a selective α2-adrenergic receptor agonist, such as brimonidine, is thought to have its primary site of action on conjunctival venules ().Citation9,Citation32 Selective constriction of venules has the potential advantage of sustaining the availability of oxygen to the surrounding tissue, which would otherwise be decreased with arteriole constriction, and has implications with respect to the potential for ocular redness rebound, discussed next. Moreover, given that veins in the conjunctiva are known to be more numerous than arteries,Citation33 and, by analogy to other tissues, veins in the conjunctiva are likely located more superficially as opposed to arteries, it follows that preferential constriction of conjunctival venules is expected to have a significant impact on redness even with little change in arterial caliber. Studies involving imaging and/or oximetry would be useful towards understanding vasoconstriction by adrenergic receptor agonists in conjunctival tissue.
Figure 2 Current understanding of the difference in mechanism of action of α-adrenergic receptor agonists for relief of ocular redness. (A) Blood vessels in the conjunctiva under normal conditions (without ocular redness). (B) Ocular redness results from the dilation of arterioles and venules. (C) α1-adrenergic receptor agonists primarily constrict arterioles to reduce redness, whereas mixed α1/α2-adrenergic receptor agonists can impact both arterioles and venules. (D) Selective α2-adrenergic receptor agonists primarily constrict the venules to reduce redness. Of relevance to potential side effects, constriction of arterioles through use of α1- or mixed α1/α2-adrenergic receptor agonists can reduce oxygen delivery to the conjunctiva and lead to ischemia, which, in turn, can be associated with rebound redness following discontinuation. Selective α2-adrenergic receptor agonists effect venular constriction thereby preventing ischemia and subsequent rebound redness. Further research is needed on vascular tone in conjunctival tissue to gain a better understanding of the change(s) in caliber of conjunctival arterioles and venules in ocular redness and their differential response to ocular decongestants.
Tachyphylaxis and Rebound Redness
Although ocular decongestants that act via stimulation of α1-adrenergic receptors are effective for the temporary relief of ocular redness, sustained use of α1- or mixed α1/α2-adrenergic receptor agonists has been associated with tachyphylaxis (tolerance or loss of effectiveness).Citation34,Citation35 Tachyphylaxis is thought to be due to a tolerance-related reduction of the α1-adrenergic receptor response, possibly through sequestration and internalization of α1-adrenergic receptors and subsequent downregulation of surface α1-adrenergic receptors.Citation34,Citation36 While it is unknown whether the distribution of ocular α2-adrenergic receptors changes with continued use of α2-adrenergic agonists, the available literature on vasoconstriction in other tissues suggests that tachyphylaxis is driven primarily by changes in α1-adrenergic receptors.Citation9,Citation36–Citation41 It is notable that studies of the selective α2-adrenergic receptor agonist brimonidine did not show evidence of tachyphylaxis of effect with long-term use at the high doses investigated for lowering intraocular pressure in patients with glaucoma (0.2%) or for treatment of facial erythema of rosacea (0.5%).Citation42,Citation43
Use of α1- or mixed α1/α2-adrenergic receptor agonists has also been associated with rebound redness upon treatment discontinuation.Citation35,Citation44 As α1-adrenergic receptors are preferentially expressed in arterial vessels,Citation9 rebound redness may be mediated by vasoconstrictor-induced tissue ischemia and subsequent release of vasodilators.Citation41,Citation44 Less rebound redness would be expected after discontinuation of selective α2-adrenergic receptor agonists because they produce vasoconstriction through a venular mechanism of action.Citation11,Citation32,Citation41,Citation45,Citation46 There is some speculation, based on data from studies of non-ocular tissues that rebound congestion after discontinuation may also be mediated, in part, by downregulation and uncoupling of α1-adrenergic receptors, which decrease vascular tone (eg, by decreasing sensitivity to endogenous circulating norepinephrine) resulting in rebound vasodilation and congestion,Citation41,Citation47 although this is far from clear. Ongoing research, specifically in conjunctival tissue, may provide a better understanding of the potential for tachyphylaxis and rebound redness of current ocular decongestants.
Clinical Efficacy and Safety
Selective α1-Adrenergic Receptor and Mixed α1/α2-Adrenergic Receptor Agonists
The effectiveness of tetrahydrozoline for reducing ocular redness was evaluated in an unmasked, controlled clinical studyCitation48 and a large case seriesCitation49 of patients with conjunctivitis of varying etiology. In the controlled clinical study, therapeutic response was evaluated in 65 patients with mild to severe conjunctival redness resulting from a variety of ophthalmic conditions (ie, chronic hyperemia, chemical and physical trauma, blepharitis, actinic conjunctivitis, thyrotropic exophthalmos, recurrent chalazia, keratitis) after study medication was instilled in 1 eye.Citation48 Tetrahydrozoline 0.1% (2 drops, 3 times per day for 2‒30 days) provided rapid (< 1 min) and enduring (up to 4 hrs) conjunctival blanching.Citation48 A weaker solution of tetrahydrozoline (0.05%) produced similar results when assessed in additional 62 patients.Citation48 Although this was a controlled study in that tetrahydrozoline was administered in only 1 eye, the study report does not include evaluation of therapeutic response in treated versus untreated eyes.Citation48 In the case series, tetrahydrozoline 0.05% (1 drop, 2‒4 times per day for 2 weeks to 2 months) was evaluated in 1156 patients (age range, 3‒91 years) with allergic or chronic conjunctivitis.Citation49 In the 348 patients with allergic conjunctivitis, tetrahydrozoline 0.05% was an effective decongestant whether with (97.4%) or without (93.5%) concomitant oral antihistamine therapy. Effectiveness of tetrahydrozoline 0.05% was also observed in 88.7% of the 808 patients with chronic conjunctivitis.Citation49 Across patient groups, improvement in redness was typically noted immediately, with duration of effect between 1 and 4 hrs.Citation49 In these studies, minimal side effects were observed; tetrahydrozoline 0.05% did not alter pupil size or raise intraocular pressure and was apparently safe for use in patients with cataracts.Citation48,Citation49
Naphazoline was evaluated as an ocular decongestant in a case series of more than 100 patients with redness of various etiologies (eg, allergic conjunctivitis; chronic conjunctivitis; keratitis; corneal pannus; bulbar congestion due to chemical burns, trauma, chronic iridocyclitis, endophthalmitis, or post-operative states).Citation50 Naphazoline 0.1% (various dosing regimens) produced constriction of superficial conjunctival and corneal capillaries resulting in reduced redness; this effect had an immediate onset and lasted for several hours.Citation50 However, increases in intraocular pressure and pupil dilation were observed in some cases.Citation50 Of note, the concentration of naphazoline used in this case series (0.1%) was substantially greater than that of currently marketed OTC naphazoline products (0.012–0.03%). Additional studies (reported in Gossel, 1983Citation51) demonstrated that pre-treatment with naphazoline (in one eye versus no treatment in the other eye, dosing regimen not reported) protected against vasodilation caused by exposure to chlorinated water and that effectiveness was comparable for naphazoline 0.012% and 0.1%.
Subsequent studies of first-generation ocular decongestants, including 3 head-to-head comparisons of tetrahydrozoline and naphazoline, used doses consistent with current OTC products.Citation4,Citation52,Citation53 In a small, double-masked study of 6 healthy adults with no ocular disease, effects on histamine-induced erythema were evaluated for naphazoline (0.1%, 0.05%, 0.02%, 0.012%), tetrahydrozoline (0.05%), and phenylephrine (0.12%).Citation53 Naphazoline 0.02% demonstrated significantly more blanching of the conjunctiva compared with lower-dose naphazoline (0.012%), tetrahydrozoline, and phenylephrine (data combined for analysis); higher doses of naphazoline (0.1% and 0.05%) did not differ significantly from the 0.02% dose.Citation53 In another small double-masked study of healthy adults (n=8; baseline ocular redness not reported), naphazoline (0.02%) instilled in one eye and tetrahydrozoline (0.05%) instilled in the other eye were evaluated after single-use (1 drop) and exaggerated-use (8 times per day for 9 days, and 4 times on the morning of Day 10) administration.Citation52 Ocular redness, as judged based on photographs taken at various times up to 24 hrs after the last treatment, was significantly reduced from baseline for up to 8 hrs post-treatment after single-dose tetrahydrozoline or naphazoline, with significantly greater improvement for naphazoline versus tetrahydrozoline at some time points; in the exaggerated use condition, significant redness reduction was observed (for up to 6 hrs) only with naphazoline.Citation52 In a safety study of naphazoline 0.012%, tetrahydrozoline 0.05%, and phenylephrine 0.12% conducted in 40 adults with no apparent ocular disease (reported in Adamczyk & Jaanus, 2008Citation4), no significant changes in pupil size or anterior chamber depth were observed. Phenylephrine produced minimal effects on intraocular pressure, assessed 30 mins after administration; compared with phenylephrine, average intraocular pressure was significantly lowered with tetrahydrozoline and somewhat increased with naphazoline.Citation4
As indicated above, use of α1- or mixed α1/α2-adrenergic receptor agonists can be associated with tachyphylaxis and/or rebound redness upon treatment discontinuation. As such, evidence of tachyphylaxis and rebound redness associated with sustained use of first-generation OTC ocular decongestants (ie, α1- or mixed α1/α2-adrenergic receptor agonists) are based on reported FDA safety data, in addition to other, smaller published studies.Citation35,Citation44,Citation52,Citation54 Tachyphylaxis has been documented after repeated daily use of tetrahydrozoline over as few as 5 to 10 days.Citation35,Citation52 In a case series of 5 patients diagnosed with conjunctivitis medicamentosa, rebound redness was observed upon discontinuation of naphazoline or tetrahydrozoline after days, weeks, or months of continuous use.Citation35 In a case series of 70 patients who used OTC decongestant eyedrops (naphazoline, tetrahydrozoline, phenylephrine) daily (mean 3.7 ± 2.2 times per day) for a median of 3 years, conjunctival redness was noted in 50 patients (the remainder were diagnosed with either follicular conjunctivitis [17 patients] or eczematoid blepharoconjunctivitis [3 patients]).Citation44 These 50 cases of conjunctival redness, despite the use of first-generation ocular decongestants, provided evidence of loss of efficacy and/or rebound dilation of conjunctival blood vessels.Citation44 Between 1969 and 1984, there were approximately 280 adverse events reported to the FDA from OTC use of tetrahydrozoline, with more than 90 reported as conjunctivitis and described by consumers as “eyes turned redder” or “eyes redder than before.”Citation54 Another 45 reports listed “no drug effect” with comments such as “eyes still red.” In 1988, the FDA made the decision to include the warning “overuse of this product may produce increased redness of the eye” for all ophthalmic OTC products containing vasoconstrictors in use at that time (eg, tetrahydrozoline, naphazoline).Citation54
Accidental ingestion is an additional concern with first-generation ocular decongestants. Data collected between 1985 and 2012 from the FDA Adverse Event Reporting System showed that 53 out of 96 cases (55.2%) of accidental ingestion of products containing tetrahydrozoline, oxymetazoline, or naphazoline by children aged 1 month to 5 years resulted in hospitalization.Citation55 In 2012, the FDA warned the public that accidental ingestion by young children of a small amount (1‒2 mL) of OTC eye drops (tetrahydrozoline, oxymetazoline, or naphazoline) may cause serious adverse events (eg, coma, decreased heart rate, sedation).Citation55
Selective α2-Adrenergic Receptor Agonist
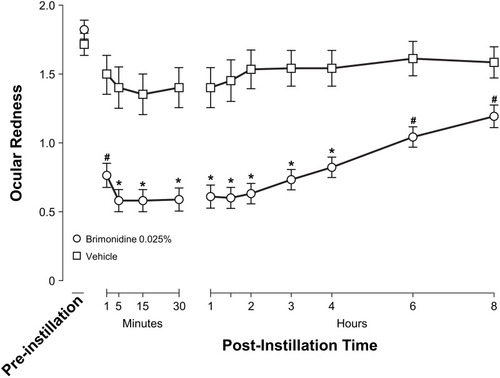
Clinical trials of low-dose brimonidine (0.025%) ophthalmic solution used rigorous study design methodology (randomized, double-masked, vehicle-controlled).Citation56,Citation57 In these studies, a total of 117 adults (≥ 18 years) with ocular redness of undetermined etiology were randomly assigned (in a 2:1 ratio) to bilateral treatment with low-dose brimonidine or vehicle, instilled 4 times daily for 4 weeks.Citation56–Citation58 Compared with vehicle, low-dose brimonidine demonstrated a significant reduction at all post-dose time points in both investigator- and subject-assessed ocular redness, evaluated using the Ora Calibra® Ocular Hyperemia Scale.Citation56–Citation58 Low-dose brimonidine had a rapid (< 1 min) onset of action and was effective for up to 8 hrs ().Citation56 Investigator ratings of ocular redness on Day 15 and Day 29 were examined for signs of tachyphylaxis.Citation56–Citation58 Mean decrease in redness score from pre-dose to 5 mins post-dose was significantly greater for low-dose brimonidine compared with vehicle on Day 15 and Day 29, as it was on Day 1, indicating that there was no evidence of tachyphylaxis.Citation56–Citation58 Across investigator assessments, there was 1 brimonidine-treated participant (1.3%) with rebound redness.Citation58 Based on these results, brimonidine 0.025% currently does not carry the “overuse” warning mandated for ocular products containing other vasoconstrictors.
Figure 3 Investigator-evaluated ocular redness (0- to 4-unit scale) on Day 1 of a randomized clinical trial of brimonidine ophthalmic solution 0.025%. *P<0.0001 vs vehicle at the indicated time point (last observation carried forward). #P≤0.01 vs vehicle at the indicated time point (observed data only). Error bars represent standard error of the mean. Reprinted with permission from McLaurin E, Cavet ME, Gomes PJ, Ciolino JB. Brimonidine ophthalmic solution 0.025% for reduction of ocular redness: a randomized clinical trial. Optom Vis Sci. 2018;95(3):264–271. Available from: https://journals.lww.com/optvissci/Fulltext/2018/03000/Brimonidine_Ophthalmic_Solution_0_025__for.13.aspx.Citation56
Brimonidine also demonstrated redness reduction in a study of 60 patients with a history of allergic conjunctivitis in which brimonidine (0.01%, 0.025%), oxymetazoline (0.025%), and vehicle (placebo) were compared using a conjunctival allergen challenge model.Citation59 Both doses of brimonidine significantly reduced mean conjunctival redness scores at 7, 15, and 20 mins post-instillation compared with placebo and oxymetazoline (all P < 0.05). Interestingly, mean conjunctival redness scores for oxymetazoline were not significantly different compared with placebo at any time point.
The safety of low-dose (0.025%) brimonidine ophthalmic solution was evaluated in 4 clinical studies conducted in 635 pediatric, adult, and geriatric participants.Citation58 An integrated analysis of the safety data indicated that the incidence of ocular adverse events was low and similar between treatment groups, with the most common adverse events (occurring in ≥ 2% of participants in either group) being reduced visual acuity (low-dose brimonidine, 4.0%; vehicle, 4.3%) and conjunctival redness (low-dose brimonidine, 2.6%; vehicle, 2.9%).Citation58 Fatigue and drowsiness have been observed during treatment with brimonidine 0.2% ophthalmic solution in patients with glaucoma or ocular hypertension,Citation60–Citation63 with reports of severe fatigue or somnolence in some pediatric patients.Citation64,Citation65 However, no clinically significant systemic adverse events were observed in either adults or children in studies of low-dose brimonidine, with mild fatigue reported in only 1 adult participant (0.2% of participants); there were no reports of somnolence and no meaningful changes in heart rate or blood pressure.Citation58 Similarly, ocular allergic reactions have been reported in studies of brimonidine 0.2%Citation43,Citation66 and 0.15%Citation67 but allergic conjunctivitis was not reported in the analysis of the 4 safety studies of brimonidine 0.025%, suggesting a dose-dependent relationship between allergic responses and brimonidine treatment, although further study is needed.Citation58 In this safety analysis, which excluded patients with elevated intraocular pressure (> 22 mm Hg) and/or a diagnosis of glaucoma, mean intraocular pressure was unchanged (within 1 mm Hg of baseline) during 4 weeks of treatment with low-dose brimonidine.Citation58
The toxicity of brimonidine in young children (≤ 5 years of age) was evaluated based on 176 unintentional exposures, nearly all (89.2%) via accidental ingestion, to brimonidine solutions (0.1‒0.2%) used for lowering intraocular pressure and compiled by the US national Toxic Exposure Surveillance System.Citation68 Such exposure resulted in hospitalization in 15.7% of cases.Citation68 Based on the mean dose associated with brimonidine toxicity in children (1.11 mg/kg) obtained from this analysis,Citation68 the small amount of brimonidine contained in a full 7.5 mL bottle of 0.025% solution for ocular use (ie, 1.88 mg) is unlikely to result in significant effects in children if accidentally ingested.
Discussion
For ocular redness due to minor irritations or without an apparent cause, patients may consider OTC products for redness relief and seek advice on such products from various health-care providers, including pharmacists, primary care physicians and eyecare practitioners. Before recommending an OTC ocular decongestant, the health-care provider should make recommendations for the management of ocular redness that address the underlying cause, where known.Citation51,Citation61 At least a detailed patient history, and a comprehensive eye exam (by an eyecare practitioner) when possible, assists in differentiating the underlying cause(s) of ocular redness so that appropriate treatment can be initiated.Citation1,Citation2 Mild infectious and noninfectious conjunctivitis may be managed by a primary care provider, but more serious cases (eg, persistent or recurring, cornea involvement, marked pain, decreased visual acuity) warrant management by an eye care practitioner.Citation1,Citation69 Appropriate treatment regimens vary based on the differential diagnosis of the patient’s ocular redness. Treatment of conjunctivitis resulting from a viral infection is usually supportive (eg, cold compresses, artificial tears); management of bacterial conjunctivitis may include topical antibiotics.Citation1 Initial treatment options for management of allergic conjunctivitis accompanied by mild-to-moderate itching include topical antihistamines and mast-cell stabilizers;Citation1,Citation3,Citation22,Citation70 these products are reviewed elsewhere.Citation22
When ocular redness is deemed to be due to minor irritations, an OTC ocular decongestant may be recommended for the management of the redness. Understanding the mechanism of action, efficacy, and safety of currently US-marketed over-the-counter (OTC) decongestants is helpful when making the decision on which product(s) to recommend.
The active ingredient in currently US-marketed OTC ocular decongestants is an α-adrenergic receptor agonist that exerts its effect by stimulating α1- and/or α2-adrenergic receptors on vascular smooth muscle, resulting in vasoconstriction of conjunctival blood vessels and reduction in ocular redness. Ophthalmic solutions of tetrahydrozoline 0.05% (a selective α1 receptor agonist), naphazoline 0.012% to 0.03% (a mixed α1/α2 receptor agonist), and brimonidine 0.025% (a selective α2 receptor agonist) all provide rapid reduction in ocular redness with sustained relief for hours after instillation. In general, ocular adverse events with these products were minimal, mild, and transient in clinical studies of OTC ocular decongestants, with no reported evidence of adverse systemic effects when administered at the relatively low concentrations required for relief of ocular redness. While various efficacy studies have been conducted and reported in the literature, only more-recent clinical trials, conducted on the selective α2-adrenergic receptor agonist brimonidine, used rigorous study design methodologies to evaluate efficacy and safety. In contrast to older ocular decongestants that act via the α1-adrenergic receptor, brimonidine showed no evidence of tachyphylaxis in clinical studies. Brimonidine also was rarely associated with rebound redness in randomized controlled trials. Well-designed comparative studies are needed to evaluate the effects of recently approved (brimonidine) versus older (tetrahydrozoline, naphazoline) agents for ocular redness reduction.
Acknowledgments
Medical writing assistance was provided by Synchrony Medical Communications, LLC, West Chester, PA, USA. The authors thank Christine M. Sanfilippo and Heleen H. DeCory from Bausch + Lomb for editorial assistance.
Disclosure
LOH and CS are employees of Bausch Health US, LLC. The authors report no other conflicts of interest in this work.
References
- Alfonso SA, Fawley JD, Alexa Lu X. Conjunctivitis. Prim Care. 2015;42(3):325–345. doi:10.1016/j.pop.2015.05.00126319341
- Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310(16):1721–1729. doi:10.1001/jama.2013.28031824150468
- Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician. 2010;81(2):137–144.20082509
- Adamczyk DT, Jaanus SD. Antiallergy drugs and decongestants In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. 5th ed. St Louis, MO: Butterworth-Heinemann; 2008.
- US Food and Drug Administration. 21CFR349.75 Labeling of ophthalmic vasoconstrictor drug products; 2019 Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=349.75. Accessed 615, 2020.
- Leibowitz HM. The red eye. N Engl J Med. 2000;343(5):345–351. doi:10.1056/NEJM20000803343050710922425
- Angus JA, Cocks TM, Satoh K. The α adrenoceptors on endothelial cells. Fed Proc. 1986;45(9):2355–2359.3015689
- Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305(5935):627–630. doi:10.1038/305627a06621711
- Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53(2):319–356.11356987
- Rudner XL, Berkowitz DE, Booth JV, et al. Subtype specific regulation of human vascular α1-adrenergic receptors by vessel bed and age. Circulation. 1999;100(23):2336–2343. doi:10.1161/01.CIR.100.23.233610587338
- Corboz MR, Mutter JC, Rivelli MA, et al. α2-adrenoceptor agonists as nasal decongestants. Pulm Pharmacol Ther. 2007;20(2):149–156. doi:10.1016/j.pupt.2006.03.01216809058
- Gavin KT, Colgan MP, Moore D, Shanik G, Docherty JR. α2C-adrenoceptors mediate contractile responses to noradrenaline in the human saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(3):406–411. doi:10.1007/PL000049619089673
- Mishra RC, Rahman MM, Davis MJ, Wulff H, Hill MA, Braun AP. Alpha1-adrenergic stimulation selectively enhances endothelium-mediated vasodilation in rat cremaster arteries. Physiol Rep. 2018;6(9):e13703. doi:10.14814/phy2.1370329756401
- Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol. 2018;10(1):17–28.29593847
- Sorriento D, Trimarco B, Iaccarino G. Adrenergic mechanism in the control of endothelial function. Transl Med UniSa. 2011;1:213–228.23905034
- Meyer SM, Fraunfelder FT. 3. Phenylephrine hydrochloride. Ophthalmology. 1980;87(11):1177–1180. doi:10.1016/S0161-6420(80)35108-77243210
- Vizi ES. Compounds acting on alpha1- and alpha2-adrenoceptors: agonists and antagonists. Med Res Rev. 1986;6(4):431–449. doi:10.1002/med.26100604032877125
- Breakey AS, Cinotti AA, Hirshman M, Skowron RA, Samson CR, Danzig MR. A double-blind, multi-centre controlled trial of 0.25% oxymetazoline ophthalmic solution in patients with allergic and non-infectious conjunctivitis. Pharmatherapeutica. 1980;2(6):353–356.7433476
- Duzman E, Anderson J, Vita JB, Lue JC, Chen CC, Leopold IH. Topically applied oxymetazoline. Ocular vasoconstrictive activity, pharmacokinetics, and metabolism. Arch Ophthalmol. 1983;101(7):1122–1126. doi:10.1001/archopht.1983.010400201240226347152
- Duzman E, Warman A, Warman R. Efficacy and safety of topical oxymetazoline in treating allergic and environmental conjunctivitis. Ann Ophthalmol. 1986;18(1):28–31.3513687
- Samson CR, Danzig MR, Sasovetz D, Thompson HS. Safety and toleration of oxymetazoline ophthalmic solution. Pharmatherapeutica. 1980;2(6):347–352.7433475
- Ackerman S, Smith LM, Gomes PJ. Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management. Ther Adv Chronic Dis. 2016;7(1):52–67. doi:10.1177/204062231561274526770669
- Ruffolo RR. α-adrenoceptors. Monogr Neural Sci. 1984;10:224–253.6142415
- Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996;41(Suppl 1):S9–18. doi:10.1016/S0039-6257(96)82027-38970245
- Piwnica D, Rosignoli C, de Ménonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective α-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75(1):49–54. doi:10.1016/j.jdermsci.2014.04.00224802713
- OTC brimonidine (Lumify) for ocular redness. Med Lett Drugs Ther. 2018;60(1558):176.30335046
- Norden RA. Effect of prophylactic brimonidine on bleeding complications and flap adherence after laser in situ keratomileusis. J Refract Surg. 2002;18(4):468–471.12160159
- Pasquali TA, Aufderheide A, Brinton JP, Avila MR, Stahl ED, Durrie DS. Dilute brimonidine to improve patient comfort and subconjunctival hemorrhage after LASIK. J Refract Surg. 2013;29(7):469–475. doi:10.3928/1081597X-20130617-0523820229
- Ucar F, Cetinkaya S. The results of preoperative topical brimonidine usage in pterygium surgery. J Ocul Pharmacol Ther. 2020;36(4):234–237. doi:10.1089/jop.2019.008532105500
- McAuliffe-Curtin D, Buckley C. Review of alpha adrenoceptor function in the eye. Eye (Lond). 1989;3(Pt 4):472–476. doi:10.1038/eye.1989.712575042
- Bartels SP. Adrenergic agents In: Albert DM, Jakobiec FA, editors. Principles and Practice of Opthalmology. Philadelphia: Basic Sciences; 1994:993–1012.
- Corboz MR, Rivelli MA, Varty L, et al. Pharmacological characterization of postjunctional α-adrenoceptors in human nasal mucosa. Am J Rhinol. 2005;19(5):495–502. doi:10.1177/19458924050190051316270605
- Song BJ, Lee NG, Haq SM, Zieske JD, Trocme SD. Duane’s Ophthalmology. Tasman W, Jaeger EA, eds. Philadelphia. PA: Lippincott Williams & Wilkins; 2013.
- Insel PA. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors–evolving concepts and clinical implications. N Engl J Med. 1996;334(9):580–585. doi:10.1056/NEJM1996022933409078569827
- Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994;94(1):134–136. doi:10.1016/0091-6749(94)90081-78027493
- Fratelli M, De Blasi A. Agonist-induced α1-adrenergic receptor changes. Evidence for receptor sequestration. FEBS Lett. 1987;212(1):149–153. doi:10.1016/0014-5793(87)81575-23026851
- Eason MG, Liggett SB. Subtype-selective desensitization of alpha 2-adrenergic receptors. Different mechanisms control short and long term agonist-promoted desensitization of alpha 2C10, alpha 2C4, and alpha 2C2. J Biol Chem. 1992;267(35):25473–25479.1334095
- Izzo NJ Jr., Seidman CE, Collins S, Colucci WS. α1 adrenergic receptor mRNA level is regulated by norepinephrine in rabbit aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87(16):6268–6271. doi:10.1073/pnas.87.16.62682166953
- Kurose H, Lefkowitz RJ. Differential desensitization and phosphorylation of three cloned and transfected alpha 2-adrenergic receptor subtypes. J Biol Chem. 1994;269(13):10093–10099.7908287
- Toews ML, Prinster SC, Schulte NA. Regulation of alpha-1B adrenergic receptor localization, trafficking, function, and stability. Life Sci. 2003;74(2–3):379–389. doi:10.1016/j.lfs.2003.09.02414607266
- Vaidyanathan S, Williamson P, Clearie K, Khan F, Lipworth B. Fluticasone reverses oxymetazoline-induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med. 2010;182(1):19–24. doi:10.1164/rccm.200911-1701OC20203244
- Moore A, Kempers S, Murakawa G, et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol. 2014;13(1):56–61.24385120
- Schuman JS, Horwitz B, Choplin NT, David R, Albracht D, Chen K. A 1-year study of brimonidine twice daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Chronic brimonidine study group. Arch Ophthalmol. 1997;115(7):847–852. doi:10.1001/archopht.1997.011001600170029230823
- Soparkar CN, Wilhelmus KR, Koch DD, Wallace GW, Jones DB. Acute and chronic conjunctivitis due to over-the-counter ophthalmic decongestants. Arch Ophthalmol. 1997;115(1):34–38. doi:10.1001/archopht.1997.011001500360049006422
- Corboz MR, Rivelli MA, Mingo GG, et al. Mechanism of decongestant activity of α2-adrenoceptor agonists. Pulm Pharmacol Ther. 2008;21(3):449–454. doi:10.1016/j.pupt.2007.06.00717869148
- Corboz MR, Varty LM, Rivelli MA, et al. Effects of an α2-adrenoceptor agonist in nasal mucosa. Arch Physiol Biochem. 2003;111(4):335–336. doi:10.3109/1381345031233133753115764068
- Hein P, Michel MC. Signal transduction and regulation: are all α1-adrenergic receptor subtypes created equal? Biochem Pharmacol. 2007;73(8):1097–1106. doi:10.1016/j.bcp.2006.11.00117141737
- Grossmann EE, Lehman RH. Ophthalmic use of tyzine; a clinical study of this new vasoconstrictor. Am J Ophthalmol. 1956;42(1):121–123. doi:10.1016/0002-9394(56)90021-613339915
- Menger HC. New ophthalmic decongestant, tetrahydrozoline hydrochloride: clinical use in 1156 patients with conjunctival irritation. JAMA. 1959;170(2):178–179. doi:10.1001/jama.1959.03010020036011
- Hurwitz P, Thompson JM. Uses of naphazoline (Privine®). Arch Ophthal. 1950;43(4):712–717. doi:10.1001/archopht.1950.00910010723008
- Gossel TA. Ophthalmic Decongestants. U S Pharmacist; 1983:1–4.
- Abelson MB, Butrus SI, Weston JH, Rosner B. Tolerance and absence of rebound vasodilation following topical ocular decongestant usage. Ophthalmology. 1984;91(11):1364–1367. doi:10.1016/S0161-6420(84)34140-96514304
- Abelson MB, Yamamoto GK, Allansmith MR. Effects of ocular decongestants. Arch Ophthalmol. 1980;98(5):856–858. doi:10.1001/archopht.1980.010200308500097378008
- Young F. 21 CFR parts 349 and 369: ophthalmic drug products for over-the-counter human use; final monograph. Fed Regist. 1988;53(43):7076–7093.
- US Food and Drug Administration. FDA drug safety communication: serious adverse events from accidental ingestion by children of over-the-counter eye drops and nasal sprays; 2012 Available from: https://www.fda.gov/Drugs/DrugSafety/ucm325257.htm. Accessed 923, 2018.
- McLaurin E, Cavet ME, Gomes PJ, Ciolino JB. Brimonidine ophthalmic solution 0.025% for reduction of ocular redness: a randomized clinical trial. Optom Vis Sci. 2018;95(3):264–271. doi:10.1097/OPX.000000000000118229461408
- Torkildsen GL, Sanfilippo CM, DeCory HH, Gomes PJ. Evaluation of efficacy and safety of brimonidine tartrate ophthalmic solution, 0.025% for treatment of ocular redness. Curr Eye Res. 2018;43(1):43–51. doi:10.1080/02713683.2017.138126929120262
- Ackerman SL, Torkildsen GL, McLaurin E, Vittitow JL. Low-dose brimonidine for relief of ocular redness: integrated analysis of four clinical trials. Clin Exp Optom. 2019;102(2):131–139. doi:10.1111/cxo.1284630525235
- Chapin M, Horn G, Gomes G. Evaluation of brimonidine tartrate for prevention of hyperemia associated with allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2013;54(15):2556.
- Al-Shahwan S, Al-Torbak AA, Turkmani S, Al-Omran M, Al-Jadaan I, Edward DP. Side-effect profile of brimonidine tartrate in children. Ophthalmology. 2005;112(12):2143. doi:10.1016/j.ophtha.2005.06.03516225927
- Cantor LB. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000;1(4):815–834. doi:10.1517/14656566.1.4.81511249518
- Derick RJ, Robin AL, Walters TR, et al. Brimonidine tartrate: a one-month dose response study. Ophthalmology. 1997;104(1):131–136. doi:10.1016/S0161-6420(97)30349-29022117
- Lee DA, Gornbein J, Abrams C. The effectiveness and safety of brimonidine as mono-, combination, or replacement therapy for patients with primary open-angle glaucoma or ocular hypertension: a post hoc analysis of an open-label community trial. Glaucoma trial study group. J Ocul Pharmacol Ther. 2000;16(1):3–18. doi:10.1089/jop.2000.16.310673126
- Bowman RJ, Cope J, Nischal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond). 2004;18(1):24–26. doi:10.1038/sj.eye.670052014707960
- Enyedi LB, Freedman SF. Safety and efficacy of brimonidine in children with glaucoma. J AAPOS. 2001;5(5):281–284. doi:10.1067/mpa.2001.11757111641636
- LeBlanc RP. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Brimonidine study group 2. Ophthalmology. 1998;105(10):1960–1967. doi:10.1016/S0161-6420(98)91048-X9787370
- Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma. 2002;11(2):119–126. doi:10.1097/00061198-200204000-0000711912359
- Lai Becker M, Huntington N, Woolf AD. Brimonidine tartrate poisoning in children: frequency, trends, and use of naloxone as an antidote. Pediatrics. 2009;123(2):e305–311. doi:10.1542/peds.2008-195119124581
- Dunlop AL, Wells JR. Approach to red eye for primary care practitioners. Prim Care. 2015;42(3):267–284. doi:10.1016/j.pop.2015.05.00226319338
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc. 2013;34(5):408–420. doi:10.2500/aap.2013.34.369523998237
