Abstract
Hypercoagulable state and disorganized angiogenesis are two conspicuous characteristics during tumor progression. There are a considerable number of clinical trials focusing on the effects of anticoagulant and antiangiogenic drugs on the survival of cancer patients. Favorable outcomes have been observed. Excessive blood coagulation not only causes cancer-associated thrombosis, which is a common complication and is the second leading cause of death in patients, but also decreases intratumoral perfusion rates and drug delivery by reducing the effective cross-sectional area of blood vessels. Meanwhile, structural and functional abnormalities of the tumor microvasculature also compromise convective drug transport and create a hypoxic and acidic microenvironment. Vascular normalization strategy can temporarily recover the abnormal state of tumor vasculature by improving blood density, dilation, and leakiness, resulting in enhanced penetration of chemotherapies and oxygen within a short time window. In this article, we first review the evidence to support the opinion that anticoagulant and antiangiogenic therapy can improve cancer survival through several underlying mechanisms. Next, we speculate on the feasibility and value of the combined strategy and discuss whether such a combination has a synergistic antineoplastic effect in cancer patients by way of increasing blood vessel perfusion and drug distribution.
Introduction
Since the French physician Armand Trousseau first described the occurrence of thromboembolic disease in cancer patients nearly 150 years ago, the close relationship between malignancy and excessive blood coagulation has slowly become well recognized. The hypercoagulable state is a universal phenomenon in malignant tumors, which not only seriously impacts local tumor microenvironment but also leads to systemic venous and arterial thrombosis.Citation1,Citation2 Beyond biological factors, clinical factors, such as bed rest, chemoradiotherapy, central venous catheters, infection, and surgery, are all contributory causes that will determine whether an asymptomatic phenotype manifests clinically.Citation3 In fact, >90% of cancer patients commonly present with various signs and symptoms of the prothrombotic state that range from one or more asymptomatic coagulation indicators, to clinically defined thromboembolism and disseminated intravascular coagulation.Citation4 A growing body of studies have proposed that thrombosis is a common complication of malignancy that provokes disease progression and poor prognosis, interferes with patients’ quality and expectancy of life, and increases health care costs considerably.Citation5,Citation6 Hence, anticoagulant therapy plays an important role in tumor treatment, even in patients with advanced disease.
During tumor progression, aberrant angiogenesis is another significant feature. Formation of a new vascular network or development of a preexisting vascular bed is initiated by the well-known angiogenesis inducer vascular endothelial growth factor (VEGF). The binding of VEGF to VEGF receptor (VEGFR) stimulates autophosphorylation in certain tyrosine residues. The signal activates downstream pathways and regulates endothelial cell survival and proliferation.Citation7 Thus, targeting the VEGF–VEGFR pathway has become a considerable therapeutic strategy for cancer treatment. Presently, three primary pharmacological strategies targeting angiogenesis have been created in cancer therapy: 1) the monoclonal antibodies (moAbs) binding VEGF directly such as the anti-VEGF antibody bevacizumab; 2) targeting angiogenesis receptors either with moAbs such as the anti-VEGFR2 antibody or with the inhibitors of tyrosine kinase receptors such as sunitinib and sorafenib; and 3) inhibiting VEGF signaling by recombinant receptor–antibody fusion proteins such as aflibercept.Citation8 Nevertheless, the fundamentals of these antiangiogenic drugs has not been determined conclusively to this day.
We first summarize the recent studies of tumor microenvironment, including both hypercoagulability and abnormal angiogenesis, in cancer. Next, we elaborate on current clinical evidence and several underlying mechanisms of anticoagulant and antiangiogenic therapy during tumor progression. Finally, we hypothesize whether both the important treatment strategies have a synergistic antineoplastic effect in clinical application that improves the prognosis of the patients, especially by increasing blood vessel perfusion and drug distribution. Furthermore, we give future perspectives in the field of coagulation and angiogenesis in cancer.
Anticoagulant therapy
The association between anticoagulant therapy and tumor disease progression is controversial. Data from different randomized clinical trials suggest a conflicting outcome of low-molecular weight heparin (LMWH) on survival in patients with several types of cancer (). Therapeutic effects of LMWH display a moderate impact on certain types of cancers, such as small-cell lung cancer.Citation9–Citation11 It possibly implies that small-cell lung cancer is more sensitive than other cancer types for LMWH treatment. In addition, researchers also notice that LMWH appears to be more effective in patient populations with limited-stage disease, or with a longer estimated life expectancy.Citation10–Citation12 This may explain the poor results that frustrated researchers on clinical trials that recruited more patients with metastatic or locally advanced cancer. These participants might not theoretically benefit from any antineoplastic effects of LMWH. Despite the fact that the benefit of LMWH therapy on cancer patients’ survival is still inconclusive, it is surely associated with a clinically significant reduction in venous thromboembolic event (VTE). Accordingly, with enough consideration in terms of cancer type and stage, anticoagulant therapy, especially LMWH, is a remarkable adjunctive therapy for cancer patients. It is worth noting that these beneficial influences are not completely explained by the prevention and treatment of thrombosis. Several possible interrelated pathology mechanisms have been raised by researchers, including both coagulation-dependent and coagulation-independent activities.
Table 1 Overview of randomized clinical trials of LMWHs on survival of cancer patients
Prevention and treatment of cancer-associated thrombosis
Cancer-associated thrombosis is a prevalent complication in tumor progression and is associated with a poor prognosis.Citation13 It is estimated consistently that cancer is related to 20%–30% of all first VTE.Citation14 Due in part to increasing cancer prevalence, improved oncology outcomes, and the use of more effective but prothrombotic therapy regimens (ie, chemotherapy, radiotherapy, hormone therapy, angiogenesis inhibitors, and surgery), a steady increase in the incidence of cancer-associated thrombosis has been observed, during the past 2 decades.Citation15 Although both venous and arterial thrombosis can be established in cancer patients, the venous thrombotic occlusions have been studied more extensively, including deep venous thrombosis and pulmonary embolism. A retrospective cohort study of over a million inpatients with cancer found an overall prevalence VTE rate of 4.1%, with prevalence incidence enhanced by 28% from 1995 to 2003.Citation16 A recent systematic review on the absolute risk of VTE in cancer patients found an overall VTE incidence rate of 43 per 1,000 person-years, with a fourfold increased risk compared with the patients with no malignancy.Citation17 VTE can occur in throughout the cancer process, especially in the first few months after the diagnosis of cancer.Citation18 Furthermore, thrombotic events are the second most common cause of death in patients with cancer, ranking after death from the cancer itself. Thrombotic event explains 9% of cancer-related deaths, at a high cost to patients and society.Citation19,Citation20 Accordingly, given the high incidence and mortality, prevention and management of thrombotic events in cancer patients are extremely urgent. Many guidelines have been published from international scientific societies. The American Society of Clinical Oncology and the National Comprehensive Cancer Network guidelines suggested that prophylaxis with anticoagulant drug is recommended for inpatients with cancer in the absence of bleeding or other contraindications to anticoagulant. By contrast, the European Society of Medical Oncology and American College of Chest Physicians requires bedridden hospital cancer patients with an acute medical complication to be considered for medical thromboprophylaxis in the form of low-dose unfractionated heparin or LMWH. Numerous guidelines acknowledge the significant incidence of VTE in cancer patients undergoing surgery, and recommend surgical patients receive perioperative VTE prophylaxis, although the specific anticoagulant scenarios differ slightly among them.Citation21–Citation24 Based on the results of several randomized controlled trials and meta-analyses, the use of LMWH is preferred over other anticoagulants in most cases in cancer patients treated for VTE.Citation25 LMWH is characterized by: lower time for coagulation monitoring, fewer major bleeding events, and once-daily dosing, which make these drugs more appropriate for cancer therapy.Citation26,Citation27 Fondaparinux is also an acceptable agent for use in initial treatment.Citation28 Furthermore, aspirin has been shown to hinder arterial thrombosis and to reduce the rate of major vascular events, but there are less data supporting the benefits of therapeutic antiplatelet agents at preventing VTE in cancer patients as well.Citation29,Citation30
Anticancer effects
The pathogenesis of the coagulation system imbalance in cancer is likely to be multifactorial, including both coagulation-dependent and -independent activities. A change in the coagulation system itself may conduce the hypercoagulable state. Tumor-specific prothrombotic mechanisms also play a significant role in the complex process. Malignant cells can influence the expression and release of procoagulant factors (ie, tissue factor and cancer procoagulant), inflammatory cytokines, and circulating microparticles, and then activate host coagulation system. The direct stimulation of blood cells, including endothelial cells, platelets, and monocytes, also causes confusion of the hemostatic system;Citation31,Citation32 vice versa, the disorder of coagula tion is closely linked to tumor progression and metastasis as well. Thrombin enlarges cancer cell binding to platelets and upgrades the metastasizing ability of cancer cells efficaciously in experimental pulmonary metastasis models.Citation33 In addition, lowering endogenous activated protein C levels will contribute to enhanced metastasis in hypercoagulable mice.Citation34 Since the first exploration of a potential beneficial antineoplastic effects of anticoagulant drugs was found in 1930, studies have actively investigated how the anticoagulant drugs affect tumor growth and metastasis through multiple mechanisms.Citation35 Many findings demonstrated that anticoagulants, in particular the LMWH, can affect the growth of primary tumors, block tumor cell heparanase, suppress selectin-mediated tumor cell invasion and metastasis, and inhibit tumor-induced angiogenesis in both in vivo and in vitro systems.Citation36–Citation39 In addition, the use of aspirin as a cancer chemopreventive agent has a long history of clinical use. Although the antineoplastic effects of aspirin have not been well explained, emerging clinic evidence has shown that constant use of aspirin effectively improves cancer patients’ prognosis, particularly those with colorectal cancer.Citation40 A randomized trial of 311 patients with colorectal adenomas and adenocarcinomas excised by endoscopy showed that the subjects treated with aspirin exhibited lower colorectal tumorigenesis and primary end points with an adjusted odds ratio of 0.60 compared with the subjects in the placebo group.Citation41 Warfarin, a kind of coumarin anticoagulant, has been known to reduce tumor metastases in mouse and rat models. But the potential mechanisms were mostly ambiguous. Recently, the study by Paolino et alCitation42 supported a coagulation-independent role of warfarin in tumor metastasis that relies on its inhibitory effect on the Cbl-b/TAM receptors in natural killer cells. It once again raised a major concern regarding the antimetastatic activity of warfarin in cancer treatment. Altogether, these preclinical studies and clinical data further contribute to supporting the proof of potential antineoplastic effects of anticoagulants. It is highly desirable that anticoagulants serve as an adjuvant therapy for cancer treatment in the future.
Increasing blood vessel perfusion and drug penetration
The influence of hypercoagulable state that hamper vessel perfusion is constantly overlooked. It could hamper blood flow in a multitude of ways. On one hand, fibrin and other plasma proteins deposited within the tumor vasculature produce a physiological barrier that reduces the effective cross-sectional area of tumor blood vessels. While on the other hand, tumors are inevitably concomitant with blood capillary ruptures and hemorrhage, resulting in blood leaking into the extracellular matrix, which becomes extravascular thrombosis. Extravascular thrombosis is a mechanical force that generates vessel compression and thus inhibits convective blood flow.Citation36,Citation43 Therefore, we assume that anticoagulant drugs improve tumor aberrant blood flow in favor of raising the effective concentration of chemotherapeutics in tumor tissue and forming closer contacts between chemotherapeutic agents and tumor cells, which ensures a better therapeutic efficacy. Phillips et alCitation44 conducted biodistribution studies to determine the effect of LMWH on uptake of paclitaxel (PACL) and doxorubicin (Dox) by breast tumor xenografts. In the MDA453/LCC6 breast tumor xenograft model, they confirmed that LMWH conducts greater [124−I]-PACL intratumoral concentration than that of [124−I]-PACL alone. Similarly, LMWH significantly increased the uptake of Dox in MCF7 Dox-resistant tumor xenografts by 1.5- to 2-fold. Importantly, although LMWH compounds selectively increased tumor uptake of Dox, the drug accumulation in heart and lung tissues, both sites of serious toxicity with this drug, were not increased but reduced. This study credibly demonstrated that LMWH administration considerably improves the efficient distribution of chemotherapeutics and overcomes tumor chemoresistance. The secondary application of anticoagulant drugs might also decrease the doses of chemotherapy required due to more efficient delivery into intratumoral region.Citation44 The possible mechanisms that explain the improvement of tumor uptake of chemotherapy agents may be complex. As stated earlier, LMWH reduces the deposition of fibrin and other plasma proteins in the blood vessel wall and the extravascular thrombosis, which reopens restricted vessels in the tumor microenvironment. Thus, LMWH and other anticoagulants may overcome the physical barrier and relieve the viscid blood flow by decreasing clotted sediments. This potential effect of anticoagulants is worth exploring thoroughly in further studies, and more direct evidence would likely increasingly support the paradigm.
Antiangiogenic therapy
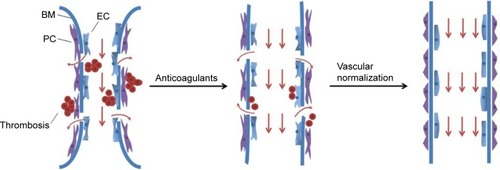
Angiogenesis, a conspicuous hallmark of malignancy, promotes tumor growth and metastasis by transferring oxygen and nutrients into the tumor mass.Citation45 In contrast to the healthy vasculature that was arranged in a coherent style, tumor vessels are aberrant in almost all aspects of their structure and function at macroscopic and microscopic levels. The dysfunctional vessels are commonly organized in a more dilated and tortuous shape, and the hyperpermeable vessels with large gaps between endothelial cells, isolated pericytes, and discontinuous or absent basement membranes.Citation46 A large quantity of blood plasma leaks into the tumor interstitial space due to the leaky vascular wall, which enhances local interstitial fluid pressure, red blood corpuscle concentration, and blood viscosity.Citation47 This greatly decreases the blood velocity in focal vascular sections and diverts blood flow to other sites. The abnormal vasculature leads to a hostile tumor microenvironment. It not only reduces tumor blood perfusion but also converts the tumor into an unfavorable hypoxic and acidic microenvironment that diminishes tumor responsiveness to treatments. These pathophysiological characteristics compromise convective transport of drug and impede the effectiveness of conventional anticancer treatments as well as the function of immune cells in tumors.Citation48–Citation50
In 1971, Judah Folkman first articulated that antiangiogenic therapy is a promising strategy for cancer and initiated the isolation of tumor angiogenesis factors.Citation51 The discovery of VEGF as a major regulator of endothelial cell growth and survival paved the way for translating the idea into clinical practice.Citation52 In 2004, bevacizumab, a humanized moAb directed against VEGF, was approved by the US Food and Drug Administration as first-line treatment for metastatic colorectal cancer, in combination with conventional chemotherapy.Citation53 The validation of the first antiangiogenic drug opened a new chapter in the development of targeted therapy. Numerous antiangiogenic drugs have been gradually applied to several tumor types over the past 11 years, in particular targeting the VEGF–VEGFR signaling axis.Citation54 The introduction of these innovative drugs for oncotherapy has resulted in moderate improvements in tumor response and progression-free survival, or overall survival of cancer patients (). Undoubtedly, the benefits in these randomized trials are not universal, most probably due to heterogeneity of tumor type and therapeutic regimen. Also, antiangiogenic agents are typically given to unselected patients for the approved indications. It is highly desirable to identify reliable predictive markers to select homogeneous groups for more clinical trials.
Table 2 Chemotherapy in combination with antiangiogenic agents in randomized trials in various malignancies
Although the improved effect has been shown in various tumor types, the principal mechanism of VEGF-targeted drugs has not been explained well to this day. It is credible that different mechanisms have a more or less significant role depending tumor type. Clinical studies have demonstrated that distinct tumor types showed inconsistent responses to different types of VEGF inhibitors or combination regimens. VEGF inhibitors are active as single agents in renal cell carcinoma, hepatocellular carcinoma, and ovarian and neuroendocrine tumors.Citation55 In these tumors, the original theory of VEGF-targeted drugs may become predominant. The classic concept was that VEGF-targeted therapy can block tumors’ blood supply, then reduce the delivery of oxygen and fundamental nutrients, and finally arrest the previously uninhibited tumor cell growth.Citation56 In contrast, for other tumors such as colorectal cancer (CRC), non-small-cell lung cancer, and breast cancer (although this efficacy is currently pending),Citation55 VEGF inhibitors are effective only when combined with cytotoxic chemotherapy, seemingly contradicting the initial principal mechanism claims. According to the classical scenario, such suppressive angiogenesis generates spatial and temporal inadequacies of blood perfusion and restrains the intratumoral delivery of coadministered drugs. Moreover, enhanced hypoxia conditions caused by the antivascular effects also render tumor cells relatively chemoradiotherapy resistant and even become more aggressive.Citation57 Accordingly, given the complexity of tumor biology, such as the heterogeneity of blood vessel growth in different stages and locations, it would be unwise to attribute a single mechanism to VEGF inhibitors in multiple tumor types. In 2001, it has been proposed first that treatment with judicious antiangiogenic agents can transiently revert the abnormal tumor vasculature toward a relatively normal status. Antiangiogenic drugs selectively prune immature blood vessels and retain relatively mature vasculature.Citation58 The favorable phenomenon, vascular normalization, remodels tumor vessels that show improved connections between adjacent endothelial cells, enhanced pericyte coverage, and tighter association between them.Citation59 These physiological changes would reduce vascular permeability and result in a drop of intratumoral interstitial fluid pressure.Citation60 The increase of tumor blood perfusion improves oxygenation and drug delivery and thus inevitably makes tumors more sensitive to chemoradiotherapy.Citation61 Recently, with positron emission tomography imaging, Chatterjee et alCitation62 demonstrated that transient antiangiogenic treatment using potent VEGFR/platelet-derived growth factor receptor inhibitor PTK787 produces a transient time window of improved tumor blood flow with reduced leakiness and improved pericyte coverage. This short-lived normalization window results in improved outcomes of erlotinib administration.Citation62 Similarly, Batchelor et alCitation63 applied magnetic resonance imaging techniques and blood biomarkers in prospective Phase II clinical trials to show that the addition of cediranib, a pan-VEGFR tyrosine kinase inhibitor, to chemoradiation improved vascular integrity and blood perfusion in 20 of 40 (50%) newly diagnosed glioblastoma patients, compared with only one of 14 (7%) patients treated with conventional chemoradiation alone, and was associated with improved tumor oxygenation status. Of note, these newly diagnosed glioblastoma patients with improved tumor blood perfusion and oxygenation status after cediranib-containing regimens treatment had improved overall survival.Citation63 These data strongly indicate that vascular normalization hypothesis theoretically and practically improves anomalous tumor vascular state. It makes the blood flow more uniform with subsequent increased uptake of cytotoxic drugs and oxygen, which result in better combined treatment outcome. There is no denying that “vascular normalization” theory is not universally recognized. A study indicated a rapid and significant reduction in perfusion and uptake of docetaxel in non-small-cell lung cancer after administration of bevacizumab.Citation64 However, this study did not look at the time course of perfusion or drug uptake and did not concern the patients’ survival benefit after treatment. Meanwhile, these patients were treated with the single dose of 15 mg/kg, and this study also did not examine whether a lower dose of bevacizumab had a diverse result. Thus, the optimal dose and scheduling of anti-VEGF agents are not yet clearly defined for the combined regimens in cancer patients. A crucial need remains for substantial research of therapeutic regimen and individual heterogeneity to support the vascular normalization theory.
The hypothesis
Given the fact that hypercoagulabale state and aberrant angiogenesis play significant roles in tumor microenvironment, we proposed combining two therapeutic strategies, anticoagulant and antiangiogenic therapy, to improve tumor patient prognosis by enhancing vessel perfusion, drug delivery, and oxygenation (). As is shown earlier, inadequate intratumor perfusion could mainly result from two aspects. On one hand, intravascular and extravascular thrombi reduce effective cross-sectional area of tumor blood vessels. On the other hand, vessel hyperpermeability and tortuosity reduce blood flow rates in tumors because of excessive fluid loss and vessel resistance. Thus, anticoagulation strategy is based on the following progression of occurrence: solve previously existing thrombi, alleviate stress levels, enlarge vessel diameter, reopen restricted vessels, improve perfusion, and maximally result in enhanced drug delivery and oxygenation. Furthermore, the significant role of prevention and treatment of cancer-associated thrombosis and potential anticancer effects necessitates applying anticoagulant therapy clinically. Vascular normalization alters the monstrous vasculature phenotype to a relatively functional phenotype, as normal blood vessels. By using judicious dose and scheduling of antiangiogenic agents, tumor vessels restore relatively mature hierarchical structure and reduce blood content leakiness. Consequently, the interstitial fluid pressure and blood viscosity are recovered, which increase drug delivery and oxygen transport. However, the vascular normalization effect is transient. Unreasonable application of anti-VEGF treatment, including larger dose or longer duration, may destroy present vascular system, generate compensatory angiogenesis signaling pathways, and even facilitate primary tumor dissemination to distant organs.Citation65,Citation66 In brief, anticoagulant and antiangiogenic therapy alleviate the problem of tumor perfusion abnormity from respective angles. Anticoagulant therapy improves abnormal high-coagulate blood, while antiangiogenic therapy modifies aberrant angiogenesis by vascular normalization. Thromboembolic events, especially arterial thrombosis, are remarkable adverse effects of VEGF inhibitors. A pooled analysis of five randomized trials indicated that addition of bevacizumab to chemotherapy was associated with a twofold increase in arterial thromboembolic events (ATEs) compared with chemotherapy alone (3.8% vs 1.7%; P=0.031). The enhancement was further aggravated in elderly patients or those with a history of ATEs.Citation67 Thus, it is acceptable to use anticoagulant drugs as standard care in reducing the risk of ATEs in terms of VEGF inhibitors. Notably, the risk of bleeding is raised in patients treated with both anticoagulant and VEGF-targeted drugs.Citation68 The hemorrhage symptoms range from moderate mucocutaneous bleeding to fatal tumor-related bleeding. Combining the two agents with chemotherapy can further increase the incidence and severity of bleeding events. It is essential that each patient should undergo elaborate risk–benefit assessment and management. Particular attention must be paid to those patients with risk factors, or with a history of bleeding. The effective benefit of the two strategies depends on the tumor microenvironment and particularly on whether tumor blood is hypercoagulable, vessels are aberrant, both of these, or neither. Thus, the paradigms that underlie anticoagulant and vascular normalization strategies are still open for improvement. We should consider the pathological process of tumor cells of every patient.
Figure 1 Strategies to enhance blood vessel perfusion and drug penetration.
Abbreviations: EC, endothelial cell; BM, basement membrane; PC, pericyte.
Conclusion
The tumor microenvironment is entirely antithetical to normal tissues, characterized by prothrombotic state, monstrous vasculature structure, and low perfusion rates. In order to gain better oncotherapy efficiency, we need to get the abnormal tumor microenvironment back on track. In this article, we hypothesized that two complementary therapeutic strategies, the recuperation of hypercoagulabale state with anticoagulant therapy and the normalization of the tumor vessel with anti-VEGF agents, may be ideal candidate methods that normalize the tumor microenvironment. Though the direct evidence in support of therapeutic benefits of vascular normalization and anticoagulation in cancer is insufficient, it provides a novel perspective into the complex pharmacokinetic and pharmacodynamic impact and interactions between hemodynamics and antitumor agent transportation. Thus, many questions remain to be explained, and more preclinical evidence is needed to support the paradigm. Scientific guidelines for the judicious application of these therapeutic strategies are necessary, given the high heterogeneity of patient constitution, tumor nature, and the differences between primary tumors and metastases. We hope that this new strategy could yield a breakthrough that results in fluent tumor vessel perfusion and drug delivery and finally improves the outcomes of cancer patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 81374014), Zhejiang Provincial Medical and Healthy Science and Technology Projects (Grant No 2013KYA228), Zhejiang Provincial Science and Technology Project (Grant No 2013C33112), Science Research Fund of Taizhou (Grant Nos A121KY08, A131KY13-3, and A131KY13-12), and Enze Medical Research Fund (Grant Nos 12EZA1, 13EZA2, and 13EZB6).
Disclosure
The authors report no conflicts of interest in this work.
References
- NagyJABrownLFSengerDRPathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin depositionBiochim Biophys Acta198994833053262465781
- VarkiATrousseau’s syndrome: multiple definitions and multiple mechanismsBlood200711061723172917496204
- KhoranaAAConnollyGCAssessing risk of venous thromboembolism in the patient with cancerJ Clin Oncol200927294839484719720906
- RicklesFREdwardsRLActivation of blood coagulation in cancer: Trousseau’s syndrome revisitedBlood198362114316407544
- KhoranaAAVenous thromboembolism and prognosis in cancerThromb Res2010125649049320097409
- EltingLSEscalanteCPCooksleyCOutcomes and cost of deep venous thrombosis among patients with cancerArch Intern Med2004164151653166115302635
- TuguesSKochSGualandiLLiXClaesson-WelshLVascular endothelial growth factors and receptors: antiangiogenic therapy in the treatment of cancerMol Aspects Med20113228811121565214
- KasperSSchulerMTargeted therapies in gastroesophageal cancerEur J Cancer20145071247125824495747
- AltinbasMCoskunHSErOA randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancerJ Thromb Haemost2004281266127115304029
- KakkarAKLevineMNKadziolaZLow molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS)J Clin Oncol200422101944194815143088
- LecumberriRLópez VivancoGFontAAdjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL studyThromb Res2013132666667024491267
- LebeauBChastangCBrechotJMSubcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” GroupCancer199474138458004580
- LeeAYLevineMNVenous thromboembolism and cancer: risks and outcomesCirculation200310723 suppl 1I17I2112814981
- TimpJFBraekkanSKVersteegHHCannegieterSCEpidemiology of cancer-associated venous thrombosisBlood2013122101712172323908465
- YoungAChapmanOConnorCPooleCRosePKakkarAKThrombosis and cancerNat Rev Clin Oncol20129843744922777060
- KhoranaAAFrancisCWCulakovaEKudererNMLymanGHFrequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patientsCancer2007110102339234617918266
- HorstedFWestJGraingeMJRisk of venous thromboembolism in patients with cancer: a systematic review and meta-analysisPLoS Med201297e100127522859911
- BlomJWDoggenCJOsantoSRosendaalFRMalignancies, prothrombotic mutations, and the risk of venous thrombosisJAMA2005293671572215701913
- ChewHKWunTHarveyDZhouHWhiteRHIncidence of venous thromboembolism and its effect on survival among patients with common cancersArch Intern Med2006166445846416505267
- KhoranaAAFrancisCWCulakovaEKudererNMLymanGHThromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapyJ Thromb Haemost20075363263417319909
- KahnSRLimWDunnASAmerican College of Chest PhysiciansPrevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest20121412 suppl195S226S
- MandalàMFalangaARoilaFESMO Guidelines Working GroupVenous thromboembolism in cancer patients: ESMO Clinical Practice Guidelines for the managementAnn Oncol201021suppl5v274v27620555096
- LymanGHKhoranaAAKudererNMAmerican Society of Clinical Oncology Clinical PracticeVenous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline updateJ Clin Oncol201331172189220423669224
- StreiffMBNational Comprehensive Cancer Center NetworkThe National Comprehensive Cancer Center Network (NCCN) guidelines on the management of venous thromboembolism in cancer patientsThromb Res2010125suppl 2S128S13320433992
- CarrierMCameronCDellucACastellucciLKhoranaAALeeAYEfficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysisThromb Res201413461214121925457583
- GouldMKDembitzerADSandersGDGarberAMLow-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysisAnn Intern Med19991301078979910366368
- FalangaAVignoliADianiEMarchettiMComparative assessment of low-molecular-weight heparins in cancer from the perspective of patient outcomes and survivalPatient Relat Outcome Meas2011217518822915978
- BüllerHRDavidsonBLDecoususHMatisse InvestigatorsSubcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolismN Engl J Med2003349181695170214585937
- BrightonTAEikelboomJWMannKASPIRE InvestigatorsLow-dose aspirin for preventing recurrent venous thromboembolismN Engl J Med2012367211979198723121403
- ConnollyGCPhippsRPFrancisCWPlatelets and cancer-associated thrombosisSemin Oncol201441330231025023346
- CaineGJStonelakePSLipGYKehoeSTThe hypercoagulable state of malignancy: pathogenesis and current debateNeoplasia20024646547312407439
- FalangaAPanova-NoevaMRussoLProcoagulant mechanisms in tumour cellsBest Pract Res Clin Haematol2009221496019285272
- KlementsenBJørgensenLMechanisms involved in the early interaction between HeLa cells, platelets and endothelial cells in vitro under the influence of thrombin. Effects of acetylsalicylic acid and Na-salicylateAPMIS199710553914019201241
- HorowitzNABlevinsEAMillerWMThrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domainBlood2011118102889289521788337
- GoernerAThe influence of anticlotting agents on transplantation and growth of tumor tissueJ Lab Clin Med1931164369372
- FalangaAMarchettiMVignoliACoagulation and cancer: biological and clinical aspectsJ Thromb Haemost201311222323323279708
- FalangaAMarchettiMHeparin in tumor progression and metastatic disseminationSemin Thromb Hemost200733768869418000796
- MousaSAMohamedSInhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitorThromb Haemost200492362763315351861
- NorrbyKLow-molecular-weight heparins and angiogenesisAPMIS200611427910216519745
- McCowanCMunroAJDonnanPTSteeleRJUse of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortalityEur J Cancer20134951049105723182687
- IshikawaHMutohMSuzukiSThe preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trialGut201463111755175924488498
- PaolinoMChoidasAWallnerSThe E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cellsNature2014507749350851224553136
- McDonaldDMBalukPSignificance of blood vessel leakiness in cancerCancer Res200262185381538512235011
- PhillipsPGYalcinMCuiHIncreased tumor uptake of chemotherapeutics and improved chemoresponse by novel non-anticoagulant low molecular weight heparinAnticancer Res201131241141921378319
- CarmelietPAngiogenesis in health and diseaseNat Med20039665366012778163
- BalukPHashizumeHMcDonaldDMCellular abnormalities of blood vessels as targets in cancerCurr Opin Genet Dev200515110211115661540
- SunCJainRKMunnLLNon-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusionAnn Biomed Eng200735122121212917846892
- JainRKStylianopoulosTDelivering nanomedicine to solid tumorsNat Rev Clin Oncol201071165366420838415
- MoellerBJRichardsonRADewhirstMWHypoxia and radiotherapy: opportunities for improved outcomes in cancer treatmentCancer Metastasis Rev200726224124817440683
- HamzahJJugoldMKiesslingFVascular normalization in Rgs5-deficient tumours promotes immune destructionNature2008453719341041418418378
- FolkmanJTumor angiogenesis: therapeutic implicationsN Engl J Med197128521118211864938153
- FerraraNVEGF and the quest for tumour angiogenesis factorsNat Rev Cancer200221079580312360282
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- KooPJMorgenszternDBoyerJLTargeting vascular endothelial growth factor in patients with squamous cell lung cancerJ Clin Oncol201230101137113922355057
- JaysonGCKerbelREllisLMHarrisALAntiangiogenic therapy in oncology: current status and future directionsLancet Epub201624
- EllisLMHicklinDJVEGF-targeted therapy: mechanisms of anti-tumour activityNat Rev Cancer20088857959118596824
- PennacchiettiSMichieliPGalluzzoMMazzoneMGiordanoSComoglioPMHypoxia promotes invasive growth by transcriptional activation of the met protooncogeneCancer Cell20033434736112726861
- JainRKNormalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapyNat Med20017998798911533692
- GoelSDudaDGXuLNormalization of the vasculature for treatment of cancer and other diseasesPhysiol Rev20119131071112121742796
- JainRKMartinJDStylianopoulosTThe role of mechanical forces in tumor growth and therapyAnnu Rev Biomed Eng20141632134625014786
- KerbelRSAntiangiogenic therapy: a universal chemosensitization strategy for cancer?Science200631257771171117516728631
- ChatterjeeSWieczorekCSchöttleJTransient antiangiogenic treatment improves delivery of cytotoxic compounds and therapeutic outcome in lung cancerCancer Res201474102816282424675359
- BatchelorTTGerstnerEREmblemKEImproved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiationProc Natl Acad Sci U S A201311047190591906424190997
- Van der VeldtAALubberinkMBahceIRapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of antiangiogenic drugsCancer Cell2012211829122264790
- Pàez-RibesMAllenEHudockJAntiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasisCancer Cell200915322023119249680
- CarmelietPJainRKPrinciples and mechanisms of vessel normalization for cancer and other angiogenic diseasesNat Rev Drug Discov201110641742721629292
- ScappaticciFASkillingsJRHoldenSNArterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumabJ Natl Cancer Inst200799161232123917686822
- ChenHXCleckJNAdverse effects of anticancer agents that target the VEGF pathwayNat Rev Clin Oncol20096846547719581909
- KlerkCPSmorenburgSMOttenHMThe effect of low molecular weight heparin on survival in patients with advanced malignancyJ Clin Oncol200523102130213515699479
- van DoormaalFFDi NisioMOttenHMRichelDJPrinsMBullerHRRandomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancerJ Clin Oncol201129152071207621502549
- AgnelliGGeorgeDJKakkarAKSemuloparin for thromboprophylaxis in patients receiving chemotherapy for cancerN Engl J Med2012366760160922335737
- SaltzLBClarkeSDíaz-RubioEBevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III studyJ Clin Oncol200826122013201918421054
- GiantonioBJCatalanoPJMeropolNJBevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol200725121539154417442997
- Van CutsemETaberneroJLakomyRAddition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimenJ Clin Oncol201230283499350622949147
- TaberneroJYoshinoTCohnALRAISE Study InvestigatorsRamucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 studyLancet Oncol201516549950825877855
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAilJ Clin Oncol20092781227123419188680
- BarlesiFScherpereelARittmeyerARandomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089)J Clin Oncol201331243004301123835708
- ZhouCWuYLChenGBEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or Placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancerJ Clin Oncol201533192197220426014294
- Pujade-LauraineEHilpertFWeberBBevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trialJ Clin Oncol201432131302130824637997
- AghajanianCBlankSVGoffBAOCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancerJ Clin Oncol201230172039204522529265
- OhtsuAShahMAVan CutsemEBevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III studyJ Clin Oncol201129303968397621844504
- WilkeHMuroKVan CutsemERAINBOW Study GroupRamucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trialLancet Oncol201415111224123525240821
