Abstract
Introduction
Ankle arthrodesis is one of the treatments of choice, particularly in late-stage and unstable diabetic Charcot arthropathy. Unfortunately, poor healing capacity might play a role in the high nonunion rate (10–40%). The advancement in regenerative medicine opens a new horizon for enhancing fusion after ankle arthrodesis in patients with poor healing capacity. However, a suitable small animal model is warranted to study the effectivity of these regenerative medicine approaches. Streptozotocin (STZ)-induced diabetes models and adjuvant-induced arthritis models with complete Freund’s adjuvant are two established models. However, no study has combined those two models to make a diabetic arthritic model that more closely resembles the condition in Charcot arthropathy.
Methods
Twenty male Sprague-Dawley rats were assigned into five groups, consisting of one control group, and four diabetic groups which were induced by STZ injection and a high-fat diet. Among these diabetic rats, two groups received complete Freund’s adjuvant (CFA) injections to the left ankle of the hind limb. The control group, one of the diabetic-only groups, and one of the arthritic-diabetic-induced groups were euthanized at 4 weeks after STZ induction, and the remainder were euthanized 6 weeks after STZ induction. Clinical, radiological, and histological examinations were then compared in all five groups.
Results
Diabetic status was successfully achieved in the model, which was maintained until the completion of the study. The CFA-induced ankles were significantly larger than the contralateral ankles in all groups (p<0.05). Histopathological evaluation confirmed arthritic changes in the CFA-induced group with less variability after 4 weeks of arthritis induction.
Conclusion
This rat model of arthritic diabetic mimics the progressive and chronic nature of Charcot arthropathy in humans. This model can be further use to study treatments that might enhance the fusion rate in ankle arthrodesis in healing-defective patients such as those with diabetes.
Level of Clinical Evidence
5.
Introduction
Animal models have long been studied to support translational studies in humans. It helps clinicians understand the complexity of certain pathological conditions as it allows them to study each progressive stage of the disease in a controlled and reproducible manner. Moreover, it also helps researchers and clinicians develop better disease treatments. Arthritic models can be made in various ways to study acute diseases such as rheumatoid arthritis and chronic arthritic conditions such as osteoarthritis.Citation1,Citation2 However, to our knowledge, there is limited data on the animal model of a metabolic condition such as Charcot arthropathy. Charcot arthropathy is a progressive joint destruction due to repetitive microtrauma and the accumulation of local inflammation as a result of neuropathic dysfunction.Citation3,Citation4 Charcot arthropathy is a disabling complication of type 2 diabetes mellitus (T2DM) and has been reported as occurring between 0.3% and 0.85% annually among people within that population. Arthrodesis is a treatment of choice to provide stable gait by fusing the ankle and hind foot joints.Citation5–8 However, the potentially high rate of fusion failure (10–40%) urges efforts to augment fusion.Citation9–11
Our primary aim was to create an appropriate small animal model before conducting further study to increase the fusion rate in Charcot ankle arthrodesis. Animal models play a crucial role in understanding the pathogenesis of diseases, evaluating potential therapeutic interventions, and drug testing. The common animal models of arthritis are collagen-induced arthritis (CIA), adjuvant-induced arthritis (AIA), streptococcal cell wall (SCW) arthritis, spontaneous models, and transgenic and knockout models.Citation12–14 Meanwhile, diabetic animal models commonly made by chemical-induced, high fat diet induced, surgical pancreatectomy model, virus induced, and genetically modified models.Citation15 However, to date, there is no model that truly represents both diabetes and arthritis, such as in the Charcot ankle condition.
In our study, a T2DM animal model was induced in Sprague Dawley rats using streptozotocin (STZ) on a high-fat diet (HFD) background, which was followed by arthritis induction using Complete Freund’s adjuvant (CFA). We hypothesized that the lesion may resemble Charcot arthropathy in humans with diabetes, and the condition can be maintained throughout the study time frame better replicating the continuum model and the chronic nature of diabetic arthropathy in humans.
Materials and Methods
Animals
All procedures involving animals were performed following approval by the Institutional Animal Care and Use Committee (IACUC) of PT Bimana Indomedical (approval number R.04–22-IR). All methods were carried out in compliance to the guidelines by the IACUC, which referred to the national ethical standard and guidelines of medical research and applicable animal welfare regulations in Indonesia, as well as The Guide for the Care and Use of Laboratory Animals (8th Edition) by the US National Research Council. The manuscript was written in accordance with the ARRIVE guideline. Twenty clinically healthy male Sprague Dawley rats (age 6–8 weeks old) obtained from the National Food and Drug Agency of Indonesia (Jakarta, Indonesia) were included in this study. Animals were acclimatized and conditioned to reach a body weight of 250–300 grams. Randomization based on body weight was then performed to allocate rats into five groups. Basic characteristics were obtained before the induction process, such as body weight, ankle diameter, lipid profile, blood glucose, and ankle x-ray. Five groups were assigned based on the diabetes status and treatment of the left hind limb ankle joints: group 1 was a control group (no diabetic and arthritic induction); groups 2 and 3 were diabetic-only groups; and groups 4 and 5 were diabetic and arthritic-induced groups. Groups 1, 2, and 4 were terminated at 4 weeks after diabetic induction (ie 2 weeks after arthritic induction in group 4), and the rest were terminated at 6 weeks after diabetic induction (ie 4 weeks after arthritic induction in group 5).
Diabetic Induction
The T2DM pre-conditioning was done by administering a high-fat diet (HFD) with total calory of 40 kJ/kg consisted of 20% animal fat (lard), 45% carbohydrate (starch, sucrose, maltodextrin), and 22% protein (casein/milk protein). This HFD background was maintained until study endpoint. After two weeks of HFD consumption, a single dose (30 mg/kg) of streptozotocin or STZ (Sigma) dissolved in a solution of 0.1 M citric acid buffer (pH 4.5), was administered intraperitoneally, following a baseline fasting blood glucose evaluation. Rats were monitored by measuring fasting blood glucose levels (ie 6-hour fasting) weekly for three weeks, The rats were considered diabetic when two consecutive positive blood glucose concentration higher than 200 mg/dL was achieved, at 9 and 14 days following STZ injection. Lipid profile was also measured after diabetic condition was achieved. An emergency procedure which included a subcutaneous insulin injection (4 IU ultralente insulin) was in place for rats that experienced clinical abnormality with confirmed hyperglycemia.
Arthritis Induction
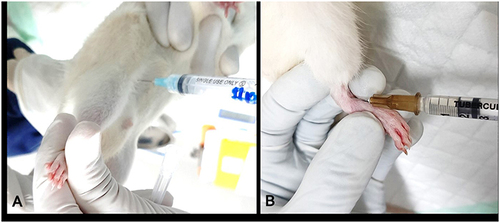
Two groups of eight diabetic-confirmed rats received an intra-articular injection of 0.2 mL of Complete Freund’s Adjuvant (CFA) containing Mycobacterium tuberculosis. The injection was given on the ankle joint in left hind limb, performed on the rats under anesthesia with isoflurane and in aseptic manner. The ankle joint was determined by one hand fixing the tarsal bones and the other hand aiming to find a soft spot medial to the tip of the lateral malleolus with a mosquito clamp. A 26G-needle was introduced into the ankle joint just lateral to the anterior tibial tendon, with some distraction of the joint by pulling the toe away from the tibia to open the joint space (). After induction, tramadol (5–20 mg/kg) was administered to reduce pain. Rats were euthanized by intracardiac injection of sodium pentobarbitone (200 mg/kg) in deep anesthesia (Ketamine 80 mg/kg and Xylazine 10 mg/kg). Ankle diameter was measured before induction (baseline) and upon study completion at 2 or 4 weeks after CFA injection, whereas body weights were measured weekly. At necropsy, bilateral hind limb ankle joints were collected for radiology and histology purposes.
Radiographs Analysis
Radiographic evaluation was performed (Indoray IR-1000, West Java, Indonesia) under 40 kV and 100 mA settings. Antero-posterior and lateral X-ray images of the ankle joints were collected after the animals were euthanized. Two independent observers blindly evaluated the images to determine the arthritic status according to the Kellgren-Lawrence (0–4) classification.
Histological Analysis
Samples were prepared by cutting each ankle 5 mm above the joint, and the skin was completely removed. The samples were then fixed in formalin for further histological processing: fixation and decalcification. The fixed ankle joint blocks were made, and the paraffin-embedded joints were cut into 0.5-mm-thick slices in a sagittal section and evaluated by optical microscopy (DM2500M-Leica, Wetzlar, Germany). Sections were deparaffinized and rehydrated before the staining process with hematoxylin–eosin (HE). Arthritic changes were evaluated in five high power fields (HPF): superior-medial, superior-lateral, central, inferior-medial, and inferior-lateral. Arthritis was classified as a universal 0–3 severity score: 0 (no arthritic changes), 1 (mild arthritis), 2 (moderate arthritis), and 3 (severe arthritis) based on synovial inflammation and cartilage erosion.
Statistical Analysis
The Shapiro–Wilk and Levene tests were applied to verify the normality of the data and the homogeneity of the variance, respectively. A student’s paired t-test was used in comparisons between baseline values and post-procedural values. Normally distributed numeric data were represented using the mean and standard deviation and analyzed using mean-based statistical tests (Student’s t-test). Statistical Package for the Social Sciences (SPSS) for Windows®, version 25.0, was used with a significance level of 5%.
Results
High Fat Diet consumption followed by STZ injection with the dose of 30 mg/kg resulted in diabetic condition in all animals, some with more marked clinical condition compared to others. The baseline characteristics are summarized in . There was no indication of infection or local inflammation. After 2 weeks of exposure to high-fat diet (HFD) and before STZ injection, all groups showed baseline glucose level of 125 ± 9.97. Most of the diabetic induction groups achieved diabetic status after one attempt of STZ induction, achieving a mean fasting glucose level of 421 ± 27.16, which was similar between experimental groups (p = 0.113) and this condition was maintained until the end of the study. All diabetic rats showed dyslipidemia with a low-density lipoprotein (LDL) level of 6.25 (4.7–12.1). One rat was euthanized due to ketoacidosis at two days after STZ induction. There were two rats that required a second dose of STZ, given at 2 weeks after the initial induction. One of those was excluded from the study after failing to achieve diabetic status.
Table 1 Subject Characteristic (n=20)
After arthritic induction with CFA, there was a significant body weight loss in the arthritic-induced groups 4 (p = 0.003) and 5 (p = 0.023) compared to the body weight before arthritic induction. Body weight reduction was not seen in other groups (p-values of 0.12, 0.18, and 0.31 for groups 1, 2, and 3, respectively). Clinically, local inflammation was seen as a marked joint swelling in the arthritic-induced group (groups 4 and 5), which showed a significantly increased ankle diameter compared to the ankle diameter before CFA injection (p<0.05) (), as well as the comparison between the arthritic ankle to the contralateral ankle and normal ankle in the control group ().
Table 2 Comparison of Left Hind Limb Ankle Diameter Before and After Induction
Table 3 Comparison to Contralateral Ankle and Control Group
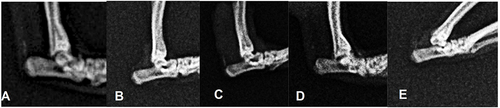
The arthritic group demonstrated radiological changes at the ankle joint, such as narrowing joint space, bony cysts, subchondral sclerosis, and osteophyte formation (). A marked arthritis change was seen in group 5 compared to group 1 (p = 0.036), with good interobserver reliability (Interclass Correlation Coefficient 0.803). There was no significant difference between groups 4 and 5 (p = 0.222).
Figure 2 Ankle radiograph. (A) initial. (B) normal ankle after induction (Kellgren-Lawrence / KL 0) (C) KL-2, (D) KL-3, (E) KL-4.
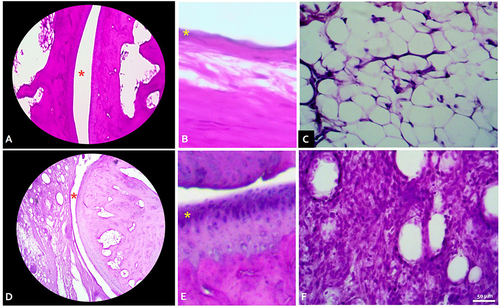
The histopathology analysis with HE staining demonstrated marked synovial hyperplasia and extensive inflammatory cell infiltration in the synovial membrane of the arthritic group compared to the control group (no inflammatory reaction) (). There was also the appearance of inflammatory cell infiltration at the contralateral ankle joint in both arthritic-induced groups and in diabetic-only groups, although it was not as extensive as in the CFA-induced ipsilateral ankle joint.
Figure 3 Histology findings in normal ankle (A–C) vs grade 3 severely arthritic ankle (C and D) with Hematoxylin-Eosin staining. (A) Talo-tibial joint space (red asterisk) in normal ankle, total magnification (TM) 40x. (B) Healthy synovium consisted of 1–2 of synovial cell linings (yellow asterix). TM 100x. (C) Synovial connective tissue showed no infiltration of inflammatory cells. TM 400x. (D) Joint space was markedly reduced with irregularity of the articular cartilage. TM 40x. (E) Synovial cells hyperplasia (yellow asterix). TM 100x. (F) Dense inflammatory cells infiltrate in synovial connective tissue. TM 400x.
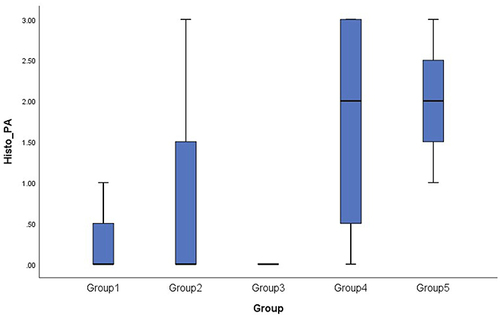
The Kruskal–Wallis test revealed a non-significant result among groups (p = 0.091). The Mann–Whitney test showed that group 5 significantly showed more arthritic changes compared to group 1 (p = 0.025) and group 3 (p = 0.026). Statistically, there was no significant difference in the histopathology profile between groups 4 and 5. The box plot graph shows that group 4 has wider variability than group 5 ().
Discussion
Charcot arthropathy, a non-infectious bone and joint progressive destruction, happens in 1% of diabetic patients. Efforts have been made to overcome fusion failure after arthrodesis in the Charcot joint ankle, such as increasing mechanical stability and utilizing regenerative medicine principles.Citation5,Citation6,Citation16,Citation17 This study was made as a preliminary study to improve the efficacy of arthrodesis in an arthritic model with poor healing capacity such as in diabetic condition, which resembled Charcot arthropathy in humans.
Diabetic induction in animals has been reported in various animal models by either chemical induction (eg alloxan, streptozotocin/STZ), spontaneous autoimmune models, genetic modification, or surgery.Citation7 STZ destroys the pancreatic cell, which leads to insulin secretion impairment. Conditioning the animal with an HFD mimics the order of the pathological events as seen in T2DM in humans, which is dyslipidemia followed by pancreatic β-cell failure. The dose of STZ itself was somewhat tricky. Reed et al used an STZ dose of 50 mg/kg in 31 rats that were fed a HFD, which resulted in a significant increase in glucose level, insulin resistance, and triglyceride compared to the control group.Citation8 Other studies used lesser dose of STZ 30 mg/kg after 4 weeks of HFD which was repeated after 1 week if diabetic state had not been achieved.Citation9,Citation10 In the pilot study (unpublished results), we initially used a 50 mg/kg dose to remain consistent with most of the literatures on diabetes induction strategy. However, all rats developed ketoacidosis, which caused 100% mortality. In this study, a 30 mg/kg dose of STZ with HFD was used and successfully induced diabetes in most of the rats, which was maintained until the end of the study. The metabolic dyslipidemia state was also significantly achieved in the HFD group compared to the control group, which further completes the entire metabolic condition that resembles T2DM.
The arthritic model has long been studied to learn the nature, basic science, and treatment of rheumatoid arthritis and osteoarthritis.Citation12 The adjuvant-induced arthritis (AIA) model is commonly used, and among the commercially available adjuvants, complete Freund’s adjuvant (CFA) is the most commonly used to develop an arthritic animal model. The CFA-induced arthritic model was first developed by Stoerk et alCitation11 to develop polyarthritis, and this model has been evolved and modified either by the dosing or the routes of injections (subcutaneous, intradermal, or intraarticular) to make various inflammatory reactions of either acute, chronic, single joint, or multiple joint.Citation18–20
The administration of CFA has been known to cause a series of inflammatory reactions after being injected into the joint space, including primary cell destruction, which releases the cell wall phospholipid, which will undergo an enzymatic process that secretes arachidonic acid. The thromboxane A2 will attract monocytes to release interleukin 1-β, which further causes cartilage destruction via matrix metalloproteinase activation.Citation21,Citation22 The synovial changes were induced by the increased expression of TNF-α and MMP3, as well as inflammatory cell infiltration into the synovium, which causes synovial hyperplasia and synovitis.Citation23
In our study, the inflammatory changes in the CFA-injected ankle confirmed the arthritic state as shown clinically, radiologically, and histologically. According to other reports, the histological exam revealed immune cell infiltration and synovial hyperplasia. In the previous study, the CFA was shown to give an earlier change compared to other substance-induced arthritis, yet still give a steadier outcome that will last up to 6 weeks. Primary lesion develops within 3–5 days and secondary lesions will develop 11–12 days after injection.Citation19 In our study, the histology showed marked joint inflammation as early as 2 weeks after arthritis induction. Although there was no significant difference in arthritic profile between the 2- and 4-week models, we chose the 4-week model as it produced a more stable outcome, as shown by the narrow box plot, which means less variation in the result. In this study, a mechanical failure of the limbs was not modeled to avoid causing more stress to the animal, morbidity and mortality. This lack of approach may be considered a limitation to the study, as a true Charcot arthropathy often involves physical trauma. More than that, the sensory impairment aspect must be further studied to allow for a more suitable model that mimics almost all pathologies in the Charcot joint.
In conclusion, we were able to develop a model of diabetic arthritis using HFD/STZ and CFA-induction in male Sprague-Dawley rats, which required an approximately 8-week process. This model has the potential to be used for further studies relating to Charcot arthropathy and its treatment approaches, such as evaluating neuropathic parameters, adding mechanical factors to prove the mechanical failure in Charcot arthropathy, as well as the application of regenerative medicine to augment healing, diabetic foot ulcer wound management, or new surgical techniques to overcome or prevent the deformity as other complications of Charcot arthropathy.
Animal Welfare
The study was performed in compliance with the guidelines by the Animal Care and Use Committee of PT Bimana Indomedical, which referred to the national ethical standard and guidelines of medical research and applicable animal welfare regulations in Indonesia, as well as The Guide for the Care and Use of Laboratory Animals (8th Edition) by the US National Research Council. The manuscript was written in accordance with the ARRIVE guideline.
Ethics Approval
Ethical approval and all experimental protocols for this study were obtained from the Institutional Animal Care and Use Committee of PT Bimana Indomedical (ACUC Number: R.04-22-IR).
Disclosure
The authors declare that there are no conflicts of interest in this work.
Additional information
Funding
References
- Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:1–14. doi:10.1155/2012/764621
- Fischer BD, Adeyemo A, O’Leary ME, Bottaro A. Animal models of rheumatoid pain: experimental systems and insights. Arthritis Res Ther. 2017;19(1):1–9. doi:10.1186/s13075-017-1361-6
- Myerson MS, Kadakia AR. Surgery for the neuropathic foot and ankle. In: Reconstructive Foot and Ankle Surgery: Management of Complications. Elsevier Health Sciences; 2019:121–140. doi:10.1016/b978-0-323-49693-3.00010-x
- Rajbhandari S, Jenkins R, Davies C, Tesfaye S. Charcot neuroarthropathy in diabetes mellitus. Diabetologia. 2002;45(8):1085–1096. doi:10.1007/s00125-002-0885-7
- Gouveri E. Charcot osteoarthropathy in diabetes: a brief review with an emphasis on clinical practice. World J Diabetes. 2011;2(5):59. doi:10.4239/wjd.v2.i5.59
- DiDomenico LA, Sann P. Posterior approach using anterior ankle arthrodesis locking plate for tibiotalocalcaneal arthrodesis. J Foot Ankle Surg. 2011;50(5):626–629. doi:10.1053/j.jfas.2011.05.007
- Kottaisamy CPD, Raj DS, Prasanth Kumar V, Sankaran U. Experimental animal models for diabetes and its related complications—a review. Lab Anim Res. 2021;37(1):1–14. doi:10.1186/s42826-021-00101-4
- Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–1394. doi:10.1053/meta.2000.17721
- Qian C, Zhu C, Yu W, Jiang X, Zhang F, Malaval L. High-fat diet/low-dose streptozotocin-induced type 2 diabetes in rats impacts osteogenesis and Wnt signaling in bone marrow stromal cells. PLoS One. 2015;10(8):e0136390. doi:10.1371/journal.pone.0136390
- Zhang F, Ye C, Li G, et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp Anim. 2003;52(5):401–407. doi:10.1538/expanim.52.401
- Stoerk H, Bielinski T, Budzilovich T. Chronic polyarthritis in rats injected with spleen in adjuvants. Am J Pathol. 1954;30(3):616.
- Cai X, Wong YF, Zhou H, et al. The comparative study of Sprague-Dawley and Lewis rats in adjuvant-induced arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(2):140–147. doi:10.1007/s00210-006-0062-5
- Daoussis D, Andonopoulos AP. The emerging role of dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. 2011;41(2):170–177. doi:10.1016/j.semarthrit.2011.01.006
- Sabreena A, Noh M, Dai T, et al. Effects of different doses of complete Freund’s adjuvant on nociceptive behaviour and inflammatory parameters in polyarthritic rat model mimicking rheumatoid arthritis. PLoS One. 2021:1–24. doi:10.1371/journal.pone.0260423
- Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res. 2013;2013:1–9. doi:10.1155/2013/149452
- Çinar M, Derincek A, Akpinar S. Tibiocalcaneal arthrodesis with posterior blade plate in diabetic neuroarthropthy. Foot Ankle Int. 2010;31(6):511–516. doi:10.3113/FAI.2010.0511
- Lowery NJ, Woods JB, Armstrong DG, Wukich DK. Surgical management of Charcot neuroarthropathy of the foot and ankle: a systematic review. Foot Ankle Int. 2012;33(2):113–121. doi:10.3113/FAI.2012.0113
- Jitta SR, Daram P, Gourishetti K, et al. Terminalia tomentosa bark ameliorates inflammation and arthritis in carrageenan induced inflammatory model and freund’s adjuvant-induced arthritis model in rats. J Toxicol. 2019;2019:1–11. doi:10.1155/2019/7898914
- Hashmi JA, Yashpal K, Holdsworth DW, Henry JL. Sensory and vascular changes in a rat monoarthritis model: prophylactic and therapeutic effects of meloxicam. Inflamm Res. 2010;59(8):667–678. doi:10.1007/s00011-010-0179-3
- Xu L, Jiang H, Feng Y, Cao P, Ke J, Long X. Peripheral and central substance P expression in rat CFA-induced TMJ synovitis pain. Mol Pain. 2019;15:1–9. doi:10.1177/1744806919866340
- Kim W, Park S, Choi C, et al. Evaluation of anti-inflammatory potential of the new ganghwaljetongyeum on adjuvant-induced inflammatory arthritis in rats. Evid Based Complement Altern Med. 2016;2016:1–10. doi:10.1155/2016/1230294
- Saccol RD, da Silveira KL, Adefegha SA, et al. Effect of quercetin on E-NTPDase/E-ADA activities and cytokine secretion of complete Freund adjuvant–induced arthritic rats. Cell Biochem Funct. 2019;37(7):474–485. doi:10.1002/cbf.3413
- Hsieh YL, Cheng YJ, Huang FC, Yang CC. The fluence effects of low-level laser therapy on inflammation, fibroblast-like synoviocytes, and synovial apoptosis in rats with adjuvant-induced arthritis. Photomed Laser Surg. 2014;32(12):669–677. doi:10.1089/pho.2014.3821