Abstract
The metastatic form of osteosarcoma is a life threatening one since it metastasizes to the lungs. The major cause of metastatic osteosarcoma is hypomethylation of numerous genes that undergo overexpression to enable the progression of the disease. In the present study, S-adenosylmethionine (SAM), a predominant methyl donor, was administered to find out its effects on osteosarcoma progression. As evidence of tumor suppression, the SAM-treated mouse tissue was analyzed histologically, which exemplifies the control that SAM has over abnormal cell proliferation, especially on primary osteosarcoma, but it lacks positive effects on metastatic osteosarcoma. At the molecular level, the successful inhibition of primary osteosarcoma was found to be associated with a lower expression of Sox2, a protein highly expressed in osteosarcoma stem cells, along with an upregulated expression of TCTP. The data suggest that the administration of SAM has a positive role in treating primary osteosarcoma, but it has no role in suppressing metastatic osteosarcoma. The decreased expression of Sox2 together with upregulation of TCTP following SAM administration indicates that SAM has a control over primary osteosarcoma.
Introduction
Osteosarcoma is the most common aggressive form of bone tumor that occurs in children, and adults >50 years.Citation1 It occurs due to abnormal osteoid production and differentiation along with genomic instability. The progression of osteosarcoma is associated with numerous genes with diverse genomic alterations.Citation2 Previously, surgical resection therapy was practiced in osteosarcoma patients, which resulted in poor prognosis. However, prominent chemotherapy was developed during the 1980s, which increased the 5-year survival rate of osteosarcoma patients to 50%–80%.Citation3,Citation4 Since then, there has been no significant progress in therapeutic modalities as well as in prognosis in patients suffering with osteosarcoma.Citation5 Especially, the patients with the metastatic form of osteosarcoma have only a 10%–30% longer survival rate,Citation6 and others exhibit less response. In order to improve the survival rate of patients, a new therapeutic target should be put forward to investigate the antimetastatic activity, or an effective drug must be required to impede the progression of osteosarcoma to metastatic stage.
Besides many reasons like deletion, translocation, amplification, and other mutations that contribute to tumor development,Citation7 epigenetic changes have also paved the way for the development of many cancers, including osteosarcoma.Citation8–Citation10 The major epigenetic changes consist of DNA methylation, histone modification, and chromosome remodeling.Citation11 S-adenosylmethionine (SAM), an important molecule in biochemistry that donates its methyl group for many biochemical reactions,Citation12 also acts as a precursor for forming higher polyamines. The polyamine helps to condense and stabilize DNA that controls the normal function of cells. It has been reported that SAM has anticancer properties and it is used as a therapeutic agent to treat cancer.Citation13,Citation14 The role of methylation especially in the promoter region was thought to repress gene expression based on the specific cell typeCitation15 and in some cases methylation-mediated gene expression, but still the more precise role of methylation in gene expression remains unclear. Similarly, there is no report that associates SAM with osteosarcoma stem cells.
TCTP is an evolutionally conserved protein from yeast to human,Citation16–Citation19 and has a vital role in cell cycle,Citation20,Citation21 apoptosis,Citation22 cytoskeleton remodeling,Citation21 protein synthesis,Citation23 immune response,Citation17 development of normal body growth and development of the organisms,Citation24 and cancer.Citation22 In addition, it was reported that TCTP expression was elevated in cancer tissues and also during the regeneration of liver;Citation25 besides the protein has a major role in tumor reversion.Citation22–Citation26 Hence, TCTP is considered as a target for cancer therapy.
In this study, we investigated the effects of exogenous SAM on cancer stem cells of the osteosarcoma mouse model. We analyzed the progression after SAM administration and we studied SAM administration counteraction using Sox2 and TCTP that are linked to osteosarcoma stem cells.
Materials and methods
Experimental animals with osteosarcoma
The animal use and care, along with the protocol carried out in this study, was approved by the institutional animal ethical committee at Binzhou Medical University Hospital. For developing an osteosarcoma mouse model, aggressive osteosarcoma-forming K7M2 cells were used.Citation27 Five-month-old male BALB/c mice were injected with K7M2 cells (106 cells/20 µL) to establish primary and metastatic forms of tumor. Following injection, one set of mice was sacrificed on the third week to obtain primary tumor and another set was allowed up to 5 weeks to obtain the metastatic form of osteosarcoma. The mice were subjected to digital radiography to confirm tumor progression before sacrifice. For determining the effects of SAM on tumor development, the mice were administered SAM (100 mg/kg/day) by oral gavage from the 15th day of injection of K7M2 cells.
Immunohistochemistry
For immunohistochemistry, the dissected tissues were initially fixed in 10% formalin solution and processed to get paraffin-embedded tissue. The microtome was set at 6 µm, and the sections obtained were fixed on a microscopic glass slide. Following deparaffinization with xylene, they were hydrated with water. To block the endogenous peroxidase activity, the sections were immersed in freshly prepared 10% H2O2 and 10% methanol in 1X phosphate-buffered saline (PBS) for 20 minutes. After washing with 1XPBS, the sections were treated with 0.1% trypsin in 0.1% CaCl2 at 37°C for 5 minutes. In order to facilitate binding of specific antibodies, the sections were incubated with the primary antibody overnight at 4°C followed by the addition of a suitable secondary antibody for 30 minutes at room temperature. After washing the tissue sections, the slides were developed using a DAB (diaminobenzidine) kit as a chromogen for detecting signals. For immunohistochemistry experiments, the following primary antibodies were used: anti-Sox2 antibody (ab97959) and anti-TCTP antibody (ab37506) from Abcam (Cambridge, UK). Similarly, the secondary antibody Goat Anti-Rabbit IgG H&L (HRP) (ab6721) from Abcam was used to detect the signals.
Western blot analysis
Cell lysates were prepared from normal tissue as well as from the primary and metastatic forms of osteosarcoma tissue as per the protocol mentioned in the literature.Citation28 The proteins were isolated and resolved in 10% SDS-PAGE gel. They were then transferred to the membrane and incubated with the primary antibody (anti-Sox2 antibody [ab97959] or anti-TCTP antibody [ab37506]) at a dilution ratio of 1:500. The unbound primary antibody was washed out and further incubated with the secondary antibody (Goat Anti-Rabbit IgG H&L [HRP] [ab6721]) at a dilution ratio of 1:5,000. Later, the signals were visualized with the DAB kit.
Results
Positive effects of SAM on the osteosarcoma mouse model
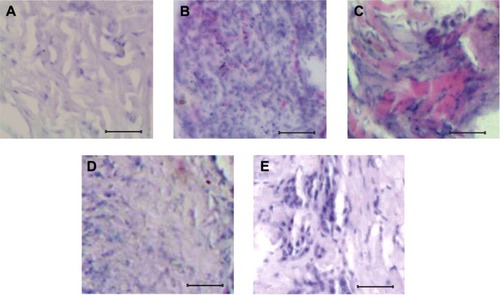
In the present study, the mouse model of osteosarcoma was successfully initiated by injecting highly tumorigenic and metastatic osteosarcoma-forming K7M2 cells. The experimental mice responded well to injected K7M2 cells, which were examined through histological observation by comparing with the control mice (). Following injection, the mice were recognized with primary tumors, which were histologically visualized on the third week after injection of K7M2 cells (). Similarly, the metastatic condition of osteosarcoma was achieved on the fifth week after injection of K7M2 cells (). When compared with the control, which had an even arrangement of cells (), the primary and metastatic osteosarcoma cells were large and deeply stained together with a disorderly arrangement of cells (). The unique feature of the metastatic form () is that it has an enlarged proliferative mass of cells with an irregular pattern of tissue arrangement compared to primary osteosarcoma (). Also, some of the cells were displaced to such an extent that the gaps between the clumps of cells were visible (). Upon SAM administration as described in the “Materials and methods” section, there was a remarkable improvement with reduction of proliferative cells, which is evident from the histological observation of primary osteosarcoma () when compared with nontreated primary osteosarcoma (). But no positive improvement was observed in SAM-treated metastatic osteosarcoma () as was analyzed with nontreated metastatic osteosarcoma (). From that, it was obvious that SAM has a positive effect in the primary stage of osteosarcoma.
Figure 1 Histological characteristics of normal and osteosarcoma tissues.
Abbreviation: SAM, S-adenosylmethionine.
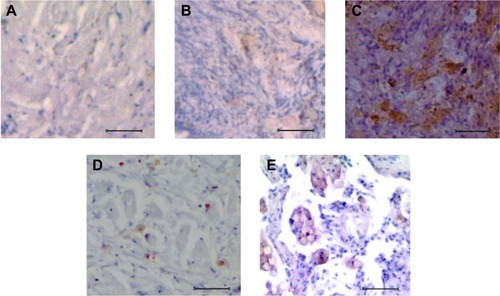
Increased expression of Sox2 in primary and metastatic osteosarcoma
The immunohistochemistry of normal bone tissue with anti-Sox2 antibody showed a minimal expression as illustrated in . But in response to osteosarcoma progression, its expression got upregulated (). In the case of primary osteosarcoma, patches of cells showed signals for Sox2 (), whereas in the metastatic form of osteosarcoma, strong signals were visualized from the background of higher proliferative mass of cells (). With SAM administration, osteosarcoma tissue showed histological improvement along with the repressed expression of Sox2 protein in the primary stage of osteosarcoma (). Following SAM treatment, the osteosarcoma development was controlled in the primary stage with a minimal expression of Sox2 along with restitution of the cellular arrangement (). But Sox2 expression was not restricted in the advanced stage of metastatic osteosarcoma ().
Figure 2 Analysis of Sox2 expression in normal and osteosarcoma tissues.
Abbreviation: SAM, S-adenosylmethionine.
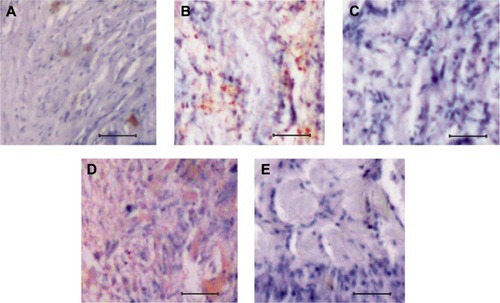
Expression studies of TCTP in primary and metastatic osteosarcoma
To understand the osteosarcoma progression and to crosscheck the results further, the expression pattern of TCTP was studied using immunohistochemistry. The control bone tissue had limited TCTP protein expression (), but upon osteosarcoma development, especially in the primary stages of osteosarcoma, there was an increased TCTP protein expression as shown in . But the advanced metastatic osteosarcoma showed only a restricted expression of TCTP with very mild signals (). After SAM treatment, interestingly, the primary osteosarcoma tissue showed an elevated expression of TCTP, which implies the fight back of cells to prevent osteosarcoma (), but in the case of metastatic osteosarcoma, the TCTP expression showed negative changes that resulted in the development of metastatic form.
Figure 3 Analysis of TCTP expression in normal and osteosarcoma tissues.
Abbreviation: SAM, S-adenosylmethionine.
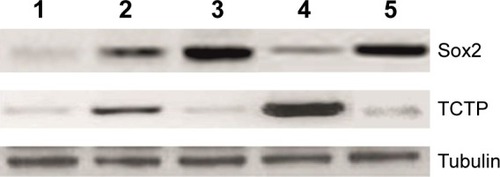
Western blotting to analyze Sox2 and TCTP expression
The results obtained using immunohistochemistry were further validated using Western blotting by comparing the Sox2 expression with that of TCTP. It is possible to precisely determine the osteosarcoma-regulating proteins in different tissues. The protein samples from normal bone tissue, primary and metastatic osteosarcoma tissues, and SAM-treated tissues were subjected to Western blotting analysis using anti-Sox2 antibody and anti-TCTP antibody (). Throughout the experiment, we observed that Sox2 was correlated with osteosarcoma progression, and upon treatment with SAM, the Sox2 level reduced along with the inhibition of tumor load in primary osteosarcoma, but SAM had no effect on metastatic osteosarcoma. The results with TCTP showed that it had an increased expression in primary osteosarcoma when compared with the normal tissue. But its expression showed some changes in metastatic osteosarcoma; on the other hand, its expression got upregulated in primary osteosarcoma after treatment with SAM ().
Figure 4 Western blotting analysis of Sox2 and TCTP expression.
Abbreviation: SAM, S-adenosylmethionine.
Discussion
The osteosarcoma mouse model has the capability to bear a resemblance to human cancer histologically that greatly helps to understand the disease biology.Citation29 In the present study, the injected K7M2 cells worked effectively in the well-established BALB/c strain of mice,Citation30 which is widely used in the osteosarcoma mouse model to study the basic science and for clinical investigations.Citation31–Citation34 In contrast, it was reported that SAM at a concentration of 150 µM through intravenous route resulted in the development of significantly smaller skeletal lesions and at the same time marked reduction in pulmonary metastasis of osteosarcoma as compared to control groups in SCID mice.Citation28 But in the present experiment, osteosarcoma-developed BALB/c mice had not shown any lesions like SCID mice. It is interesting to note that SAM had different roles in the SCID than in the BALB/c mouse strain. The experimental success of developing primary and metastatic osteosarcoma is visualized with an abnormal proliferative mass of cells together with deeply stained clusters of cells (). To understand the role of SAM in osteosarcoma, the mice were administered SAM. The role of SAM in inhibiting the primary osteosarcoma () was effective when compared with the control (). But it had no effective role in inhibiting the later stages of osteosarcoma development (), which implies that the SAM has not much potential to stop metastatic osteosarcoma.
The molecular role of SAM was identified to have an effect on many transcription factors and genes associated with growth and apoptosis.Citation35–Citation37 The function of Sox2 was also well defined in osteosarcoma that its expression is associated with triggering of the proliferative ability of normal bone tissue and the knockout of Sox2 leads to having a control over its proliferation ability.Citation38 The present study revealed the role of SAM in controlling the Sox2 expression. While the osteosarcoma progresses, the Sox2 also shows overexpression (), which implies that the development of osteosarcoma is associated with Sox2 expression. Similarly, the TCTP downregulation is also associated with osteosarcoma progression ( and ).
The regulation of Sox2 by SAM is more understandable with the experiment of SAM administration into the osteosarcoma model that inhibits tumor progression along with the downregulation of Sox2 expression (). SAM methylate-mediated downregulation of TCTP expression does not happen in primary osteosarcoma (), but similar effects occur in metastatic osteosarcoma (). On examining the results of SAM-treated metastatic osteosarcoma along with SAM-treated primary osteosarcoma, the Sox2 expression is well controlled in the primary form of osteosarcoma because SAM has a control over the initial stage of osteosarcoma but it does not happen in the advanced stage of osteosarcoma. In addition, the results of immunohistochemistry with anti-TCTP antibody illustrate that TCTP has an important role in controlling the tumor. In contrast, it was already reported that normal and transformed cells show limited expression of TCTP.Citation39 It was also reported that the TCTP levels are downregulated through activation of the tumor suppressor protein p53.Citation22,Citation40 Immunohistochemistry analysis shows elevated expression of TCTP, and the results coincide with the data illustrating. In addition to human osteosarcoma cells, other cell lines have also expressed the elevated levels of TCTP.Citation41
In conclusion, the collective results show that SAM plays a major role in controlling osteosarcoma progression. As a supportive effect of osteosarcoma reversion after SAM administration, we observed downregulation of Sox2 expression along with key upregulation of TCTP protein in primary osteosarcoma. The results obtained in this study are interesting, which promotes the prognosis of osteosarcoma for future investigation.
Acknowledgments
We thank the institutional review board approval committee and the ethical committee for the successful completion of this project.
Disclosure
The authors report no conflicts of interest in this work.
References
- PicciPOsteosarcoma (osteogenic sarcoma)Orphanet J Rare Dis20072617244349
- SmidaJBaumhoerDRosemannMGenomic alterations and allelic imbalances are strong prognostic predictors in osteosarcomaClin Cancer Res201016164256426720610556
- OttavianiGJaffeNThe epidemiology of osteosarcomaJaffeNBrulandOBielackSPediatric and Adolescent OsteosarcomaNew YorkSpringer2009313
- TanMLChoongPFDassCROsteosarcoma – conventional treatment vs gene therapyCancer Biol Ther20098210611719098456
- AllisonDCCarneySCAhlmannERA meta-analysis of osteosarcoma outcomes in the modern medical eraSarcoma2012201270487222550423
- MeyersPAMuramyl tripeptide (mifamurtide) for the treatment of osteosarcomaExpert Rev Anticancer Ther2009981035104919671023
- SzuhaiKCleton-JansenA-MHogendoornPCBovéeJVMolecular pathology and its diagnostic use in bone tumorsCancer Genet2012205519320422682618
- EstellerMCancer epigenomics: DNA methylomes and histone-modification mapsNat Rev Genet20078428629817339880
- DasPMRamachandranKVanWertJMethylation mediated silencing of TMS1/ASC gene in prostate cancerMol Cancer2006512816848908
- Al-RomaihKSadikovicBYoshimotoMWangYZielenskaMSquireJADecitabine-induced demethylation of 5′ CpG island in GADD45A leads to apoptosis in osteosarcoma cellsNeoplasia200810547148018472964
- CoteGMChoyERole of epigenetic modulation for the treatment of sarcomaCurr Treat Options Oncol201314345446423749746
- ChiangPGordonRTalJS-Adenosylmethionine and methylationFASEB J19961044714808647346
- LukaZMuddSHWagnerCGlycine N-methyltransferase and regulation of S-adenosylmethionine levelsJ Biol Chem200928434225072251119483083
- BaleSEalickSEStructural biology of S-adenosylmethionine decarboxylaseAmino Acids201038245146019997761
- SuzukiMMBirdADNA methylation landscapes: provocative insights from epigenomicsNat Rev Genet20089646547618463664
- BöhmHBenndorfRGaestelMThe growth-related protein P23 of the Ehrlich ascites tumor: translational control, cloning and primary structureBiochem Int19891922772862479380
- MacDonaldSMRafnarTLangdonJLichtensteinLMMolecular identification of an IgE-dependent histamine-releasing factorScience199526952246886907542803
- ThawPBaxterNJHounslowAMPriceCWalthoJPCravenCJStructure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperonesNat Struct Mol Biol200188701704
- YenofskyRCereghiniSKrowczynskaABrawermanGRegulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxideMol Cell Biol198337119712036577280
- BrioudesFThierryA-MChambrierPMollereauBBendahmaneMTranslationally controlled tumor protein is a conserved mitotic growth integrator in animals and plantsProc Natl Acad Sci U S A201010737163841638920736351
- GachetYTournierSLeeMLazaris-KaratzasAPoultonTBommerU-AThe growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycleJ Cell Sci199911281257127110085260
- TuynderMSusiniLPrieurSBiological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1Proc Natl Acad Sc U S A20029923149761498112399545
- CansCPasserBJShalakVTranslationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1AProc Natl Acad Sci U S A200310024138921389714623968
- ChenSHWuP-SChouC-HA knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific mannerMol Biol Cell20071872525253217475776
- ZhuW-LChengH-XHanNMessenger RNA expression of translationally controlled tumor protein (TCTP) in liver regeneration and cancerAnticancer Res2008283A1575158018630514
- ArcuriFPapaSCarducciATranslationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activityProstate200460213014015162379
- KhannaCKhanJNguyenPMetastasis-associated differences in gene expression in a murine model of osteosarcomaCancer Res20016193750375911325848
- ParasharSCheishviliDArakelianAS-Adenosylmethionine blocks osteosarcoma cells proliferation and invasion in vitro and tumor metastasis in vivo: therapeutic and diagnostic clinical applicationsCancer Med20154573274425619880
- EkETDassCRChoongPFCommonly used mouse models of osteosarcomaCrit Rev Oncol Hematol20066011816837208
- SchmidtJStraußGPSchönAEstablishment and characterization of osteogenic cell lines from a spontaneous murine osteosarcomaDifferentiation19883931511603243385
- WanXMendozaAKhannaCHelmanLJRapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcomaCancer Res20056562406241115781656
- TerabeMKhannaCBoseSCD1d-restricted natural killer T cells can down-regulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-βCancer Res20066673869387516585215
- BarkanDKleinmanHSimmonsJLInhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeletonCancer Res200868156241625018676848
- MerchantMSMelchiondaFSinhaMKhannaCHelmanLMackallCLImmune reconstitution prevents metastatic recurrence of murine osteosarcomaCancer Immunol Immunother20075671037104617149595
- LuSCMatoJMRole of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancerAlcohol200535322723416054984
- ChenHXiaMLinMRole of methionine adenosyltransferase 2A and S-adenosylmethionine in mitogen-induced growth of human colon cancer cellsGastroenterology2007133120721817631143
- LiTWZhangQOhPS-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cellsMol Pharmacol200976119220019372210
- Basu-RoyUAmbrosettiDFavaroRNicolisSKMansukhaniABasilicoCThe transcription factor Sox2 is required for osteoblast self-renewalCell Death Differ20101781345135320489730
- BommerU-AThieleB-JThe translationally controlled tumour protein (TCTP)Int J Biochem Cell Biol200436337938514687915
- NiforouKNAnagnostopoulosAKVougasKKittasCGorgoulisVGTsangarisGTThe proteome profile of the human osteosarcoma U2OS cell lineCancer Genomics Proteomics200851637818359981
- ShenJHQuCBChuHKsiRNA targeting TCTP suppresses osteosarcoma cell growth and induces apoptosis in vitro and in vivoBiotechnol Appl Biochem201663151425522670
