Abstract
Approximately a fifth of ovarian carcinoma (OC) is associated with inherited germline mutations, most commonly in the DNA repair genes BRCA1 or BRCA2 (BRCA). BRCA1- and BRCA2-associated OCs have historically been described as a single subgroup of OC that displays a distinct set of characteristics termed the “BRCAness” phenotype. The hallmarks of this phenotype are superior clinical outcome and hypersensitivity to platinum-based chemotherapy and poly-(ADP-ribose) polymerase (PARP) inhibitors. However, growing evidence suggests that BRCA1- and BRCA2-associated OCs display distinct characteristics, most notably in long-term patient survival. Furthermore, recent data indicate that the site of BRCA1 mutation is important with regard to platinum and PARP inhibitor sensitivity. Here, we summarize the body of research describing the BRCAness phenotype and highlight the differential implications of different BRCA mutations with regard to clinicopathologic features, therapy sensitivity and clinical outcome in OC.
Keywords:
Introduction
Ovarian cancer accounts for ~21% of malignancies diagnosed in the female genital tract and is responsible for >14,000 deaths per annum in the US alone.Citation1 More than 90% of cases are epithelial in origin. Ovarian carcinoma (OC) is now recognized to comprise a heterogeneous group of discrete disease entities, each displaying distinct clinical behavior and molecular landscapes.Citation2,Citation3 The current standard of care for the first-line treatment of OC comprises maximal surgical resection of the tumor mass and platinum-based chemotherapy, usually in combination with paclitaxel.Citation4 While some therapy stratification based on our understanding of disease biology is beginning to emerge in OC – most notably in the advent of poly-(ADP-ribose) polymerase (PARP) inhibitor therapy – personalization of OC treatment based on histological subtype and molecular characterization remains in its infancy.Citation4,Citation5
Hereditary OC accounts for a significant proportion of cases, with around a fifth of patients harboring germline pathogenic sequence variants.Citation6 A large proportion of these mutations occur within genes encoding components of the homologous recombination DNA repair (HRR) pathway, most notably in BRCA1 or BRCA2 (BRCA), which together account for ~10% of OC cases.Citation7 Other inherited mutations in HRR pathway-related genes include BARD1, BRIP1, CHEK2, PALB2 and RAD51C, which together account for a minority (≤5%) of cases.Citation6
Historically, BRCA-associated OC has been described as a single subtype of OC that displays a distinct set of characteristics – frequently referred to as the “BRCAness” phenotype.Citation8 However, the differential impact of BRCA1 versus BRCA2 inactivation has become increasingly apparent in recent years.Citation9 Here, we summarize the growing body of evidence describing the BRCAness phenotype and highlight the emerging evidence of the distinct implications of different BRCA mutations on the treatment and clinical outcome of OC patients.
Structure and function of BRCA genes
BRCA1
Since its identification in 1994, BRCA1 has become one of the most extensively studied tumor suppressor genes to date.Citation10 BRCA1 comprises 24 exons coding for 1863 amino acids, more than half of which are encoded by exon 11.Citation11 Its 208 kDa protein product, BRCA1, contains an N-terminal RING domain with E3 ligase activity and a phosphoprotein-binding C-terminal BRCT domain, encoded by exons 2–7 and 16–24, respectively ().Citation12–Citation16 Exons 11–13 are known to encode a region with two nuclear localization sequences (NLSs) and protein-binding domains for a multitude of proteins involved in various signaling pathways, including multiple tumor suppressors, oncogenes and DNA repair-associated proteins.Citation17,Citation18 These include portions of a coiled-coil domain, which are known to mediate interactions with PALB2, and a serine cluster domain (SCD) whose phosphorylation sites are targeted by ATM and ATR kinases in response to DNA damage.Citation11,Citation19 Cancer-predisposing BRCA1 mutations are known to occur across these three regions, indicating important tumor suppressive function in each region.Citation11
Figure 1 Structure of BRCA1 and BRCA2 genes, showing regions encoding identified protein domains, BCCRs and OCCRs.
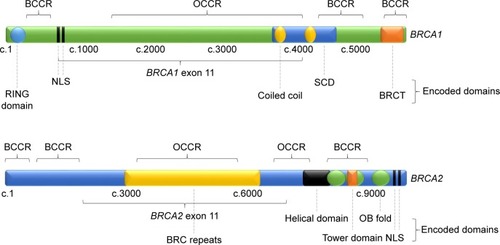
BRCA1 is multifunctional, with roles in the DNA damage response, cell cycle checkpoint maintenance and DNA repair.Citation20–Citation24 BRCA1 is known to play a role in maintaining the G1/S, S-phase and G2/M cell cycle checkpoints; however, its principally associated role is in repair of double-stranded DNA breaks (DSB), primarily through HRR.Citation20–Citation24 Briefly, BRCA1 associates with ubiquitinated histones at DSBs and facilitates break resection and subsequent recruitment of RAD51 through interaction with PALB2 and BRCA2.Citation25,Citation26 Accordingly, loss of BRCA1 expression renders cells hypersensitive to ionizing radiation and interstrand DNA crosslinking agents, consistent with loss of high fidelity DSB repair.Citation20,Citation21
BRCA2
BRCA2 comprises 27 exons encoding 3418 amino acids, which form its 384 kDa protein product, BRCA2, also involved in repair of DSBs through HRR.Citation27,Citation28 BRCA2 exon 11 contains eight highly conserved BRC repeats that are known to interact with RAD51, an essential HRR protein whose family members RAD51C and RAD51D have been identified as OC susceptibility genes ().Citation6,Citation29–Citation33 The C-terminal region of BRCA2 also interacts with RAD51 and is known to contain two NLS.Citation34
BRCA2 contains a DNA-binding domain comprising an α-helical domain, a tower domain and three oligonucleotide-binding (OB) motifs for binding single- and double-stranded DNA (ssDNA and dsDNA).Citation26,Citation35 Pathogenic mutations have been detected across the length of BRCA2, including in its BRC repeats and DNA-binding domain.Citation9
While BRCA1 is multifunctional, BRCA2 appears to function almost exclusively in HRR: it recruits RAD51 to DSB sites, a crucial step in repair.Citation28 BRCA2-mutant cells are hypersensitive to DNA damage, accumulate gross DNA damage with passage in culture and fail to recruit RAD51 to DSB sites, but do not appear to demonstrate substantial cell cycle checkpoint impairment.Citation36–Citation39
Clinicopathologic features of BRCA-associated OC
Cancer predisposition in BRCA1 and BRCA2 carriers
BRCA mutation carriers are predisposed to a number of malignancies, most notably OC and breast cancer (BC). However, the level of risk for the development of OC and BC appears dependent upon the affected gene.Citation40,Citation41 The average cumulative risk of BRCA1 carriers developing BC and OC by the age of 70 is ~50%–60% and 40%–50%, respectively, while the equivalent risk in BRCA2 carriers is substantially lower at ~40%–50% and 10%–20%, receptively.Citation40–Citation42
Growing evidence has begun to elucidate the discrete impact of the type and location of BRCA1 and BRCA2 mutations with regard to cancer predisposition.Citation43–Citation46 These studies were founded on the early observation that carriers of mutations in the central portion of BRCA1 exon 11 displayed an augmented risk of OC versus BC relative to those with mutations in other areas of the gene.Citation43,Citation44 Similarly, early observations identified increased risk of OC versus BC in those harboring mutations in exon 11 of BRCA2 versus mutations in other regions.Citation45
A recent study sought to more thoroughly investigate the relationship between BRCA mutation position and differential OC versus BC predisposition in an extensive cohort of BRCA carriers.Citation46 Analysis of BRCA1 mutation positions revealed three regions associated with increased BC versus OC risk relative to mutations in other areas of the gene. These conferred a relative hazard ratio (HR) of BC versus OC (BC-RHR) ranging from 1.34 to 1.46. A cluster region within BRCA1 exon 11 conferring increased risk of OC versus BC development, relative to other areas of the gene, was also identified (BC-RHR =0.62, 95% CI, 0.56–0.70).Citation46 This is consistent with previous reports of BRCA1 exon 11 mutations with augmented OC risk.Citation43,Citation44 Such BC cluster regions (BCCRs) and OC cluster regions (OCCRs) were also identified in BRCA2: multiple BRCA2 BCCRs and OCCRs were identified with BC-RHRs ranging from 1.63 to 2.31 and 0.51 to 0.57, respectively.Citation46
Age at disease onset
As with many cancer predisposition syndromes, BRCA-linked OC is associated with earlier age at diagnosis.Citation47–Citation49 Interestingly, BRCA1 carriers appear to develop OC at an average of ~7 years earlier versus nonhereditary OC patients, while BRCA2 carriers do not display a strong trend for earlier diagnosis ().Citation47,Citation50–Citation53 BRCA1 mutations account for over 80% of BRCA-associated OC diagnosed below the age of 50, while BRCA2 carriers account for ~60% of BRCA-associated OC diagnosed at >60 years old, despite the higher prevalence of BRCA1 versus BRCA2 mutations in OC.Citation54
Table 1 Characteristics of BRCA1-associated, BRCA2-associated, and BRCA wild-type OC
Histological subtype of OC
OC is largely grouped into five core histologically defined subtypes (histotypes): high-grade serous (HGS), endometrioid, clear cell (CC), low-grade serous (LGS) and mucinous OC, which together represent over 95% of presenting cases.Citation55 HGS OC represents the bulk (~70%) of cases, while the endometrioid, CC, LGS and mucinous histotypes are reported to account for ~10%, 10%, <5% and 3% of OC, respectively.Citation2,Citation55,Citation56 These histotypes represent inherently different tumors, displaying differential chemosensitivity and survival, and are now acknowledged to have discrete developmental origins.Citation57–Citation62 Indeed, a wealth of evidence now illustrates that these represent separate disease entities at both the genomic and transcriptomic levels.Citation3,Citation62–Citation65
While a minority of BRCA-mutant CC and endometrioid OC have also been identified, BRCA mutations are associated predominantly with HGS OC.Citation8,Citation47,Citation66 Germline BRCA mutations account for ~15% of HGS OC, with an additional 5%–10% displaying somatic BRCA mutations.Citation3,Citation63,Citation67
Metastasis to the viscera
Although the vast majority of OC are diagnosed at advanced stage, disease is frequently confined to the peritoneal cavity, even at recurrence.Citation68 Even when distant metastases are present, the majority involve nonvisceral sites.
BRCA-linked OC has been associated with an increased frequency of visceral metastasis, most notably to the liver: approximately three in four patients with germline BRCA mutations who develop OC display visceral metastasis, while the rate in nonhereditary OC patients is estimated at less than 20%.Citation69 BRCA1 mutation carriers appear to have a particular propensity to develop visceral metastases: while investigations to date have been limited, current data suggest that almost all BRCA1 carriers develop disease at visceral sites, compared to only around half of BRCA2 carriers.Citation69,Citation70 Furthermore, BRAC1-associated OC has also been shown to display an increased rate of brain metastasis specifically.Citation70
Chemosensitivity
Platinum-based chemotherapy
A predominant characteristic of the BRCAness phenotype is their sensitivity to platinum-based DNA-damaging agents, even upon repeated exposure at disease recurrence.Citation8,Citation49,Citation71 Tan et alCitation8 demonstrated that the majority of BRCA-associated OC patients experience partial or complete response to platinum-based agents in the second- and third-line settings, compared to less than half and less than one-tenth of matched controls, respectively. However, they did not compare rates in a BRCA1- and BRCA2-mutant gene-specific manner. The superior sensitivity of BRCA-associated OC to platinum agents was confirmed in later studies of BRCA-associated versus nonhereditary OC.Citation49,Citation71
Yang et alCitation53 compared the frequency of primary platinum sensitivity of BRCA1 versus BRCA2-associated HGS OC. They observed a significantly superior primary platinum sensitivity in the BRCA2- versus BRCA1-mutant population: 100% of BRCA2-associated OC (25 of 25 in their cohort) displayed primary platinum sensitivity versus 80% (24 of 30 in their cohort) of BRCA1-associated OC.Citation53 They also observed a 5.5-month superior platinum-free interval in BRCA2 versus BRCA1 carriers and a “mutator phenotype” indicative of high genome instability in BRCA2-associated OC.Citation53 Similarly, Vencken et alCitation71 reported prolonged treatment-free intervals in BRCA2- versus BRCA1-associated OCs, although no significantly superior primary response rate was detected.
While investigations are beginning to dissect the differential implications of BRCA1 versus BRCA2 mutations with regard to chemosensitivity, less is known about the implications of the exact mutation site within each of the two genes. Recent work has begun to elucidate the distinct implication of frameshift-inducing mutations that occur in exon 11 of BRCA1.Citation72
In vitro, cells harboring BRCA1 exon 11 frameshifting mutations (E11mut) were found to express a BRCA1 isoform missing the majority of exon 11 (BRCA1Δ11q). While wild-type cells and cells harboring mutations outside of exon 11 (OE11mut) displayed resistance and sensitivity to cisplatin, respectively, E11mut cells displayed partial platinum resistance.Citation72 E11mut cells were able to form RAD51 and BRCA1 foci in response to ionizing radiation, indicating at least partial HRR proficiency. Interestingly, a recent investigation of OC patients harboring BRCA1 exon 11 mutations revealed no significantly superior platinum response rate versus the wild-type population.Citation73
While the functional characterization of BRCA1 exon 11 remains poor, shrouding the mechanisms that underpin the partial HRR proficiency of E11mut cells, mutations in better characterized portions of the gene have also been correlated with chemosensitivity.Citation74,Citation75 Recent investigations suggest that while BRCA1 RING domain function appears important for tumor suppression, hypomorphic BRCA1 isoforms lacking RING domain function display platinum resistance.Citation74,Citation75 Introduction of the missense brca1C61G mutation into murine models demonstrated the poor efficacy of platinum agents against brca1C61G breast carcinomas in a study by Drost et al.Citation74 They later compared the effects of two BRCA1 truncating mutations, reflecting two known founder mutations in the Ashkenazi Jewish population, on chemosensitivity.Citation75–Citation77 This study demonstrated that introduction of brca1185stop, reflective of the BRCA1185delAG founder mutation, led to production of a RING-less BRCA1, which mediated resistance to cisplatin.Citation75
Together, these data demonstrate a clear differential impact for different BRCA mutations. While both BRCA1 and BRCA2 mutations confer superior sensitivity to platinum-based chemotherapy, this phenotype may be exaggerated in BRCA2-associated OC. This is perhaps because BRCA2-associated OC is rendered HRR defective to a greater extent than BRCA1-associated tumors, manifesting as extensive genomic instability and exquisite sensitivity to DNA damage.Citation53 Furthermore, evidence that not all BRCA1 mutations are equal is beginning to emerge. Specifically, mutations in exon 11 and mutations that abrogate RING domain function appear to result in the production of hypomorphic BRCA1 isoforms that mediate resistance to platinum agents but still predispose carriers to OC development.Citation72–Citation74 This is consistent with the multifunctional role of BRCA1 in tumor suppression and suggests that multiple aspects of BRCA1 functionality, particularly RING domain function, appear dispensable for HRR function.
Taxanes
Taxanes are typically used in combination with platinum agents in the treatment of OC but can also be used as single agents, usually in the context of platinum resistance.Citation4,Citation78–Citation80 They are distinct from DNA-damaging agents in their mechanism of action, primarily functioning via induction of cell cycle arrest at the spindle assembly checkpoint through disruption of microtubule disassembly.Citation81 Paclitaxel sensitivity may therefore be dependent on intact cell cycle checkpoint regulation. Indeed, paclitaxel treatment has been shown to induce acute G2/M arrest in the context of BRCA1 expression.Citation82 Given both the known function of BRCA1 in cell cycle checkpoint regulation and the suggestion that there may be an inverse relationship between paclitaxel and cisplatin sensitivity in a range of malignancies, cells may be expected to demonstrate paclitaxel resistance in the absence of BRCA1 function.Citation23,Citation24,Citation83
A number of in vitro studies have provided evidence that BRCA1 may play a role in modulating paclitaxel sensitivity.Citation82,Citation84–Citation89 BRCA1-defective BC and head and neck squamous cell carcinoma (HNSCC) cells are more resistant to paclitaxel treatment versus BRCA1-proficient cells, suggesting BRCA1-associated OC may display paclitaxel resistance.Citation82,Citation84–Citation87 Additionally, BRCA1 loss appears to modulate microtubule dynamics rendering them less susceptible to the action of paclitaxel.Citation88 However, some in vitro studies have reported conflicting results on the role of BRCA1 in modulating taxane sensitivity.Citation90
In line with the notion that BRCA1 deficiency may mediate taxane resistance, expression of BRCA1 was associated with longer time to progression in a taxane-treated cohort of BC.Citation91 However, clinical data regarding the sensitivity of BRCA-linked OC to taxane monotherapy are severely limited, with most data described in the context of combination with platinum agents. There has been a suggestion that OC expressing high BRCA1 mRNA levels may benefit from addition of taxanes to platinum, while those with low levels do not, though these data are yet to be confirmed in a comprehensive cohort of OC.Citation92 It has been shown that BRCA-linked OC can benefit from paclitaxel monotherapy in both the platinum-sensitive and platinum-resistant relapsed disease settings (response rate 60%, 9 of 15 patients and 33%, 3 of 9 patients, respectively); however, meaningful comparison of taxane monotherapy efficacy between BRCA-linked and BRCA wild-type OC has not been conducted.Citation93 Critically, the existing data have examined BRCA-associated OC as a single entity.
While the current data suggest that BRCA1-associated OC may be more resistant to paclitaxel, further studies are required to investigate this relationship in the clinical setting.Citation71 Given the preclinical evidence suggesting that BRCA1 mutation specifically may mediate taxane resistance, a comprehensive comparison of BRCA-mutant versus BRCA wild-type OC in a gene-specific manner, is now needed to elucidate the implication of BRCA status with regard to taxane monotherapy. Because BRCA2 appears to function almost exclusively in HRR, and the mechanism of action of taxanes does not seem to involve induction of DNA damage, there is no clear rationale for differential paclitaxel sensitivity between BRCA2-associated and BRCA wild-type OC. This represents a potential pitfall for therapeutic stratification of taxanes while all BRCA-associated OCs continue to be considered as a single clinical entity. Future stratification within this population specifically will require a wider appreciation of the distinction between “BRCA1ness” and “BRCA2ness” in clinical practice.
Nonplatinum DNA-damaging agents
Nonplatinum nontaxane chemotherapies are also used in the treatment of OC, primarily in the platinum-resistant relapsed disease setting.Citation94,Citation95 Pegylated liposomal doxorubicin (PLD) represents one such drug whose mechanism of action involves DNA damage.
Retrospective studies examining differential response rate to PLD have reported superior response and superior clinical outcome after PLD treatment in BRCA-associated OC versus nonhereditary disease.Citation96,Citation97 Differential sensitivity to nonplatinum DNA-damaging agents between BRCA1-and BRCA2-mutated OC may be expected to reflect those observed for platinum agents; however, these comparisons are yet to be made in the context of PLD monotherapy. Similarly, mutations in BRCA1 exon 11 or mutations that affect RING domain function may be expected to confer differential sensitivity phenotypes versus other BRCA1 mutations.
Intraperitoneal chemotherapy administration
While the majority of OC treatment is given intravenously (IV), chemotherapy may also be administered intraperitoneally (IP).Citation4,Citation98,Citation99 IP chemotherapy achieves higher concentrations of drug within the peritoneum compared to IV administration, delivering dose intense chemotherapy to the tumor.Citation99–Citation101
Multiple randomized trials have shown a survival benefit for IP administration in advanced-stage OC, particularly in the context of optimal surgical debulking.Citation102–Citation106 Although uptake of IP administration has increased, IV therapy remains the predominant treatment protocol in many centers.Citation107 Cost and resource implications for IP administration, as well as increased therapy-associated gastrointestinal toxicity, pain, and infection among IP-treated patients, have undoubtedly contributed to variable uptake of treatment regimens.Citation108 Thus, identification of OC subgroups who are likely to benefit most from IP administration is an area of keen research interest.
Because BRCA-mutant OC is hypersensitive to platinum agents, it is plausible that BRCA status modulates the efficacy of this dose intense administration route. This hypothesis has in part been explored in the GOG 172 study: this phase III trial comparing IP versus IV cisplatin and paclitaxel reported greater clinical benefit for OC in the IP arm whose patients expressed low levels of BRCA1 protein.Citation109
These data suggest an interaction between BRCA status and administration route: the higher concentrations of chemotherapy achieved locally during IP treatment may well be particularly effective in treating HRR-defective tumors. Importantly, these data were limited to immunohistochemistry of BRCA1 protein, and we therefore await translational analysis of IP-treated OC with matched sequencing data for both BRCA1 and BRCA2 in order to fully overlap these genomic features with IP chemotherapy outcome. Analysis of IP chemotherapy efficacy in BRCA wild-type OC will undoubtedly shed light on whether the clinical benefit, if any, experienced in this patient group is outweighed by excessive toxicity.
Neoadjuvant chemotherapy
Historically, standard OC treatment begins with primary debulking surgery (PDS) of the tumor mass followed by adjuvant platinum-based or platinum-taxane combination chemotherapy.Citation4 However, neoadjuvant chemotherapy (NAC) followed by interval debulking surgery (IDS) is increasingly used in OC management and is thought to reduce postsurgical mortality and morbidities.Citation110,Citation111 Two large trials have demonstrated NAC as noninferior to PDS in the treatment of advanced stage OC.Citation110,Citation112 However, a recent multi-institutional study reported inferior OS in NAC-treated OC with stage IIIC disease who achieved optimal primary surgical debulking, and there is a clear need to dissect exactly which OC patients will benefit most from NAC versus PDS.Citation113
Although there has been no prospective comparison of NAC versus PDS in BRCA-associated OC specifically, early data are suggesting that BRCA-mutant OC may be associated with improved response to NAC.Citation114 These findings are consistent with the association between BRCA mutation and hypersensitivity with platinum.Citation8,Citation49,Citation71
Alarmingly, and in keeping with the concern that NAC may promote platinum resistance, the limited data available suggest that NAC may provide a selection pressure toward BRCA-proficient cells.Citation114 NAC may therefore compromise the exquisite platinum sensitivity of BRCA-associated OC by exposing a clonally diverse mass to the selection pressure of DNA-damaging agents.Citation115,Citation116 Thus, BRCA carriers may benefit most from PDS followed by adjuvant chemotherapy directed at residual disease, in the hope that HRR-proficient subclones representing a route of chemoresistance may have been surgically removed prior to application of a selection pressure.
Sensitivity to PARP inhibition
Cells harboring BRCA1 or BRCA2 mutation are heavily reliant upon PARP-mediated DNA repair of ssDNA breaks.Citation117 PARP-inhibited cells are thought to accumulate ssDNA damage, which is converted to DSBs during subsequent cellular replication, whether through defective ssDNA damage repair or PARP trapping at DNA damage sites.Citation117–Citation120 In the context of HRR deficiency, accumulation of unrepaired DSBs results in cytotoxicity and cell death, and BRCA mutations therefore exhibit synthetic lethality with PARP inhibition.Citation121 Indeed, the PARP inhibitors olaparib, rucaparib and niraparib have shown marked antitumor activity in monotherapy or maintenance phase II and phase III trials of OC patients with particularly marked efficacy demonstrated in patients with germline BRCA defects.Citation122–Citation130 Olaparib and rucaparib are now licensed by the FDA as a monotherapy for recurrent OC in this patient population and olaparib is licensed by the European Medicines Agency as a maintenance therapy following a response to chemotherapy in patients with germline or somatic BRCA mutations.
While both BRCA1 and BRCA2 mutations sensitize cells to PARP inhibition, the affected gene appears to have a modulating effect on sensitivity: BRCA1-defective cells demonstrate ~60-fold increase in sensitivity to olaparib versus BRCA wild-type cells, while the corresponding increase in sensitivity in BRCA2-defective cells is ~130-fold.Citation121 However, data regarding differential response rates of BRCA1 versus BRCA2 carriers to PARP inhibition in the clinical setting are currently limited. Some data suggest a trend for slightly superior response rate in BRCA2-associated OC treated with PARP inhibitors, while others report no difference in sensitivity or PFS, and the consensus remains that BRCA-associated OC is considered as a single clinical entity with regard to PARP inhibitor sensitivity.Citation122–Citation130
While the distinction in sensitivity between BRCA1-and BRCA2-associated OCs remains unclear in the clinical setting, emerging in vitro data suggest that the location of BRCA1 mutation may influence the efficacy of PARP inhibitors.Citation72,Citation74,Citation75 Consistent with the notion that the hypomorphic BRCA1 isoform BRCA1Δ11q can mediate partial HRR function and consequentially platinum resistance, cells harboring BRCA1 E11mut cells also appear to display an intermediate partially PARP inhibitor-resistance phenotype.Citation72 Similarly, loss of BRCA1 RING domain function appears insufficient to fully sensitize cells to PARP inhibition, while still predisposing to cancer development.Citation74,Citation75
Given the financial implications of targeted therapy use in routine clinical practice, identifying patients most likely to benefit from these drugs is of great importance. Comparison of PARP inhibitor sensitivity in patients harboring BRCA1 exon 11 and RING domain mutations with BRCA wild-type patients is warranted to determine whether these patients represent a truly HRR-deficient population that benefit from PARP inhibition.
BRCA mutations in acquired therapy resistance
In recent years, secondary BRCA mutations have been implicated in platinum and PARP inhibitor resistance.Citation131 These mutations restore BRCA function and HRR proficiency by restoring open-reading frames, reverting mutant alleles back to wild type or removing premature stop codons.Citation132–Citation138 Such mutations are a known mechanism of cisplatin and PARP inhibitor resistance when deriving drug-resistant clones in vitro.Citation133–Citation135 In keeping with the notion that these secondary events are associated with acquired therapy resistance, secondary BRCA2 mutations have been detected in cell lines derived from patients subsequent to chemotherapy, and these cells are reported to display platinum resistance.Citation133,Citation134,Citation139,Citation140
Mutational analysis of clinical specimens has also revealed the presence of secondary BRCA sequence events.Citation132–Citation138 Secondary mutations have been detected in both BRCA1 and BRCA2 and correlated with resistance to platinum-based chemotherapy.Citation132–Citation135,Citation138 Analysis of BC and OC with acquired PARP inhibitor resistance has also uncovered secondary BRCA reversion events and demonstrated their potential to predict platinum and PARP inhibitor resistance at recurrence in BRCA-associated OC.Citation136,Citation137
Clinical outcome
Progression-free survival
Multiple studies have investigated the prognostic significance of BRCA mutations on PFS and OS within OC.Citation8,Citation47,Citation53,Citation66,Citation71,Citation141–Citation146 It has become clear that, together, BRCA-associated disease represents a subgroup of OC that experiences superior PFS, with studies reporting BRCA-mutant patients experience PFS around twice that of their BRCA wild-type counterparts.Citation71,Citation144–Citation146 Although many studies have failed to analyze PFS in a gene-specific manner, others have suggested that BRCA1-associated OC may experience inferior PFS versus BRCA2-associated OC.Citation53,Citation71,Citation147 Indeed, some investigators have suggested that BRCA1-associated OC may not experience a PFS benefit compared to BRCA wild-type OC.Citation53,Citation148
A recent meta-analysis of over 18,000 OC patients reported superior PFS in both BRCA1- and BRCA2-associated OCs.Citation149 They reported HRs for PFS in BRCA1- and BRCA2-associated versus BRCA wild-type OC of 0.68 (95% CI, 0.52–0.89) and 0.48 (95% CI, 0.30–0.75), respectively. Interestingly, a recent study of BRCA1 exon 11 mutation-associated OC revealed no PFS benefit versus the wild-type population, suggesting an interaction between mutation site and PFS.Citation73
Overall survival
A fundamental characteristic of the BRCAness phenotype is superior OS.Citation8,Citation47,Citation53,Citation141,Citation146,Citation150–Citation154 Recent work has begun to elucidate the distinction between BRCA1 and BRCA2 mutations with regard to survival.Citation53,Citation66,Citation143,Citation155 The current consensus is that both BRCA1- and BRCA2-mutated OCs experience superior short-term OS; however, this survival advantage seems exaggerated in BRCA2- versus BRCA1-mutant disease.Citation66,Citation143,Citation147,Citation155,Citation156 Five-year survival in BRCA1- and BRCA2-mutant OC is estimated at ~44% and 52%–61%, respectively, versuŝ25%–42% in BRCA wild-type OC.Citation53,Citation66,Citation143
While BRCA2 carriers continue to experience superior long-term OS, the survival of BRCA1-mutant OC patients appears limited to ~5 years, with investigators reporting no 10-year OS advantage in this group.Citation143,Citation155 Hyman et alCitation155 reported long-term survival benefit in BRCA2-associated serous OC versus the BRCA wild-type population, with no such benefit in the BRCA1-mutant population. Later, Candido-dos-Reis et alCitation143 reported 10-year OS in BRCA1-associated, BRCA2-associated and BRCA wild-type OC of 25%, 35% and 30%, respectively, in a large cohort of OC. Their study showed an increasingly detrimental effect for BRCA1 mutation after ~5 years compared to both BRCA2-mutated and BRCA wild-type populations.
The recent meta-analysis by Xu et alCitation149 reported HRs for OS in BRCA1- and BRCA2-associated versus BRCA wild-type OC of 0.73 (95% CI, 0.63–0.86) and 0.57 (95% CI, 0.45–0.73), respectively. The study by Dimitrova et alCitation73 of BRCA1 exon 11-associated OC revealed no 5-year OS benefit in this population versus the wild-type population, suggesting that all BRCA1 mutations are not equal in conveying survival advantage.
Key future research avenues
Dissecting BRCA1ness from BRCA2ness
A key aim of future research is to continue to dissect the distinct phenotypes of BRCA1- and BRCA2-associated OCs, both from one another and from BRCA wild-type OC. Critically, this will rely on investigators conducting gene-specific analyses. It is becoming clear that patients with BRCA2-associated OC experience an exaggerated BRCAness phenotype, displaying superior long-term OS in comparison to BRCA1-associated OC, and emerging data suggest that superior PFS and platinum sensitivity may also be exaggerated in this patient group.Citation53,Citation66,Citation71,Citation143,Citation147,Citation148,Citation155,Citation156
Future studies should aim to elucidate the differential sensitivity, if any, of BRCA1- and BRCA2-associated OCs to nonplatinum agents, including nonplatinum DNA-damaging agents, taxanes and PARP inhibitors. It has been suggested that BRCA1-associated OC may be more resistant to paclitaxel, and we await data from independent cohorts investigating the potential impact of BRCA1 and BRCA2 mutations with regard to taxane monotherapy sensitivity.Citation71,Citation82,Citation84–Citation89,Citation91,Citation92 While in vitro data suggest that BRCA2-mutant cells are more sensitive to PARP inhibition compared to BRCA1-mutant cells, this comparison is yet to be made in the clinical setting.Citation122–Citation130 Similarly, characterization of how BRCA1 and BRCA2 mutations may modulate clinical outcome in the context of NAC and IP chemotherapy administration is now warranted. An appreciation of the distinction between BRCA1ness and BRCA2ness by both researchers and clinicians will be paramount in the translation of findings from these studies into clinical practice.
Correlating mutation site and type to chemosensitivity and clinical outcome
While some studies have investigated the impact of BRCA1 and BRCA2 mutation site on chemosensitivity and OC versus BC predisposition, the differential impact of distinct BRCA mutation sites remains largely understudied.Citation40–Citation46
Growing data suggest that BRCA1 E11mut cells display a distinct partially platinum- and PARP inhibitor-resistant phenotype, and OC patients harboring BRCA1 mutations in exon 11 may not experience a BRCAness survival benefit.Citation72,Citation73 Similarly, BRCA1 mutations affecting RING domain function may also not display hypersensitivity to platinum or PARP inhibition.Citation74,Citation75 Further investigation of these findings in well clinically annotated OC datasets is now warranted to elucidate whether these groups of patients represent a non-BRCAness, partially HRR proficient subgroup of OC. It may transpire that after removal of these patient groups, the characteristics of the remaining “true” BRCA1-mutant HRR-deficient population may be more BRCA2 like.
While some progress has been made investigating site-specific implications of BRCA1 mutation, correlation of BRCA2 mutation site with platinum sensitivity, PARP inhibitor efficacy and survival is yet to be drawn. These investigations are likely to be hindered by the relative rarity of BRCA2 versus BRCA1 mutation and will require large multinational retrospective cohorts of OC. Furthermore, while BRCA1 is multifunctional – providing a rationale for differential modulation of HRR activity with varying mutation site – BRCA2 appears to function almost exclusively in HRR, and phenotypic differences between mutation sites may therefore be subtle. Indeed, BRCA2 mutation site may not influence chemosensitivity or survival.
Characterizing secondary BRCA mutations and their implications for treatment failure
Increasingly, research efforts have turned to characterizing mechanisms of acquired chemoresistance in BRCA-associated OC. Emergence of disease displaying secondary BRCA sequence changes that restore protein function has now been demonstrated in both the preclinical and clinical settings and has been correlated with therapy resistance.Citation132–Citation138 Whether these changes arise de novo or through selection of preexisting subclones already present at diagnosis remains an area of keen interest and could influence the selection of NAC versus PDS. Furthermore, investigation into whether different mutation types display differential propensity for reversion – and indeed whether these correlate with prolonged sensitivity to platinum and PARP inhibitors – is yet to be undertaken. Collection of temporally and spatially separated biopsies throughout the disease journey in BRCA-associated OC will be invaluable in correlating acquisition of reversion events with clinical outcome, particularly with regard to platinum and PARP inhibitor sensitivity. Studies should aim to identify the frequency at which clinically relevant secondary BRCA mutations arise, the potential therapeutic options to rescue resistance in BRCA-reverted patients and whether these mutations arise de novo or are present in subclonal populations at diagnosis.
Conclusion
Clearly, substantial advances in defining the characteristics of BRCA-associated OC have been made in the past decade. Emerging data are beginning to illuminate the distinction between BRCA1- and BRCA2-associated OCs, highlighting distinctions between BRCA1ness and BRCA2ness, consistent with the discrete functions of the BRCA1 and BRCA2 gene products. However, dissecting the characteristics of these two distinct OC patient populations from one another is an area of ongoing research.
Perhaps most intriguingly, it is becoming clear that not all BRCA1 mutations are equal and that mutations at particular sites – most notably within exon 11 and those affecting BRCA1 RING domain function – may not confer a BRCA-ness phenotype. Instead, their role may be confined to compromising the tumor suppressive function of BRCA1, rather than inducing HRR deficiency, and thus chemosensitivity. We await further clinical data on the implications of mutations at these sites, particularly with regard to sensitivity to platinum-based agents and the efficacy of PARP inhibitors. Investigation of the impact, if any, of other BRCA1 mutation sites and of different BRCA2 mutations is eagerly anticipated.
Acknowledgments
We would like to extend our thanks to the Nicola Murray Foundation for their generous support of the Nicola Murray Centre for Ovarian Cancer Research.
Disclosure
RLH and MC report no conflicts of interest in this work. CG discloses the following conflicts of interest: AstraZeneca (advisory board attendance, lecture fees and research funding); Clovis (advisory board attendance); Tesaro (advisory board attendance), Novartis (research funding), Nucana (advisory board attendance and research funding) and Aprea (research funding).
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- PratJNew insights into ovarian cancer pathologyAnn Oncol201223Suppl 10x111x11722987944
- HollisRLGourleyCGenetic and molecular changes in ovarian cancerCancer Biol Med201613223624727458531
- LedermannJARajaFAFotopoulouCGonzalez-MartinAColomboNSessaCNewly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201324Suppl 6vi24vi3224078660
- SymeonidesSGourleyCOvarian cancer molecular stratification and tumor heterogeneity: a necessity and a challengeFront Oncol2015522926557500
- WalshTCasadeiSLeeMKMutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencingProc Natl Acad Sci U S A201110844180321803722006311
- PratJRibeAGallardoAHereditary ovarian cancerHum Pathol200536886187016112002
- TanDSRothermundtCThomasK“BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutationsJ Clin Oncol200826345530553618955455
- LiuGYangDSunYDiffering clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancerPharmacogenomics201213131523153523057551
- MikiYSwensenJShattuck-EidensDA strong candidate for the breast and ovarian cancer susceptibility gene BRCA1Science1994266518266717545954
- ClarkSLRodriguezAMSnyderRRHankinsGDBoehningDStructure-function of the tumor suppressor BRCA1Comput Struct Biotechnol J201211e20120400522737296
- HashizumeRFukudaMMaedaIThe RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutationJ Biol Chem200127618145371454011278247
- MankeIALoweryDMNguyenAYaffeMBBRCT repeats as phosphopeptide-binding modules involved in protein targetingScience2003302564563663914576432
- YuXChiniCCHeMMerGChenJThe BRCT domain is a phosphoprotein binding domainScience2003302564563964214576433
- MezaJEBrzovicPSKingMCKlevitREMapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1J Biol Chem199927495659566510026184
- WilliamsRSGreenRGloverJNCrystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1Nat Struct Biol200181083884211573086
- ThakurSZhangHBPengYLocalization of BRCA1 and a splice variant identifies the nuclear localization signalMol Cell Biol19971714444528972225
- DengCXBrodieSGRoles of BRCA1 and its interacting proteinsBioessays200022872873710918303
- CortezDWangYQinJElledgeSJRequirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaksScience199928654421162116610550055
- AbbottDWThompsonMERobinson-BenionCTomlinsonGJensenRAHoltJTBRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repairJ Biol Chem199927426188081881210373498
- MoynahanMECuiTYJasinMHomology-directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutationCancer Res200161124842485011406561
- MoynahanMEChiuJWKollerBHJasinMBrca1 controls homology-directed DNA repairMol Cell19994451151810549283
- FabbroMSavageKHobsonKBRCA1-BARD1 complexes are required for p53Ser-15 phosphorylation and a G1/S arrest following ionizing radiation-induced DNA damageJ Biol Chem200427930312513125815159397
- XuBKimSKastanMBInvolvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiationMol Cell Biol200121103445345011313470
- WangBMatsuokaSBallifBAAbraxas and RAP80 form a BRCA1 protein complex required for the DNA damage responseScience200731658281194119817525340
- RoyRChunJPowellSNBRCA1 and BRCA2: different roles in a common pathway of genome protectionNat Rev Cancer20121216878
- ShahidTSorokaJKongEHStructure and mechanism of action of the BRCA2 breast cancer tumor suppressorNat Struct Mol Biol2014211196296825282148
- MoynahanMEPierceAJJasinMBRCA2 is required for homology- directed repair of chromosomal breaksMol Cell20017226327211239455
- WongAKPeroROrmondePATavtigianSVBartelPLRAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2J Biol Chem19972725131941319449405383
- ChenPLChenCFChenYXiaoJSharpZDLeeWHThe BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatmentProc Natl Acad Sci U S A1998959528752929560268
- BorkPBlombergNNilgesMInternal repeats in the BRCA2 protein sequenceNat Genet199613122238673099
- BignellGMicklemGStrattonMRAshworthAWoosterRThe BRC repeats are conserved in mammalian BRCA2 proteinsHum Mol Genet19976153589002670
- DaviesAAMassonJYMcIlwraithMJRole of BRCA2 in control of the RAD51 recombination and DNA repair proteinMol Cell20017227328211239456
- EsashiFGalkinVEYuXEgelmanEHWestSCStabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2Nat Struct Mol Biol200714646847417515904
- YangHJeffreyPDMillerJBRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structureScience200229755881837184812228710
- YoshidaKMikiYRole of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damageCancer Sci2004951186687115546503
- YuanSSLeeSYChenGSongMTomlinsonGELeeEYBRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivoCancer Res199959153547355110446958
- AbbottDWFreemanMLHoltJTDouble-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cellsJ Natl Cancer Inst199890139789859665145
- ForayNRandrianarisonVMarotDPerricaudetMLenoirGFeunteunJGamma-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2Oncogene199918517334734210602489
- KingMCMarksJHMandellJBNew York Breast Cancer Study GroupBreast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2Science2003302564564364614576434
- ChenSParmigianiGMeta-analysis of BRCA1 and BRCA2 penetranceJ Clin Oncol200725111329133317416853
- PenningtonKPSwisherEMHereditary ovarian cancer: beyond the usual suspectsGynecol Oncol2012124234735322264603
- GaytherSAWarrenWMazoyerSGermline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlationNat Genet19951144284337493024
- ThompsonDEastonDBreast Cancer Linkage ConsortiumVariation in BRCA1 cancer risks by mutation positionCancer Epidemiol Biomarkers Prev200211432933611927492
- GaytherSAMangionJRussellPVariation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 geneNat Genet19971511031058988179
- RebbeckTRMitraNWanFAssociation of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancerJAMA2015313131347136125849179
- BoydJSonodaYFedericiMGClinicopathologic features of BRCA-linked and sporadic ovarian cancerJAMA2000283172260226510807385
- GarberJEOffitKHereditary cancer predisposition syndromesJ Clin Oncol200523227629215637391
- AlsopKFeredaySMeldrumCBRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study GroupJ Clin Oncol201230212654266322711857
- RamusSJGaytherSAThe contribution of BRCA1 and BRCA2 to ovarian cancerMol Oncol20093213815019383375
- The Breast Cancer Linkage ConsortiumCancer risks in BRCA2 mutation carriersJ Natl Cancer Inst199991151310131610433620
- TakahashiHChiuHCBanderaCAMutations of the BRCA2 gene in ovarian carcinomasCancer Res19965612273827418665505
- YangDKhanSSunYAssociation of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancerJAMA2011306141557156521990299
- RischHAMcLaughlinJRColeDEPrevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancerAm J Hum Genet200168370071011179017
- PratJOvarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological featuresVirchows Arch2012460323724922322322
- GilksCBPratJOvarian carcinoma pathology and genetics: recent advancesHum Pathol20094091213122319552940
- KindelbergerDWLeeYMironAIntraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationshipAm J Surg Pathol200731216116917255760
- LeeYMironADrapkinRA candidate precursor to serous carcinoma that originates in the distal fallopian tubeJ Pathol20072111263517117391
- KurmanRJShih IeMThe origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theoryAm J Surg Pathol201034343344320154587
- PiekJMvan DiestPJZweemerRPDysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancerJ Pathol2001195445145611745677
- SomiglianaEViganoPParazziniFStoppelliSGiambattistaEVercelliniPAssociation between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidenceGynecol Oncol2006101233134116473398
- MarquezRTBaggerlyKAPattersonAPPatterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colonClin Cancer Res200511176116612616144910
- Cancer Genome Atlas Research NetworkIntegrated genomic analyses of ovarian carcinomaNature2011474735360961521720365
- TothillRWTinkerAVGeorgeJNovel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcomeClin Cancer Res200814165198520818698038
- ZornKKBonomeTGangiLGene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancerClin Cancer Res200511186422643016166416
- BoltonKLChenevix-TrenchGGohCAssociation between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancerJAMA2012307438239022274685
- KanchiKLJohnsonKJLuCIntegrated analysis of germline and somatic variants in ovarian cancerNat Commun20145315624448499
- CormioGRossiCCazzollaADistant metastases in ovarian carcinomaInt J Gynecol Cancer200313212512912657111
- GourleyCMichieCORoxburghPIncreased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotypeJ Clin Oncol201028152505251120406939
- SekineMYoshiharaKKomataDHainoKNishinoKTanakaKIncreased incidence of brain metastases in BRCA1-related ovarian cancersJ Obstet Gynaecol Res201339129229622889437
- VenckenPMKriegeMHoogwerfDChemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patientsAnn Oncol20112261346135221228333
- WangYBernhardyAJCruzCThe BRCA1-Delta11q alternative splice isoform bypasses germline mutations and promotes therapeutic resistance to PARP inhibition and cisplatinCancer Res20167692778279027197267
- DimitrovaDRuscitoIOlekSGermline mutations of BRCA1 gene exon 11 are not associated with platinum response neither with survival advantage in patients with primary ovarian cancer: understanding the clinical importance of one of the biggest human exons. A study of the Tumor Bank Ovarian Cancer (TOC) ConsortiumTumour Biol2016379123291233727297669
- DrostRBouwmanPRottenbergSBRCA1 RING function is essential for tumor suppression but dispensable for therapy resistanceCancer Cell201120679780922172724
- DrostRDhillonKKvan der GuldenHBRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1J Clin Invest201612682903291827454287
- StruewingJPAbeliovichDPeretzTThe carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individualsNat Genet19951121982007550349
- RoaBBBoydAAVolcikKRichardsCSAshkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2Nat Genet19961421851878841191
- TinkerAVGebskiVFitzharrisBPhase II trial of weekly docetaxel for patients with relapsed ovarian cancer who have previously received paclitaxel – ANZGOG 02-01Gynecol Oncol2007104364765317079006
- KatsumataNTsunematsuRTanakaKA phase II trial of docetaxel in platinum pre-treated patients with advanced epithelial ovarian cancer: a Japanese cooperative studyAnn Oncol200011121531153611205459
- Gynecologic Oncology Group; MarkmanMBlessingJPhase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group studyGynecol Oncol2006101343644016325893
- WeaverBAHow taxol/paclitaxel kills cancer cellsMol Biol Cell201425182677268125213191
- QuinnJEKennedyRDMullanPBBRCA1 functions as a differential modulator of chemotherapy-induced apoptosisCancer Res200363196221622814559807
- StordalBDaveyRA systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: role of BRCA1Curr Cancer Drug Targets20099335436519442054
- SaikiYOgawaTShigaKSunamuraMKobayashiTHoriiAA human head and neck squamous cell carcinoma cell line with acquired cis-diamminedichloroplatinum-resistance shows remarkable upregulation of BRCA1 and hypersensitivity to taxaneInt J Otolaryngol2011201152185222046189
- GilmorePMMcCabeNQuinnJEBRCA1 interacts with and is required for paclitaxel-induced activation of mitogen-activated protein kinase kinase kinase 3Cancer Res200464124148415415205325
- TassonePTagliaferriPPerricelliABRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cellsBr J Cancer20038881285129112698198
- ChabalierCLamareCRaccaCPrivatMValetteALarminatFBRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistanceCell Cycle2006591001100716639080
- SungMGiannakakouPBRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signalingOncogene201433111418142823524581
- FedierASteinerRASchwarzVALenherrLHallerUFinkDThe effect of loss of Brca1 on the sensitivity to anticancer agents in p53-deficient cellsInt J Oncol20032251169117312684687
- ZhouCSmithJLLiuJRole of BRCA1 in cellular resistance to paclitaxel and ionizing radiation in an ovarian cancer cell line carrying a defective BRCA1Oncogene200322162396240412717416
- KurebayashiJYamamotoYKurosumiMLoss of BRCA1 expression may predict shorter time-to-progression in metastatic breast cancer patients treated with taxanesAnticancer Res2006261b69570116739340
- QuinnJEJamesCRStewartGEBRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapyClin Cancer Res200713247413742018094425
- TanDSYapTAHutkaMImplications of BRCA1 and BRCA2 mutations for the efficacy of paclitaxel monotherapy in advanced ovarian cancerEur J Cancer20134961246125323265709
- PisanoCCecereSCDi NapoliMClinical trials with pegylated liposomal doxorubicin in the treatment of ovarian cancerJ Drug Deliv2013201389814623577259
- ShawHMHallMEmerging treatment options for recurrent ovarian cancer: the potential role of olaparibOnco Targets Ther201361197120624043945
- AdamsSFMarshEBElmasriWA high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancerGynecol Oncol2011123348649121945552
- SafraTBorgatoLNicolettoMOBRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicinMol Cancer Ther201110102000200721835933
- FujiwaraKArmstrongDMorganMMarkmanMPrinciples and practice of intraperitoneal chemotherapy for ovarian cancerInt J Gynecol Cancer200717112017291226
- HowellSBPharmacologic principles of intraperitoneal chemotherapy for the treatment of ovarian cancerInt J Gynecol Cancer200818suppl 1202518336394
- YanTDCaoCQMunkholm-LarsenSA pharmacological review on intraperitoneal chemotherapy for peritoneal malignancyWorld J Gastrointest Oncol20102210911621160929
- DedrickRLMyersCEBungayPMDeVitaVTJrPharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancerCancer Treat Rep1978621111626987
- AlbertsDSLiuPYHanniganEVIntraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancerN Engl J Med199633526195019558960474
- ArmstrongDKBundyBWenzelLIntraperitoneal cisplatin and paclitaxel in ovarian cancerN Engl J Med20063541344316394300
- MarkmanMBundyBNAlbertsDSPhase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology GroupJ Clin Oncol20011941001100711181662
- PolyzosATsavarisNKosmasCA comparative study of intraperitoneal carboplatin versus intravenous carboplatin with intravenous cyclophosphamide in both arms as initial chemotherapy for stage III ovarian cancerOncology199956429129610343192
- YenMSJuangCMLaiCRChaoGCNgHTYuanCCIntraperi-toneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancerInt J Gynaecol Obstet2001721556011146078
- WrightAACroninAMilneDEUse and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancerJ Clin Oncol201533262841284726240233
- GourleyCWalkerJLMackayHJUpdate on intraperitoneal chemotherapy for the treatment of epithelial ovarian cancerAm Soc Clin Oncol Educ Book20163514315127249695
- LesnockJLDarcyKMTianCBRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group StudyBr J Cancer201310861231123723462720
- KehoeSHookJNankivellMPrimary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trialLancet2015386999024925726002111
- SatoSItamochiHNeoadjuvant chemotherapy in advanced ovarian cancer: latest results and place in therapyTher Adv Med Oncol20146629330425364394
- VergoteITropeCGAmantFNeoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancerN Engl J Med20103631094395320818904
- MeyerLACroninAMSunCCUse and effectiveness of neo-adjuvant chemotherapy for treatment of ovarian cancerJ Clin Oncol Epub201696
- GorodnovaTVSokolenkoAPIvantsovAOHigh response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutationCancer Lett2015369236336726342406
- SchwarzRFNgCKCookeSLSpatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysisPLoS Med2015122e100178925710373
- BlagdenSPHarnessing pandemonium: the clinical implications of tumor heterogeneity in ovarian cancerFront Oncol2015514926175968
- KyleSThomasHDMitchellJCurtinNJExploiting the Achilles heel of cancer: the therapeutic potential of poly(ADP-ribose) polymerase inhibitors in BRCA2-defective cancerBr J Radiol200881Spec1S6S1118820000
- McCabeNTurnerNCLordCJDeficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP- ribose) polymerase inhibitionCancer Res200666168109811516912188
- MuraiJHuangSYDasBBTrapping of PARP1 and PARP2 by clinical PARP inhibitorsCancer Res201272215588559923118055
- StromCEJohanssonFUhlenMSzigyartoCAErixonKHelledayTPoly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediateNucleic Acids Res20113983166317521183466
- FarmerHMcCabeNLordCJTargeting the DNA repair defect in BRCA mutant cells as a therapeutic strategyNature2005434703591792115829967
- FongPCBossDSYapTAInhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriersN Engl J Med2009361212313419553641
- FongPCYapTABossDSPoly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free intervalJ Clin Oncol201028152512251920406929
- KaufmanBShapira-FrommerRSchmutzlerRKOlaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutationJ Clin Oncol201533324425025366685
- LedermannJHarterPGourleyCOlaparib maintenance therapy in platinum-sensitive relapsed ovarian cancerN Engl J Med2012366151382139222452356
- SwisherEMLinKKOzaAMRucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trialLancet Oncol2017181758727908594
- MirzaMRMonkBJHerrstedtJNiraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian CancerN Engl J Med2016375222154216427717299
- LedermannJHarterPGourleyCOlaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trialLancet Oncol201415885286124882434
- ColemanRLSillMWBell-McGuinnKA phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation – an NRG Oncology/Gynecologic Oncology Group studyGynecol Oncol2015137338639125818403
- LedermannJAHarterPGourleyCOverall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trialLancet Oncol2016
- DhillonKKSwisherEMTaniguchiTSecondary mutations of BRCA1/2 and drug resistanceCancer Sci2011102466366921205087
- SwisherEMSakaiWKarlanBYWurzKUrbanNTaniguchiTSecondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistanceCancer Res20086882581258618413725
- SakaiWSwisherEMKarlanBYSecondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancersNature200845171821116112018264087
- SakaiWSwisherEMJacquemontCFunctional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinomaCancer Res200969166381638619654294
- EdwardsSLBroughRLordCJResistance to therapy caused by intragenic deletion in BRCA2Nature200845171821111111518264088
- BarberLJSandhuSChenLSecondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitorJ Pathol2013229342242923165508
- NorquistBWurzKAPennilCCSecondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomasJ Clin Oncol201129223008301521709188
- PatchAMChristieELEtemadmoghadamDWhole-genome characterization of chemoresistant ovarian cancerNature2015521755348949426017449
- LangdonSPLawrieSSHayFGCharacterization and properties of nine human ovarian adenocarcinoma cell linesCancer Res19884821616661723167863
- StordalBTimmsKFarrellyABRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutationMol Oncol20137356757923415752
- Ben DavidYChetritAHirsh-YechezkelGEffect of BRCA mutations on the length of survival in epithelial ovarian tumorsJ Clin Oncol200220246346611786575
- RubinSCBenjaminIBehbakhtKClinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1N Engl J Med199633519141314168875917
- Candido-dos-ReisFJSongHGoodeELGermline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancerClin Cancer Res201521365265725398451
- BigliaNSgandurraPBounousVEOvarian cancer in BRCA1 and BRCA2 gene mutation carriers: analysis of prognostic factors and survivalEcancermedicalscience20161063927350785
- HarterPJohnsonTBerton-RigaudDBRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 studyGynecol Oncol2016140344344926740259
- ZhongQPengHLZhaoXZhangLHwangWTEffects of BRCA1-and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysisClin Cancer Res201521121122025348513
- VenckenPMReitsmaWKriegeMOutcome of BRCA1-compared with BRCA2-associated ovarian cancer: a nationwide study in the NetherlandsAnn Oncol20132482036204223543211
- SynowiecAWcisloGBodnarLClinical features and outcomes of germline mutation BRCA1-linked versus sporadic ovarian cancer patientsHered Cancer Clin Pract201614126753012
- XuKYangSZhaoYPrognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysisOncotarget20178128530227690218
- StricklandKCHowittBEShuklaSAAssociation and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancerOncotarget2016712135871359826871470
- McLaughlinJRRosenBMoodyJLong-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2J Natl Cancer Inst2013105214114823257159
- ChetritAHirsh-YechezkelGBen-DavidYLubinFFriedmanESadetzkiSEffect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancerJ Clin Oncol2008261202518165636
- SunCLiNDingDThe role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysisPLoS One201495e9528524788697
- LacourRAWestinSNMeyerLAImproved survival in non- Ashkenazi Jewish ovarian cancer patients with BRCA1 and BRCA2 gene mutationsGynecol Oncol2011121235836321276604
- HymanDMZhouQIasonosAImproved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancerCancer2012118153703370922139894
- PalTPermuth-WeyJKapoorRCantorASutphenRImproved survival in BRCA2 carriers with ovarian cancerFam Cancer20076111311917160431
