Abstract
In the past few years, nanomaterial-based drug delivery systems have been applied to enhance the efficacy of therapeutics and to alleviate negative effects through the controlled delivery of targeting and releasing agents. However, few drug carriers can achieve high targeting efficacy, even when targeting modalities and surface markers are introduced. Immunological problems have also limited their wide applications. Biological drug delivery systems, such as erythrocytes, platelets, and albumin, have been extensively investigated because of their unique properties. In this review, erythrocytes, platelets, and albumin are described as efficient drug delivery systems. Their properties, applications, advantages, and limitations in disease treatment are explained. This review confirms that these systems can be used to facilitate a specific, biocompatible, and smart drug delivery.
Introduction
Drug delivery systems aim to improve the pharmacological properties of drugs and to achieve maximal therapeutic efficacy and minimal side effects by directing therapeutic cargos to target cells and tissues.Citation1,Citation2 Novel drug delivery systems have been developed because conventional and new drugs provide intrinsic disadvantages, such as poor stability, poor solubility, gastrointestinal reactions, and unwanted toxicity.
With remarkable advancements in nanotechnology, nanomaterials and nanostruc-tures have been widely applied. Nanotechnology- and nanoscience-related studies have been extensively conducted over the past decades. Nanomaterial-based drug delivery systems have also been rapidly developed.Citation3,Citation4 Various nanomaterials, including nanoparticles,Citation5 polymeric micelles,Citation6 lipsomes,Citation7 and nanotubesCitation8 have been utilized as drug carriers. Nanocarrier-delivered drugs provide several advantages over free drugs; for instance, nanocarriers can prolong circulation time, slow down metabolism, induce controlled drug release, and improve patient convenience and compliance.Citation9 When used for chemotherapy, nanocarriers can enhance drug targeting to tumors because of their selective accumulation in tumors through enhanced permeability and retention (EPR) effects. EPR effects or passive targeting effects that often occur in solid tumors involve nanocarriers or macromolecules that preferentially accumulate in tumors rather than in normal tissues, because of leaky vasculature and poor lymphatic drainage in tumor cells and tissues.Citation10 However, some hypoxic regions of solid tumors may fail to elicit EPR effects because of poor angiogenesis.Citation11 Thus, active targeting strategies, including coupling with homing peptides,Citation12 antibodies,Citation13,Citation14 and growth factors,Citation15 have been employed to overcome the limitations of passive targeting. Although major advancements have been observed in this field, neither passive targeting nor active targeting can achieve specific nonrandom targeting that remains chance dependent. Human bodies are equipped with innate immune defenses, such as the reticuloendothelial system (RES), which rapidly recognizes and destroys foreign objects. As such, nanocarriers may be engulfed by macrophages of the mononuclear phagocyte system before these carriers reach target sites.Citation16 As the immune system catches up in the game of hide and seek, biological drug carriers have been developed to bypass immune surveillance.Citation16,Citation17
Biological carriers, such as erythrocytes, platelets, and albumin, can bypass immune surveillance and provide several advantages, including long circulation time, good biodegradability, abundant surface ligands, and flexible morphology. Therefore, these carriers are optimum biological delivery systems and potential solutions to overcome the limitations of nanomaterial-based drug carriers.Citation18,Citation19 This review describes erythrocytes, platelets, and albumin as promising biological drug carriers and their applications in various diseases.
Erythrocytes as drug delivery systems
Properties of erythrocytes
Erythrocytes or red blood cells (RBCs) are enucleated biconcave disk-shaped cells in humans. RBCs are characterized by a diameter of ~7 μm, a thickness of ~2 μm, and a plasma membrane surface area of ~160 μm2. Erythrocytes are the most abundant blood components, and 1 μL of human blood contains ~4–5 million RBCs. The half-life of human erythrocytes ranges from 100 days to 120 days. Various receptors, proteins, and functional groups on the membrane of erythrocytes provide binding sites for antibodies, specific ligands, and drug.Citation20–Citation22
The complex and unique membranes of RBCs are involved in the delivery of oxygen from lungs to different tissues.Citation23 RBCs travel ~250 km through the cardiovascular system containing capillary networks, which can be as narrow as one-third of a diameter of cells.Citation24 The cytoskeleton, especially hexagonal actin–spectrin lattice underlying the plasmalemma, supports the membrane of RBCs, and the integrity of the cytoskeleton is based on a series of “horizontal” and “vertical” linkages between membrane protein complexes within the fluid phospholipid membrane bilayer. These structures ensure that the erythrocyte membrane simultaneously undergoes reversible changes between expanded and tight networks to maintain integrity and to avoid intra-vascular hemolysis.Citation25 These damaged and aged RBCs are eliminated by tissue macrophages in the liver and spleen, and RBCs continuously circulate within the bloodstream but pass through the interstitium in splenic follicles and hepatic sinusoid. Thus, RBCs are suitable for intravascular delivery to treat blood, or endothelium-related diseases.Citation1,Citation26 Erythrocytes can be supplied as ideal drug delivery systems because of these important features; since the discovery of RBCs as potential carrier systems, numerous substances have been bound to these blood cells or used to encapsulate them for disease treatment.Citation27–Citation32
Preparation approaches for RBCs as drug delivery systems
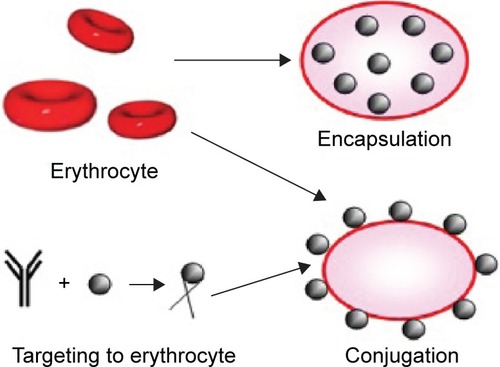
Encapsulation
Several methods have been used to encapsulate cargos in RBCs. Osmosis-based methods developed in the 1970s have been considered standard methods, because erythrocytes can act as an osmometer. In a hypotonic solution, erythrocytes reversibly swell to ~25% of their original volume, and pores ranging from 200 Å to 500 Å are temporarily formed in the membrane. Thus, substances can enter erythrocytes through these pores. In an isotonic solution, the shape of RBCs is regained, and their pores close; substances are then encapsulated within erythrocytes.Citation33 This mechanism exhibits some variations, including hypotonic hemolysis, hypotonic pre-swelling, and hypotonic dilution. Considered as the most simple and rapid method of encapsulation, hypotonic dilution is the first process in which therapeutic moieties are loaded into erythrocytes.Citation34–Citation36
Another encapsulation method is electroporation or electro-insertion; in this process, erythrocytes are exposed to a strong external electrical field that causes irreversible changes in the RBC membrane.Citation37,Citation38 However, Kinosita and TsongCitation39 suggested that desirable membrane permeability can be produced through transient electrolysis. Kitao et alCitation40 pioneered the successful application of chemical perturbation in human and mouse RBCs in 1980. However, this method has been rarely employed because it induces irreversible destructive changes in the membrane. Alternative approaches, such as lipid fusion and endocytosis, have also been applied to encapsulate agents into erythrocytes.Citation41,Citation42
Membrane coupling or binding
Among conventional strategies, reversible or irreversible coupling of a therapeutic moiety to RBCs is the most extensively utilized strategy. Nonspecific chemical cross-linkers have been used to bind molecules to the surface of erythrocytes.Citation43,Citation44 However, implementation of these strategies has been impeded by specific cross-links, such as biotin–avidin pair, which allow biotinylated molecules, including nucleic acids, to couple to sulfhydryl groups, amino acids, and other specific groups expressed on the membrane of RBCs. Through these methods, various agents, such as viral antigens and immunoglobulins, have been coupled to the RBC surface.Citation21,Citation45 In addition to biotin–avidin, various chemical linkers have been utilized to attach different molecules, including small therapeutic molecules, such as daunorubicin,Citation46 and large molecules, such as hyperbranched chemical linkers.Citation47 Furthermore, single or combined molecules can conjugate with the surface of RBCs. In another approach, site-specific drug binding occurs as follows.Citation48 Antibodies, peptides, or other affinity ligands are initially anchored to the surface proteins of erythrocytes; therapeutic agents bind to and conjugate with these ligands.Citation48 Agents can also be attached to RBC surfaces through physical interactions; for instance, polystyrene particles can adhere to the surface of erythrocytes via nonspecific van der Waals and hydrogen bonding, which work through electrostatic and hydrophobic forces, respectively, between particles and erythrocytes.Citation49
illustrates the two main preparation strategies for RBCs as a drug delivery system.
Application of erythrocytes as a drug delivery system
Erythrocytes encapsulate and protect L-asparaginase (L-ASNase) from degradation; the encapsulated L-ASNase exhibits a longer half-life and lower incidence and severity of side effects, such as allergic reactions than native L-ASNase. Encapsulated L-ASNase is a promising product with a good safety profile to treat children and adults with refractory or relapsing acute lymphoblastic leukemia.Citation50,Citation51 For instance, the toxic effect of erythrocyte-delivered etoposide on macrophages is greater than that of free chemotherapeutic drugs; this phenomenon indicates the usefulness of RBCs in the delivery of this cytotoxic agent to target macrophages.Citation52 Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), an autosomal recessive disease, is caused by mutations in the nuclear gene ECGF1 coding for thymidine phosphorylase (TP); thus, high deoxythymidine and deoxyuridine levels accumulate in the body and induce gastrointestinal motility disorders, such as stomachache, progressive external ophthalmoplegia, hearing loss, and peripheral sensorimotor polyneuropathy. Moran et alCitation53 found that deoxythymidine and deoxyuridine levels decrease significantly, and symptoms have been ameliorated in patients with MNGIE when TP is carried by RBCs. Levene et al concluded that serious toxicities likely preclude a clinical trial of TP carried by RBCs in patients with MNGIE.Citation54 Harisa et alCitation32 demonstrated that human erythrocytes can be successfully loaded with pravastatin, and relatively high drug loading and encapsulation efficiency can be obtained. Moreover, no significant loading parameters and morphological changes in erythrocytes have been observed in entrapping pravastatin; this finding indicates that erythrocytes are potential carriers for pravastatin. Methotrexate (MTX), an antimetabolite and antifolate agent used in solid tumors and hematological diseases, can be encapsulated by erythrocytes, and the average survival time of rat hepatoma cells is enhanced with MTX-loaded erythrocyte treatment compared with that of cells treated with native MTX.Citation55 Biagiotti et alCitation56 confirmed that immunosuppressants can be encapsulated into erythrocytes in the presence of corresponding target proteins, and RBCs can serve as a promising delivery system for immunosuppressive agents.Citation56 The use of RBCs as a drug delivery system for chemotherapeutic agents, especially in vitro and in vivo use of antineoplastic agents, has been widely investigated. Other therapeutic drugs delivered by RBCs include gentamicin for bacterial infection,Citation57 δ-aminolevulinate dehydratase for lead poisoning,Citation58 β-glucocerebrosidase for enzyme replacement therapy in Gaucher’s disease,Citation59 adenosine deaminase for adenosine deaminase deficiencies,Citation60 enalaprilat for hypertension management and congestive heart failure,Citation61 and heparin for thromboembolismCitation62 and carrier for thrombolytic agents.Citation63 provides some examples of therapeutic moieties loaded by erythrocytes.Citation28,Citation46,Citation64–Citation75
Table 1 Examples of therapeutic moieties loaded by erythrocytes
Advantages
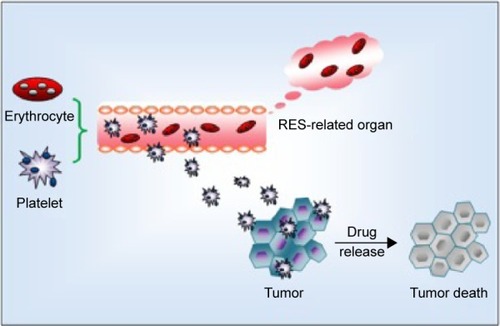
Erythrocytes applied as drug delivery systems have been extensively investigated because of several factors. For instance, erythrocyte sources are abundant, and the structure and properties of erythrocytes are well understood. RBCs also possess good biocompatibility and biodegradability without inducing immunological reactions and producing toxic by-products. The membrane properties of RBCs permit relatively high drug loading and slow molecular release. Furthermore, their circulation time in the bloodstream is prolonged. RBCs are phagocytosed by macrophages in the liver and spleen. Thus, RBCs introduce cargos into the RES of cells, thus they can be beneficial for the treatment of macrophage-related hepatic diseasesCitation22,Citation23,Citation48,Citation76 ().
Drawbacks
Similar to other drug delivery systems, carrier erythrocytes are limited by various factors. For example, encapsulation may cause an osmotic stress-induced damage to the RBC membrane. Coupling therapeutic moieties to RBCs can lead to the loss of the mechanical stability and plasticity of erythrocytes. These morphological and physiological changes in erythrocytes may trigger an unwanted removal of RBCs by RES; as a consequence, their circulation time in the bloodstream is decreased. Molecules encapsulated in or coupled with RBCs may induce erythrocyte leakage and thus elicit toxic effects. Furthermore, preparation strategies for erythrocyte carriers have yet to be standardized; these carriers are also more varied than synthetic carrier systems. The storage of erythrocyte carriers and risk of blood contamination before, during, and after drug loading should also be highly considered.Citation27,Citation77
Advancements
Drug selection and RBC–drug relationship have been improved because of various limitations. Prodrugs, such as corticosteroid prodrugs for prolonged circulation and nucleoside/nucleotide prodrugs for macrophage targeting, can improve the performance of RBC-based drug carriers. If an encapsulated or coupled molecule is inactive, erythrocytes can transform and release prodrugs in their active forms. This strategy can reduce off-target effects and prolong the half-life of drugs. Kwon et alCitation78 introduced a new strategy of protein loading by using membrane-translocating low molecular weight protamine, which causes negligible changes in RBC membrane. Other novel engineered substances, such as nanoparticles and polymeric multilayered microcapsules, combined with RBCs are also currently used.Citation22,Citation79,Citation80
Platelets as drug delivery systems
Properties of platelets
Platelets, with an average diameter of 2–3 μm, are discoid fragments from megakaryocytes of the bone marrow and the smallest components of the blood. Approximately 108 cells can be produced by megakaryocytes daily. Thus, 2×1011 to 5×1011 platelets can be generated; when demands are high, this number increases tenfold. The platelet concentration in the circulating blood is ~150×109/L to 350×109/L with an average life span of 7–10 days; after this period, platelets are removed by reticuloendothelial cells from the liver and spleen.Citation81,Citation82
Platelets participate in several physiological and pathological processes, including hemostasis, wound healing, thrombosis, inflammation, and atherosclerosis.Citation83 In hemostasis, damaged small blood vessels and capillaries promote vasoconstriction to prevent blood loss. In addition, platelets adhere to the site of injury by interacting with von Willebrand factor and collagen; some mediators work with various potent functional molecules, including adenosine diphosphate and thromboxane A2. Thus, platelets act as a storage “depot” and trafficking “vehicle” in blood vessels.Citation84
Pathological conditions occur because of nonspecific activity of platelets, stimulation of agonist secretion, and platelet aggregation in atheromatous plaques; these conditions decrease the number of platelets in circulation and cause ischemia.Citation85 The mechanism by which platelets engulf small molecules is different from conventional phagocytosis, in which an engulfed substance is metabolized. Compared with other carriers, encapsulated substances in the platelet remain intact, and are thus referred to as covercytes.Citation86
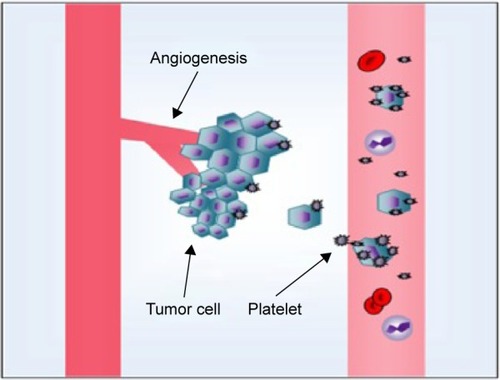
The relationship between platelets and malignancy was first discovered in 1865 by Armand Trousseau, a French clinician who reported that migratory thrombophlebitis can be an indicator of occult malignancies, including pancreatic carcinoma. Named after this clinician, Trousseau syndrome is characterized by a hypercoagulable state with advanced malignancy. Seven years later, RiessCitation87 described a “massive increase of platelets” in patients with carcinoma. In 1878,Citation88 Billroth found that platelets not only participate in tumor growth but also contribute to metastatic spread. A century later, researchers found that thrombocytopenia alleviates metastasis in mice. Tumor cells induce platelet aggregation, and active platelets protect or cloak circulatory tumor cells from physical factors such as shear stress within the vasculature, and help them escape from immune surveillance. Subsequently, platelets cause tumor cells to transfer to secondary sites on the vascular wall; platelets also secrete proteases and cytokines that promote the formation of new blood vessels, which are necessary for tumor-associated angiogenesis and growth. Platelet count is increased by tumor cells. Therefore, the correlation between platelets and tumors can be described as a vicious cycle: tumor increases platelet count; the increased platelet count promotes tumor growth and metastasis; as a consequence, platelet count is further increased ().Citation89,Citation90 The phenomenon by which tumor cells cause platelet aggregation was first discovered in 1968 and referred to as tumor-induced platelet aggregation (TCIPA).Citation91 TCIPA is considered the fundamental to utilize platelets as drug delivery systems for malignant tumor treatment. The mechanism of TCIPA is illustrated in .
Figure 3 The relationship between platelets and malignancy.
Application of platelets as a drug delivery system
Sarkar et alCitation92 showed that platelets can take up drugs at a relatively high drug loading concentration, and the efficiency of inducing cytotoxicity is significantly increased when platelets are used as drug delivery carriers in vitro and in vivo. They concluded that platelets can be potential drug carriers when a low drug concentration is used in a targeted mode. Anti-thrombotic agents are commonly utilized to manage patients at a high risk of thrombosis and hemorrhage. Platelets have also been effectively exploited as carriers for antithrombotic agents, especially for arterial clots.Citation93,Citation94 Davies et alCitation95 constructed a strategy to deliver europium luminescent complex and gold nanoparticles (AuNPs) into human platelets by using a low-pH insertion peptide. The radiolabeling of platelets to assess survival and recovery has been developed for more than 4 decades. However, this technique has been banned for use in clinical studies. By contrast, nonradioactive labeling of platelets has been used frequently in clinical studies, including evaluation of the survival of transfused platelets and analysis of the pathogenesis of stroke.Citation96 In our study, doxorubicin is loaded into platelets, and high drug loading and encapsulation efficacy are detected. Doxorubicin is also released by a pH-based trigger. Inhibition and apoptosis rates are higher in the group that received drug-loaded platelets than in the untreated group.
Nanoparticles mimicking platelets have been proposed for drug and gene delivery to treat various diseases. Kona et alCitation97 formulated drug-loaded poly(lactic-co-glycolic acid) nanoparticles and conjugated the external fraction of platelet glycoprotein Ib (GPIb) via carbodiimide chemistry. They confirmed that the GPIb-conjugated poly(lactic-co-glycolic acid) nanoparticles can act as a controlled and targeted drug carrier under flow conditions at vascular injury sites.Citation97 Lin et al obtained similar results.Citation98 Modery-Pawlowski et alCitation99 found that platelet mimetics is a promising method in the treatment of metastasized tumor. Researchers prepared polymeric nanoparticles enclosed in the plasma membrane of human platelet; they verified that platelet membrane-cloaked nanoparticles exhibit less uptake by macrophage-like cells than uncoated nanoparticles do, and particle-induced complement activation is reduced. Furthermore, therapeutic efficacy is enhanced when vancomycin and docetaxel are applied to an experimental mouse model of systemic bacterial infection and a rat model of coronary restenosis via platelet-mimetic nanoparticles.Citation100
Other applications of platelets as drug carriers are summarized in .Citation92,Citation101
Table 2 Examples of therapeutic moieties loaded by platelets
Advantages
Biocompatibility, which is defined as “expression of the benignity of the relation between a material and its biological environment”, is considered a fundamental property of a carrier. The biocompatibility of platelets is superior to that of other drug delivery systems because platelets are components of the human body. In theory, ~500 molecules can be loaded on 1×10−15/L nanoparticles; in reality, the amount is much lower. The loading efficacy of platelets is relatively high, with an average of 50,000–70,000 molecules encapsulated by the same number of platelets.Citation86,Citation102 Another property of platelets is that targets are well defined: these targets are mostly sites with high density of proliferating cells or injured sites. Thus, side effects caused by nonspecific targeting are decreased because of the inherent targeting capacity of platelets. Furthermore, platelet-encapsulated drugs are protected from physical stress and immunological reactions; as such, the systemic clearance of the encapsulated drugs is similar to that of single platelet, and circulation time is prolonged. Controlled drug delivery is quite important, and platelets can facilitate controlled drug release by artificially exposing drugs to agonists. According to Sarkar et al, lethal effects are exacerbated by enhanced apoptosis when cytotoxic drugs are carried by platelets; this phenomenon indicates the synergetic effect of platelets and drugs. As such, low amounts of drugs can be used to achieve the same treatment efficacy, and few side effects are induced. Another advantage of platelets over other carriers is that the method can be individualized because a patient’s own platelets are the ones used to deliver drugs.Citation92 The method is also appropriate for patients who need transfusion.
Drawbacks
The main drawbacks of platelets are similar to those of RBCs. These limitations include proper storage and contamination. In genetic therapy, whether the platelet-specific expression of transgenes affects the expression of endogenous proteins remains unknown. Furthermore, whether the usage of platelets as drug carriers causes thrombogenesis should be further investigated.
Albumin as a drug delivery system
Properties of albumin and its preparation
Albumin, which is the most abundant plasma protein, acts as a molecular taxi for various small insoluble substances, such as hormones, nutrients, and toxins. The albumin concentration in an adult ranges from 35 g/L to 50 g/L.Citation103 Albumin, with an average half-life of 19 days, is synthesized in the liver and its molecular weight is 66.5 kDa.Citation104
Babson and WinnickCitation105 demonstrated for the first time that tumor cells can trap plasma proteins and utilize their degradation products for proliferation. Since then, other researchers have revealed the ability of albumin to accumulate in malignant tissues via the EPR effect. The leakiness of the defective blood vessels of tumor tissues enables macromolecules to pass the endothelial barrier, but normal tissues are permeable only to small molecules.Citation71,Citation104 As such, the proper size of macromolecules that can extravasate into tumor tissues but not into healthy tissues ranges from 2 nm to 10 nm, and the diameter of serum albumin is 7.2 nm. In addition, Stehle et al proposed that albumin is a major nutrient and energy source for tumor cell growth.Citation106 In 2003, Wunder et alCitation107 showed that albumin accumulates in the arthritic paws of mice. Therefore, albumin is a potential carrier that can be used to deliver drugs to the inflamed joints of patients with rheumatoid arthritis.Citation104
Albumin as a drug delivery system is prepared via two primary ways: 1) encapsulation of drugs into albumin nanoparticles, which are dependent on physical interaction between drugs and albumin, and 2) coupling of drugs to endogenous or exogenous albumin and conjugation with bioactive proteins.
Application of albumin as a drug delivery system
The MTX bound to human serum albumin (MTX-HSA) is the first albumin conjugate evaluated in Phase I/II clinical studies. In the assessment of antitumor activity in preclinical in vivo models, complete remission/cure is achieved in soft-tissue sarcoma SXF 1301 by using MTX-HSA; free MTX lasts for a short period. In the prostate cancer model PRXF PC3M, MTX-HSA induces a greater growth inhibition than native MTX dose. These results demonstrate that antitumor effectiveness can be enhanced when MTX is conjugated with albumin.Citation108 MTX-HSA can be combined with cisplatin, and the toxicity of the antitumor activity against urothelial carcinomas is tolerable.Citation109 One of the successful applications of albumin as a drug delivery system is demonstrated by the albumin–paclitaxel (PAC) nanoparticle Abraxane, which was first approved in 2005 by the US Food and Drug Administration to treat metastatic breast cancer; this drug was also approved in 41 countries in the same year. Teneriello et alCitation110 verified that nanoparticle albumin-bound PAC is active in patients with recurrent ovarian, peritoneal, or fallopian tube cancer, and toxicity is manageable, as revealed by Phase II evaluation. HSA has been proposed as a carrier for renal targeting drugs, but glomeruli are not entirely permeable to HSA because of the molecular weight of albumin. Therefore, Yuan et alCitation111 used three peptide fragments (PFs), namely PF-A1-123, PF-A124-298, and PF-A299-585, obtained through the cyanogen bromide degradation of HSA to carry agents and selectively accumulate in kidneys; they verified that PFs can be applied as potential drug delivery systems for renal targeting, and PF-A299-585 may be an optimal carrier. Cationic bovine serum albumin as a gene delivery system has also been demonstrated as a promising strategy to treat lung and metastatic cancers.Citation112 Asmatulu et alCitation113 fabricated albumin-based drug-carrying microcomposite spheres loaded with cyclophosphamide and 5-fluorouracil; they subsequently investigated the potential of these spheres for breast cancer treatment in vitro by using fibroblast cells (3T3) and breast cancer cells (MDA-486). Nude mice have also been used for in vivo evaluation. The results showed that chemotherapeutic efficacy is enhanced when the mice are exposed to other drug delivery systems possibly because of the albumin-induced increase in uptake of microspheres by malignant cells. Peralta et alCitation114 revealed that gold nanorods combined with the chemotherapeutic drug PAC are successfully encapsulated into HSA nanoparticles (HSAPs) to form PAC–AuNP–HSAPs. They verified that PAC–AuNP–HSAPs are more efficient than free PAC in inhibiting the proliferation of 4T1 mouse breast cancer cells and inducing the apoptosis of these cancer cells. Zhang et alCitation115 demonstrated that cytotoxicity is enhanced by two to five times in tumor cells when ruthenium-based anticancer complexes [RuCl5(ind)]2− bind to HSA; no side effects are detected in normal cells in vitro. Compared with the use of single drugs, the use of the HSA–[RuCl5(ind)]2− complex promotes tumor cell apoptosis. Albumin drug nanoparticles combined with phospholipid perifosine or the antibodies trastuzumab (Herceptin) and bevacizumab (Avastin) can effectively treat patients with head, neck, or breast cancer.Citation116 Thus, albumin is a potential drug delivery system.Citation117 Other uses of albumin as a drug carrier are listed in .Citation112,Citation118–Citation127
Table 3 Examples of therapeutic moieties loaded by albumin
Advantages
Albumin is an optimal candidate for drug delivery because of several advantages, including abundant sources, nontoxicity, biodegradability, nonimmunogenicity, and preferential uptake in tumor and inflamed tissues. Albumin is also stable at various temperatures (it can be heated at 60°C for up to 10 hours without deleterious effects), pH (it is stable at pH 4–9), and in various organic solvents.Citation117 Therefore, albumin can enhance the solubility of poorly water-soluble molecules, prolong the circulation time of drugs, and minimize side effects by improving targeting. Furthermore, albumin provides a depot for various drug binding sites and high binding capacity for different drugs.Citation117,Citation128
Drawbacks
In preparing albumin–drug conjugates with or without additional targeting ligands, the structure of albumin may be disrupted; as a result, these conjugates may accumulate in nontarget tissues and may be eradicated by the mononuclear phagocyte sysetm.Citation128 Furthermore, the performances of nanomaterials and albumin in vitro differ from the interaction of albumin and nanomaterials in vivo; undesirable results, such as the risk of capillary blockage because of increased size and potential toxicity caused by conformational changes in albumin, may also be obtained.Citation9
Others
In addition to erythrocytes, platelets, and albumin as drug delivery systems, apoferritin has been used to encapsulate cisplatin and carboplatin.Citation129 Ivy nanoparticles also exhibit great potential for the delivery of chemotherapeutic drugs in cancer therapy.Citation130 Wang et alCitation131 suggested that tea nanoparticles can be useful to prevent multidrug resistance in tumor cells and to enhance the chemotherapeutic efficacy in tumor treatment. Yi et alCitation132 also confirmed that tea nanoparticles can function as a multifunctional nanocarrier for cancer therapy. Fungal nanoparticles have also been proposed as nanocarriers for cancer treatment.Citation133 Low-density lipoprotein,Citation134 nonpathogenic bacteria,Citation135,Citation136 and dendritic and eukaryotic cellsCitation137 have also been used to deliver drugs, enzymes, and genes to specific sites.
Conclusion
Biological agents have been investigated as drug delivery systems in various research and clinical applications, especially for antineoplastic agent delivery, because of their excellent properties. The advantages of biological delivery carriers have been verified by numerous studies. Nevertheless, further research should be conducted to clarify the following questions: 1) Can thrombus be formed through the reduplicative application of platelets as drug carriers? 2) Can carrier erythrocytes deliver oxygen from lungs to different tissues? 3) Can carrier platelets still participate in physiological and pathological processes; if so, what is their role in human bodies?
Acknowledgments
We would like to thank all the volunteers who took part in this study. This work was supported by the National Natural Science Foundation of China (81400162, 81570174), the Natural Science Foundation of Jiangsu Province (BK20140100), the Technique Development Foundation of Nanjing (Outstanding Youth Foundation, JQX15004), the Innovation Program of Jiangsu Province (SJZZ15_0029), and the Medical Science and Technology Development Program of Nanjing (Ykk14069, 201402066).
Disclosure
The authors report no conflicts of interest in this work and have received no payment in preparation of this manuscript.
References
- YooJWIrvineDJDischerDEMitragotriSBio-inspired, bioengineered and biomimetic drug delivery carriersNat Rev Drug Discov201110752153521720407
- LaiJYBiodegradable in situ gelling delivery systems containing pilocarpine as new antiglaucoma formulations: effect of a mercaptoacetic acid/N-isopropylacrylamide molar ratioDrug Des Devel Ther2013712731285
- MarkmanJLRekechenetskiyAHollerELjubimovaJYNanomedicine therapeutic approaches to overcome cancer drug resistanceAdv Drug Deliv Rev20136513–141866187924120656
- HouLYangXMRenJXA novel redox-sensitive system based on single-walled carbon nanotubes for chemophotothermal therapy and magnetic resonance imagingInt J Nanomedicine20161160762426917960
- FukumoriYIchikawaHNanoparticles for cancer therapy and diagnosisAdv Powder Technol2006171128
- ChengQDuLLMengLWThe promising nanocarrier for doxorubicin and siRNA co-delivery by PDMAEMA-based amphiphilic nanomicellesACS Appl Mater Interfaces2016874347435626835788
- ShigehiroTZhaiWVaidyanathAEvaluation of glycosylated docetaxel-encapsulated liposomes prepared by remote loading under solubility gradientJ Microencapsul201633217218226885749
- WuPPLiSZhangHJDesign real-time reversal of tumor multidrug resistance cleverly with shortened carbon nanotubesDrug Des Devel Ther2014824312438
- PengQMuHThe potential of protein-nanomaterial interaction for advanced drug deliveryJ Control Release201622512113226812004
- KimJKimPHKimSWYunCOEnhancing the therapeutic efficacy of adenovirus in combination with biomaterialsBiomaterials20123361838185022142769
- FangJNakamuraHMaedaHThe EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effectAdv Drug Deliv Rev201163313615120441782
- YaoXLYoshiokaYMorishigeTTumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapyMol Ther20111991619162521673661
- BachtarziHStevensonMUbrVSeymourLWFisherKDE-selectin is a viable route of infection for polymer-coated adenovirus retargeting in TNF-α-activated human umbilical vein endothelial cellsJ Drug Target201119869070021309681
- WillemsenRAPecharMCarlisleRCMulti-component polymeric system for tumour cell-specific gene delivery using a universal bungarotoxin linkerPharm Res201027112274228220300804
- ParkJWMokHParkTGEpidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirusBiochem Biophys Res Commun2008366376977418083120
- SuYXieZKimGBDongCYangJDesign strategies and applications of circulating cell-mediated drug delivery systemsACS Biomater Sci Eng20151420121725984572
- FangRHHuCMZhangLNanoparticles disguised as red blood cells to evade the immune systemExpert Opin Biol Ther201212438538922332936
- HamidiMZarrinAForoozeshMMohammadi-SamaniSApplications of carrier erythrocytes in delivery of biopharmaceuticalsJ Control Release2007118214516017270305
- ZhangFXuCLLiuCMDrug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of gliomaDrug Des Devel Ther2015920892100
- AllenTMLigand-targeted therapeutics in anticancer therapyNat Rev Cancer200221075076312360278
- MuzykantovVRTaylorRPAttachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complementAnal Biochem199422311421487695090
- BhateriaMRachumalluRSinghRBhattaRSErythrocytes based synthetic delivery systems: transition from conventional to novel engineering strategiesExpert Opin on Drug Deliv201411812191236
- MuzykantovVRDrug delivery by red blood cells: vascular carriers designed by mother natureExpert Opin Drug Deliv20107440342720192900
- SosaJMNielsenNDVignesSMChenTGShevkoplyasSSThe relationship between red blood cell deformability metrics and perfusion of an artificial microvascular networkClin Hemorheol Microcirc201357327528923603326
- VillaCHPanDCZaltsevSCinesDBSiegelDLMuzykantoyVRDelivery of drugs bound to erythrocytes: new avenues for an old intravascular carrierTher Deliv20156779582626228773
- OldenborgPAZheleznyakAFangYFLagenaurCFGreshamHDLindbergFPRole of CD47 as a marker of self on red blood cellsScience200028854732051205410856220
- MillanCGMarineroMLCastanedaAZLanaoJMDrug, enzyme and peptide delivery using erythrocytes as carriersJ Control Release2004951274915013230
- WangGPGuanYSJinXRDevelopment of novel 5-fluorouracil carrier erythrocyte with pharmacokinetics and potent antitumor activity in mice bearing malignant ascitesJ Gastroenterol Hepatol201025598599020546454
- AlanaziFKHariaGel-DMaqboulAAbdel-HamidMNeauSHAlsarraIABiochemically altered human erythrocytes as a carrier for targeted delivery of primaquine: an in vitro studyArch Pharm Res201134456357121544721
- StaedtkeVBrahlerMMullerAIn vitro inhibition of fungal activity by macrophage-mediated sequestration and release of encapsulated amphotericin B nanosuspension in red blood cellsSmall2010619610319882684
- ShaviGVDoijadRCDeshpandePBErythrocytes as carrier for prednisolone: in vitro and in vivo evaluationPak J Pharm Sci201023219420020363699
- Harisa GelDIbrahimMFAlanaziFKCharacterization of human erythrocytes as potential carrier for pravastatin: an in vitro studyInt J Med Sci20118322223021448309
- KwantWSeemanPThe erythrocyte ghost is a perfect osmometerJ Gen Physiol19705522082195413078
- IhlerGMGlewRHSchnureFWEnzyme loading of erythrocytesProc Natl Acad Sci U S A1973709266326664354859
- DeLoachJHarrisRIhlerGAn erythrocyte encapsulator dialyzer used in preparing large quantities of erythrocyte ghosts and encapsulation of a pesticide in erythrocyte ghostsAnal Biochem198010212202276766688
- TajerzadehHHamidiMEvaluation of hypotonic preswelling method for encapsulation of enalaprilat in intact human erythrocytesDrug Dev Ind Pharm200026121247125711147125
- LizanoCPerezMTPinillaMMouse erythrocytes as carriers for coencapsulated alcohol and aldehyde dehydrogenase obtained by electroporation in vivo survival rate in circulation, organ distribution and ethanol degradationLife Sci200168172001201611388702
- DongQJinWMonitoring diclofenac sodium in single human erythrocytes introduced by electroporation using capillary zone electrophoresis with electrochemical detectionElectrophoresis200122132786279211545409
- KinositaKTsongTHemolysis of human erythrocytes by transient electric fieldProc Natl Acad Sci U S A197774519231927266714
- KitaoTHattoriKTakeshitaMAgglutination of leukemia cells and daunomycin entrapped erythrocytes with lectin in vitro and in vivoExperientia19783419495620752
- NicolauCGersondeKIncorporation of inositol hexaphosphate into intact red blood cellsNaturwissenschaften19796611563566514370
- SchrieiSBenschKJohnsonMJungaIEnergized endocytosis in human erythrocyte ghostsJ Clin Invest1975561822124748
- Page FaulkWHoubaVImmunological reactions with chromic chloride-treated erythrocytesJ Immunol Methods19733187984126015
- BoydenSVThe adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein seraJ Exp Med195193210712014803635
- CorintiSChiarantiniLDominiciSLaguardiaMEMagnaniMGirolomoniGErythrocytes deliver Tat to interferon-gamma-treated human dendritic cells for efficient initiation of specific type 1 immune responses in vitroJ Leukoc Biol200271465265811927652
- SkorokhodOAGarmaevaTTVitvitskyVMPharmacokinetics of erythrocyte-bound daunorubicin in patients with acute leukemiaMed Sci Monit2004104PI55PI6415039656
- RossiNAConstantinescuIKainthanRKBrooksDEScottMDKizhakkedathuJNRed blood cell membrane grafting of multi-functional hyperbranched polyglycerolsBiomaterials201031144167417820172604
- HuCMFangRHZhangLErythrocyte-inspired delivery systemsAdv Healthcare Mater201215537547
- ChambersEMitragotriSProlonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytesJ Control Release2004100111111915491815
- DomenechCThomasXChabaudSL-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trialBr J Haematol20111531586521332712
- AgrawalVWooJHBorthakurGKantarjianHFrankelAERed blood cell-encapsulated L-asparaginase: potential therapy of patients with asparagine synthetase deficient acute myeloid leukemiaProtein Pept Lett201320439240223016580
- LoteroLAOlmosGDiezJCDelivery to macrophages and toxic action of etoposide carried in mouse red blood cellsBiochim Biophys Acta2003162016016612595085
- MoranNFBainMDMuqitMMBaxBECarrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIENeurology200871968668818725595
- LeveneMColemanDGKilpatrickHCPreclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and beagle dogs: an enzyme-replacement therapy for mitochondrial neurogastrointestinal encephalomyopathyToxicol Sci2013131131132422977166
- KruseCAFreehaufCLPatelKRBaldeschwielerJDMouse erythrocyte carriers osmotically loaded with methotrexateBiotechnol Appl Biochem1987921231403593543
- BiagiottiARossiLBianchiMImmunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugsJ Control Release2011154330631321640771
- EichlerHGasicSBauerKKornABacherSIn vivo clearance of antibody-sensitized human drug carrier erythrocytesClin Pharmacol Ther19864033003033091309
- DelCBatlleAMBustosNLStellaAMEnzyme replacement therapy in porphyrias – IV. First successful human clinical trial of δ-aminolevulinate dehydratase-loaded erythrocyte ghostsInt J Biochem19831510126112656628828
- BeutlerEDaleGGuintoDKuhlWEnzyme replacement therapy in Gaucher’s disease: preliminary clinical trial of a new enzyme preparationProc Natl Acad Sci U S A1977741046204623200923
- BaxBEFairbanksLDBainMDSimmondsHAChalmersRAThe entrapment of polyethylene glycol-bound adenosine deaminase (Pegademase) in human carrier erythrocytesBiochem Soc Trans1996243442442S8736780
- HamidiMTajerzadehHDehpourASInhibition of serum angiotensin-converting enzyme in rabbits after intravenous administration of enalaprilat-loaded intact erythrocytesJ Pharm Pharmacol20015391281128611578111
- EichlerHGSchneiderWRabergerGBacherSPabingerIErythrocytes as carriers for heparin. Preliminary in vitro and animal studiesRes Exp Med19861866407412
- MurcianoJCMedinillaSEslinDAtochinaECinesDBMuzykantovVRProphylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytesNat Biotechol2003218891896
- AnneseVLatianoARossiLErythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients – a pilot uncontrolled studyAm J Gastroenterol200510061370137515929772
- BossaFAnneseVValvanoMRErythrocytes-mediated delivery of dexamethasone 21-phosphate in steroid-dependent ulcerative colitis: a randomized, double-blind Sham-controlled studyInflamm Bowel Dis20131991872187923714676
- AnneseVLatianoARossiLThe polymorphism of multi-drug resistance 1 gene (MDR1) does not influence the pharmacokinetics of dexamethasone loaded into autologous erythrocytes of patients with inflammatory bowel diseaseEur Rev Med Pharmacol Sci2006101273116494108
- RossiLSerafiniSCeneriniLErythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary diseaseBiotechnol Appl Biochem200133Pt 2858911277860
- RossiLCastroMD’OrioFLow doses of dexamethasone constantly delivered by autologous erythrocytes slow the progression of lung disease in cystic fibrosis patientsBlood Cells Mol Dis2004331576315223012
- ChessaLLeuzziVPlebaniAIntra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trialOrphanet J Rare Dis20149524405665
- SkorokhodOAKulikovaEVGalkinaNMDoxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded erythrocytesHaematologica200792457057117488674
- RossiLPierigèFCarducciCErythrocyte-mediated delivery of phenylalanine ammonia lyase for the treatment of phenylketonuria in BTBR-Pahenu2 miceJ Control Release2014194374425151978
- GodfrinYHorandFFrancoRMeeting highlights: international seminar on the red blood cells as vehicles for drugsExpert Opin Biol Ther201212112713322023703
- KosenkoEAVenediktovaNIKudryavtsevAAEncapsulation of glutamine synthetase in mouse erythrocytes: a new procedure for ammonia detoxificationBiochem Cell Biol200886646947619088795
- SinauridzeEIVuimoTAKulikovaEVShmyrevIIAtaullakhanovFIA new drug form of blood coagulation factor IX: red blood cell-entrapped factor IXMed Sci Monit201016101926
- BourgeauxVHequetOCampionYInositol hexaphosphate-loaded red blood cells prevent in vitro sicklingTransfusion201050102176218420456710
- PierigèFSerafiniSRossiLMagnaniMCell-based drug deliveryAdv Drug Deliv Rev200860228629517997501
- MagnaniMChiarantiniLVittoriaEManciniURossiLFaziARed blood cells as an antigen-delivery systemBiotechnol Appl Biochem19921621881941457052
- KwonYMChungHSMoonCL-Asparaginase encapsulated intact erythrocytes for treatment of zcute lymphoblastic leukemia (ALL)J Control Release200913918218919577600
- ZarrinAForoozeshMHamidiMCarrier erythrocytes: recent advances, present status, current trends and future horizonsExpert Opin Drug Deliv201411343344724456118
- MuraSCouvreurPNanotheranostics for personalized medicineAdv Drug Deliv Rev201264131394141622728642
- WongAKPlatelet biology: the role of shearExpert Rev Hematol20136220521223547868
- WalshTGMetharomPBerndtMCThe functional role of platelets in the regulation of angiogenesisPlatelets201526319921124832135
- MünzerPBorstOWalkerBAcid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formationArterioscler Thromb Vasc Biol2014341617124233488
- ShiQZMontgomeryRRPlatelets as delivery systems for disease treatmentsAdv Drug Deliv Rev201062121196120320619307
- GeraldoRBSathlerPCLourencoALPlatelets: still a therapeutical target for haemostatic disordersInt J Mol Sci20141510179011791925295482
- WhiteJGPlatelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular systemPlatelets200516212113115823869
- RiessLZurpathologischenanatomie des blutes [The pathological anatomy of the blood]Arch Anat Physiol Wissensch Med187239237249 German
- BillrothTLectures on Surgical Pathology and Therapeutics: A Handbook for Students and PractitionersLondonThe New Sydenham Society1878
- BuergyDWenzFGrodenCBrockmannMATumor-platelet in interaction in solid tumorsInt J Cancer2012130122747276022261860
- BambaceNMHolmesCEThe platelet contribution to cancer progressionJ Thromb Haemost20119223724921040448
- GasicGJGasicTBStewartCCAntimetastatic effects associated with platelet reductionProc Natl Acad Sci U S A196861146525246932
- SarkarSAlamMAShawJDasguptaAKDrug delivery using platelet cancer cell interactionPharm Res201330112785279423739991
- GreinederCFHowardMDCarnemollaRCinesDBMuzykantovVRAdvanced drug delivery systems for antithrombotic agentsBlood201312291565157523798715
- ChenHMoWSuHZhangYSongHCharacterization of a novel bifunctional mutant of staphylokinase with platelet-targeted thrombolysis and antiplatelet aggregation activitiesBMC Mol Biol200788817919340
- DaviesALewisDJWatsonSPThomasSGPikramenouZpH-controlled delivery of luminescent europium coated nanoparticles into plateletsProc Natl Acad Sci U S A201210961862186722308346
- AurichKSpoerlMSFurllBDevelopment of a method for magnetic labeling of plateletsNanomedicine20128553754422024199
- KonaSDongJFLiuYLTanJNguyenKTBiodegradable nanoparticles mimicking platelet binding as a targeted and controlled drug delivery systemInt J Pharm2012423251652422172292
- LinASabnisAKonaSShear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticlesJ Biomed Mater Res A200993383384219653303
- Modery-PawlowskiCLMasterAMPanVHowardGPSen GuptaAA platelet-mimetic paradigm for metastasis-targeted nanomedicine platformsBiomacromolecules201314391091923360320
- HuCMFangRHWangKCNanoparticle biointerfacing by platelet membrane cloakingNature2015526757111812126374997
- ShiQFahsSAWilcoxDASyngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunityBlood200811272713272118495954
- BuckleyMFJamesJWBrownDEA novel approach to the assessment of variations in the human platelet countThromb Haemost200083348048410744157
- BernMSandMKNilsenJSandlieIAndersenJTThe role of albumin receptors in regulation of albumin homeostasis: implications for drug deliveryJ Control Release201521114416226055641
- KratzFAlbumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticlesJ Control Release2008132317118318582981
- BabsonALWinnickTProtein transfer in tumor-bearing ratsCancer Res195414860661113199806
- StehleGSinnHWunderAPlasma protein (albumin) catabolism by the tumor itself – implications for tumor metabolism and the genesis of cachexiaCrit Rev Oncol Hematol1997262771009298326
- WunderAMuller-LadnerUStelzerEHAlbumin-based drug delivery as novel therapeutic approach for rheumatoid arthritisJ Immunol200317094793480112707361
- BurgerAMHartungGStehleGSinnHFiebigHHPre-clinical evaluation of a methotrexate-albumin conjugate (MTX-HSA) in human tumor xenografts in vivoInt J Cancer200192571872411340578
- BollingCGraefeTLubbingCPhase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinomaInvest New Drugs200624652152716699974
- TenerielloMGTsengPCCrozierMPhase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal, or fallopian tube cancerJ Clin Oncol20092791426143119224848
- YuanZXHeXKWuXJPeptide fragments of human serum albumin as novel renal targeting carriersInt J Pharm20144601–219620424184033
- HanJHWangQZhangZRGongTSunXCationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancerSmall201410352453524106138
- AsmatuluRYangSYAbedinFAnwarMDAlbumin-based micro-composite drug carriers with dual chemo-agents for targeted breast cancer treatmentJ Biomater Appl2015301384925638169
- PeraltaDHeidariZDashSTarrMAHybrid paclitaxel and gold nanorod-loaded human serum albumin nanoparticles for simultaneous chemotherapeutic and photothermal therapy on 4T1 breast cancer cellsACS Appl Mater Interfaces20157137101711125768122
- ZhangYHoAYueJPStructural basis and anticancer properties of ruthenium-based drug complexed with human serum albuminEur J Med Chem20148644945525200980
- VolkLDFlisterMJChihadeDDesaiNTrieuVRanSSynergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastasesNeoplasia201113432733821472137
- ElzoghbyAOSamyWMElgindyNAAlbumin-based nanoparticles as potential controlled release drug delivery systemsJ Control Release2012157216818221839127
- KamaliniaGKhodagholiFShaerzadehFCationic albumin-conjugated chelating agent as a novel brain drug delivery system in neurodegenerationChem Biol Drug Des20158651203121425976552
- SebakSMirzaeiMMalhotraMKulamarvaAPrakashSHuman serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysisInt J Nanomedicine2010552553220957217
- Thao leQByeonHJLeeCPharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissuesInt J Pharm20164971–226827626657273
- KuipersMESwartPJWitvrouwMAnti-HIV-1 activity of combinations and covalent conjugates of negatively charged human serum albumins (NCAs) and AZTJ Drug Targeting199965323335
- KuipersMEBergMSwartPJMechanism of anti-HIV activity of succinylated human serum albuminBiochem Pharmacol199957888989810086322
- KimIKimTHMaKSynthesis and evaluation of human serum albumin-modified exendin-4 conjugate via heterobifunctional polyethylene glycol linkage with protracted hypoglycemic efficacyBioconjug Chem20102181513151920715855
- DasSBanerjeeRBellareJAspirin loaded albumin nanoparticles by coacervation: implications in drug deliveryTrends Biomater Artif Organs2005182203212
- KufleitnerJWagnerSWorekFBriesenHKreuterJAdsorption of obidoxime onto human serum albumin nanoparticles: drug loading, particle size and drug releaseJ Microencapsul201027650651320214419
- KufleitnerJWorekFKreuterJIncorporation of obidoxime into human serum albumin nanoparticles: optimisation of preparation parameters for the development of a stable formulationJ Microencapsul201027759460120923399
- GraeserREsserNUngerHINNO-206, the (6-maleimidocaproyl hydrazone derivative of doxorubicin), shows superior antitumor efficacy compared to doxorubicin in different tumor xenograft models and in an orthotopic pancreas carcinoma modelInvest New Drugs2010281141919148580
- SleepDAlbumin and its application in drug deliveryExpert Opin Drug Deliv201512579381225518870
- YangZWangXDiaoHEncapsulation of platinum anticancer drugs by apoferritinChem Commun (Camb)20077333453345517700879
- HuangYWangYJWangYExploring naturally occurring ivy nanoparticles as an alternative biomaterialActa Biomater20152526828326219859
- WangYJHuangYJAnreddyNTea nanoparticle, a safe and biocompatible nanocarrier, greatly potentiates the anticancer activity of doxorubicinOncotarget2015755877589126716507
- YiSWangYHuangYTea nanoparticles for immunostimulation and chemo-drug delivery in cancer treatmentJ Biomed Nanotechnol20141061016102924749396
- WangYYiSSunLHuangYZhangMCharge-selective fractions of naturally occurring nanoparticles as bioactive nanocarriers for cancer therapyActa Biomater201410104269428424952072
- JinHLovellJFChenJMechanistic insights into LDL nanoparticle-mediated siRNA deliveryBioconjug Chem2011231334122142191
- WatterlotLRochatTSokolHIntragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in miceInt J Food Microbiol20101441354120452077
- LautenschlagerCSchmidtCFischerDStallmachADrug delivery strategies in the therapy of inflammatory bowel diseaseAdv Drug Deliv Rev201471587624157534
- Gutierrez MillanCColino GandarillasCISayalero MarineroMLLanaoJMCell-based drug-delivery platformsTher Deliv201231254122833931