Abstract
Esophageal squamous cell carcinoma (ESCC) is often diagnosed at late incurable stage and lacks effective treatment strategy. Bufadienolides are cardiotonic steroids isolated from the skin and parotid venom glands of the toad Bufo bufo gargarizans Cantor with novel anticancer activity. However, there is little information about the effects and action mechanisms of bufadienolides on ESCC cells. In this study, the in vitro and in vivo anti-ESCC activities of bufadienolides, including bufalin (Bu) and arenobufagin (ArBu), were examined and the underlying molecular mechanisms were elucidated. The results showed that ArBu exhibited higher anticancer efficacy than Bu against a panel of five ESCC cells, with IC50 values ranging from 0.8 μM to 3.6 μM. However, ArBu showed lower toxicity toward Het-1A human normal esophageal squamous cells, indicating its great selectivity between cancer and normal cells. Moreover, ArBu effectively induced ESCC cell apoptosis mainly by triggering caspase activation through intrinsic and extrinsic pathways. Treatment of ESCC cells also significantly activated p53 signaling by enhancing its phosphorylation. Interestingly, transfection of cells with p53 small interfering RNA significantly inhibited the ArBu-induced p53 phosphorylation and the overall apoptotic cell death. Furthermore, ArBu also demonstrated novel in vivo anticancer efficacy by inhibiting the tumor growth through activation of p53 pathway. Taken together, these results demonstrate the p53-targeting therapeutic potential of bufadienolides against ESCC.
Introduction
Toad venom (venenum bufonis, also called Chan’su) is derived from the dried skin secretions of giant toads (Bufo gargarizans Cantor or Bufo melanostictus Suhneider) and has been widely used for centuries in traditional Chinese medicine alone or in combination with other herbal ingredients.Citation1,Citation2 It is evident that amphibians, such as frogs and toads, possess various bioactive substances in their skin. The discovery of diversified biological activities of the skin extracts has encouraged further study and exploitation of novel amphibian biochemicals with cancer therapeutic application potentials. Many studies have shown that frogs and toads possess different bioactive substances in their skin. In traditional Chinese medicine, amphibian skin extract has been used for the treatment of different diseases. Previous studies have demonstrated that the major pharmacological constituents derived from toad skins are hydrosoluble indole alkaloids (bufotenine, bufotenidine, and cinobufotenine) and liposoluble steroidal cardiac glycosides mainly composed of bufadienolides.Citation3,Citation4 Bufadienolide-type cardiotonic steroids are a class of C-24 steroids with a characteristic pyrone ring at C-17 and have been identified as major active components and marker compounds of venenum bufonis.Citation5 Currently, bufadienolides, including bufotalin, bufalin, and resibufogenin, have been found to show potent cytotoxic and growth inhibitory activity against various human cancer cells.Citation6–Citation15 For instance, bufalin and cinobufagin could induce HepG2 cell apoptosis via both Fas- and mitochondria-mediated pathways.Citation9 Bufalin exerted antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cells.Citation12 These results have supported the future therapeutic potential of bufadienolides in the treatment of human cancers in clinic.
Arenobufagin (ArBu), a natural bufadienolide compound, has been reported as a novel anticancer compound from toad venom.Citation13,Citation16 For instance, Zhang et alCitation13 have shown that ArBu effectively induced apoptosis and autophagy in human hepatocellular carcinoma cells through inhibiting PI3K/Akt/mTOR pathway. Interestingly, Li et alCitation16 also found that ArBu could inhibit vascular endothelial growth factor-mediated angiogenesis through suppression of vascular endothelial growth factor receptor 2 signaling pathway. These results have demonstrated the application potential of ArBu in cancer therapy. However, the effects and mechanisms of ArBu on esophageal squamous cell carcinoma (ESCC) cells remain elusive. Therefore, in this study, the in vitro and in vivo anticancer activities of ArBu were examined by comparing with bufalin (Bu), and the underlying molecular mechanisms were also elucidated. The results demonstrated that ArBu effectively impedes the cell growth of a panel of five ESCC cells by inducing p53-mediated apoptosis. ArBu also exhibited novel in vivo anticancer efficacy in ESCC nude mice model by induction of apoptotic cell death. Taken together, these findings indicate the therapeutic potential of ArBu against ESCC.
Materials and methods
Reagents and materials
ArBu and Bu standards were provided by Tauto Biotech Co., Ltd (Shanghai, People’s Republic of China). Other reagents were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Cell lines and cell cultures
Human ESCC cells (Eca-109, EC9706, TE5, Hec2, and TE11) and a nonmalignant human esophageal squamous cell line Het-1A were obtained from American Type Culture Collection (Manassas, VA, USA) and GuangZhou Jennio Biotech Co., Ltd (Guangzhou, People’s Republic of China). The cells were cultured and maintained in RPMI 1640 and Dulbecco’s Modified Eagle’s Medium supplemented with fetal bovine serum (10%), penicillin (100 units/mL), and streptomycin (50 units/mL) at 37°C in a humid incubator with 5% CO2.
MTT assay
The effects of ArBu and Bu at different concentrations on the cell growth were determined by MTT assay according to a previous method on a microplate spectrophotometer (VERSA max; Molecular Devices LLC, Sunnyvale, CA, USA).Citation17 The IC50 values were calculated by generation of a competitive sigmoidal isoplot.
Flow cytometric analysis
The effects of ArBu and Bu on the cell cycle distribution and cell apoptosis were examined by propidium iodide flow cytometric analysis according to previous protocol.Citation18 Cell apoptosis was estimated by quantifying the sub-G1 peak in the cell cycle profile.
TUNEL assay and DAPI staining
Terminal dexynucleotidyl transferase(TdT)-mediated dUTP nick end labeling (TUNEL)–4′,6-diamidino-2-phenylindole (DAPI) co-staining assay kit (Hoffman-La Roche Ltd., Basel, Switzerland) was employed to detect the apoptotic cell death induced by ArBu.Citation19 Briefly, the cells cultured in confocal dish were fixed with 3.7% formaldehyde for 10 minutes and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS). After then, the cells were incubated with 100 μL/well TUNEL reaction mixture containing nucleotide mixture and terminal deoxynucleotidyl transferase for 1 hour and 1 μg/mL of DAPI for 15 minutes at 37°C, respectively. The cells were then washed with PBS and examined under a fluorescence microscope.
Determination of the caspase activity
The enzymatic activities of caspase-3, -8, and -9 in cells treated with ArBu were monitored by fluorometric method using specific caspase substrates (Ac-DEVD-AFC for caspase-3, Ac-IETD-AFC for caspase-8, and Ac-LEHD-AFC for caspase-9).Citation20
Western blot analysis
The cells after being treated differently were harvested and collected as cell pellets, which were lysed in lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) on ice for 1 hour. The protein concentration was determined by bicinchoninic acid assay (Sigma-Aldrich Co.).Citation21 Equal proteins of each treatment were separated on 12% sodium dodecyl sulfate denaturing polyacrylamide gel and electrophoretically transferred to polyvinylidene fluoride membranes. After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies (Cell Signaling Technology, Inc.) at 1:1,000 dilution overnight at 4°C. After washing, the membranes were incubated with corresponding secondary antibodies and visualized by Pierce ECL Western blotting substrate.
siRNA transfection
Cells were seeded at 2×105 cells per well in six-well plates and allowed to grow to 50% confluence after 24 hours. The cells were then incubated with 50 nmol/L of p53 small interfering RNA (siRNA) and transfection reagent in serum-free culture medium for 24 hours, and then 1 mL of fresh completed medium was added to each well for 24 hours with or without ArBu. Additionally, the cells were also transfected with a fluorescein-labeled nontargeted control siRNA, which allows us to monitor the efficiency of transfection.
Immunofluorescence
Immunofluorescence imaging of phosphorylated p53 was analyzed by immunofluorescence. In brief, cells after treatments were washed with PBS and fixed with 3.7% formaldehyde in PBS for 15 minutes. After rinsing several times with PBS, the cells were permeabilized with 0.2% Triton X-100 in PBS at room temperature for 5 minutes. The permeabilized cells were then blocked with 0.1% bovine serum albumin and subsequently incubated with primary antibody of p-p53 (1:200) overnight at 4°C, followed by Alexa-488-labeled anti-rabbit IgG antibody (1:250) for 1 hour at room temperature. Nuclei were stained with DAPI for 15 minutes after secondary antibody incubation. After washing with PBS, the slides were analyzed using a fluorescence microscopy.
Tumor xenograft in nude mice
Eca-109 cells (5×106) suspended in 200 μL PBS were injected subcutaneously into the right lower hind flank of each 6-week-old male nude mouse. The mice were randomly assigned into three groups with five mice in each group. After 10 days, ArBu dissolved in the solution (DMFv:Tween-80v: salinev =10:2:88) was given intraperitoneally (2 mg/kg and 4 mg/kg body weight every other day) for 20 days. Control mice received an equal volume of the vehicle (saline) only. Body weights and tumor volumes were monitored in every 2 days. Tumor xenografts were removed and weighed at the end of the experiments (day 20). Part of each tumor tissue was then subjected to further biochemical analysis.
Tumor dimensions were measured with calipers, and the volume was calculated using the formula: volume = l × w2/2, where l is the maximal length and w is the width. A portion of the tumors from control and treated groups were used for the preparation of tumor lysate and the rest were fixed in 10% formalin, dehydrated with graded ethanols, and embedded with paraffin for hematoxylin and eosin staining and immunohistochemical analyses. All animal experiments were approved by the Animal Experimentation Ethic Committee of Guangdong No 2 Provincial People’s Hospital, the study followed the Companion Animal Care Guidelines.
Immunohistochemical and immunofluorescence staining analysis
Glutaraldehyde-fixed tumor specimens were embedded with paraffin and cut into 5 μm thin sections. Each tissue section was deparaffinized and underwent hematoxylin and eosin and immunofluorescence staining analysis, respectively. Immunofluorescence staining analysis was performed with anti-p-p53 (SerCitation15) and Ki-67 antibodies (1:100) and Alexa Fluor 488-conjugated anti-rabbit IgG antibody (1:100).
Statistical analysis
In this study, all experiments were performed at least in triplicate, and the data were presented as mean ± standard error. Difference between the two groups was analyzed by two-tailed Student’s t-test and that among three or more groups was analyzed by one-way analysis of variance multiple comparisons. Difference with P<0.05 (*) or P<0.01 (**) was considered statistically significant.
Results and discussion
ArBu and Bu effectively inhibit ESCC cell growth
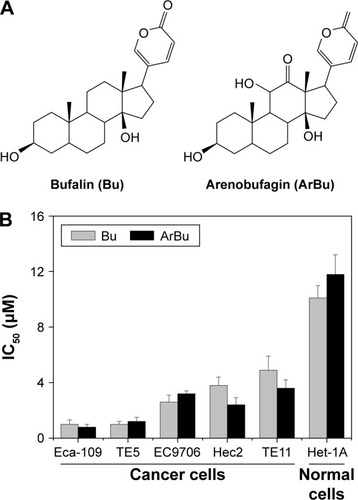
The in vitro antiproliferative activities of ArBu and Bu () were first screened against five human ESCC cancer cell lines (Eca-109, EC9706, TE5, Hec2, and TE11) and a human esophageal squamous cell line Het-1A. After 72 hours treatment with ArBu and Bu, cell viability was detected by MTT assay. As shown in , the results of IC50 value revealed that ArBu and Bu effectively inhibited the cell growth of ESCC cells. ArBu demonstrated higher anticancer efficacy, with IC50 value found at 0.8 μM, 1.2 μM, 3.2 μM, 2.4 μM, and 3.6 μM for the tested ESCC cells, which was more effective than those of Bu, with IC50 value found at 1.0 μM, 1.0 μM, 2.6 μM, 3.8 μM, and 4.9 μM, respectively. In contrast, ArBu and Bu showed lower toxicity toward Het-1A human normal esophageal squamous cells, with IC50 value found at 11.8 μM and 10.1 μM for ArBu and Bu, respectively, indicating their great selectivity between human cancer and normal cells. Taken together, these results exhibit the therapeutic potential of ArBu in ESCC treatment.
Figure 1 In vitro anticancer activities of Bu and ArBu.
Abbreviations: Bu, bufalin; ArBu, arenobufagin; ESCC, esophageal squamous cell carcinoma.
Activation of cell apoptosis by ArBu
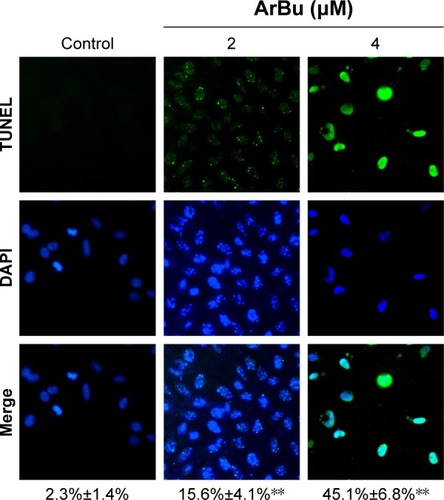
Studies have shown that the loss of control of cell apoptosis played an important role in carcinogenesis of human cancers.Citation22 Therefore, induction of cancer cell apoptosis has been regarded as an effective way to treat cancers.Citation23 Many studies have found that most anticancer drugs exhibited their anticancer activities through activating cell apoptosis.Citation24 Based on the in vitro anticancer screening, ArBu was found to demonstrate higher anticancer efficacy than Bu. Therefore, studies were also carried out to explore the anticancer mechanism through which ArBu caused cancer cell death. As shown in , from the results of TUNEL–DAPI co-staining assay, exposure of Eca-109 cells to 2 μM and 4 μM of ArBu for 24 hours triggered significant and dose-dependent apoptosis. The representative fluorescent images displayed that Eca-109 cells treated with ArBu appeared evident DNA fragmentation and nuclear condensation.
Figure 2 Induction of Eca-109 cell apoptosis by ArBu.
Abbreviations: ArBu, arenobufagin; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI, 4′,6-diamidino-2-phenylindole.
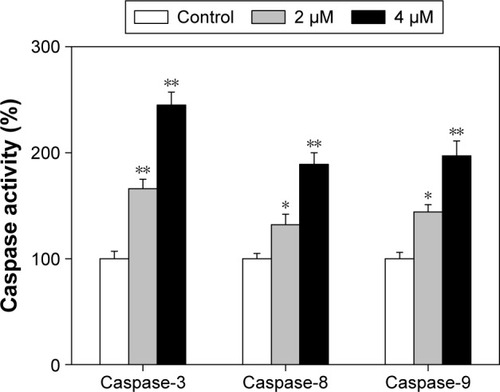
Caspases family proteases act as important factors in regulation of cancer cell apoptosis.Citation25 Activated caspases subsequently induced proteolytic cleavage of poly ADP-ribose polymerase and finally resulted in cell apoptosis.Citation25 Cell apoptosis can be initiated by two central mechanisms, the extrinsic (death receptor-mediated) and the intrinsic (mitochondrial-mediated) apoptotic pathways. In this study, we also examined the intracellular caspase activities in cells exposed to ArBu to examine the requirement of caspases for the apoptotic program. As shown in , exposure of Eca-109 cells to ArBu significantly induced the activation of caspase-3, caspase-8, and caspase-9. These results revealed that ArBu could induce ESCC cell apoptosis mainly through activation of caspase-mediated apoptosis.
Contribution of p53 pathway to cell apoptosis induced by ArBu
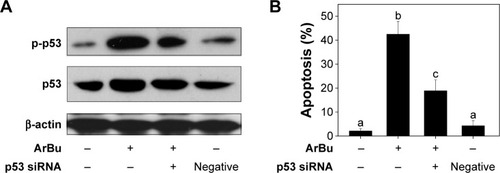
Tumor suppressor gene p53 acts as an important effector to activate cell apoptosis. Therefore, dysregulation of this process can promote tumor progression and chemoresistance during cancer therapy.Citation24,Citation26 Therefore, in this study, the levels of total p53 and phosphorylated p53 were examined by Western blot analysis and fluorescence imaging to elucidate the action mechanisms of ArBu. As shown in , ArBu moderately increased the expression of total p53 protein and significant increase in phosphorylated p-p53 at Ser15 was detected in a dose-dependent manner in Eca-109 cells. Phosphorylation of p53 was further confirmed by fluorescence imaging. As shown in , significant increase in green fluorescence intensity in Eca-109 cells demonstrated that the phosphorylation of p53 protein was induced by ArBu. The role of p53 in ArBu-induced ESCC cell apoptosis was further verified by using RNA interference to silence p53 gene. As shown in , transfection of cells with p53 siRNA significantly inhibited the ArBu-induced p53 phosphorylation and the overall cell apoptosis by measuring the sub-G1 cell population (), whereas transfection with the negative control siRNA showed no effects in the earlier events. These results suggest that ArBu could activate ESCC cell apoptosis through activation of p53-dependent pathway.
Figure 4 Activation of p53 by ArBu.
Abbreviations: ArBu, arenobufagin; DAPI, 4′,6-diamidino-2-phenylindole.
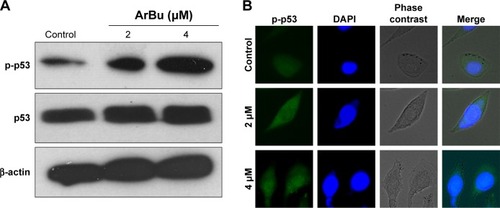
Figure 5 Downregulation of p53 by siRNA transfection (A) and the effects on cell apoptosis induced by ArBu (4 μM, 24 hours) as examined by PI flow cytometric analysis (B).
Abbreviations: siRNA, small interfering RNA; ArBu, arenobufagin; PI, propidium iodide.
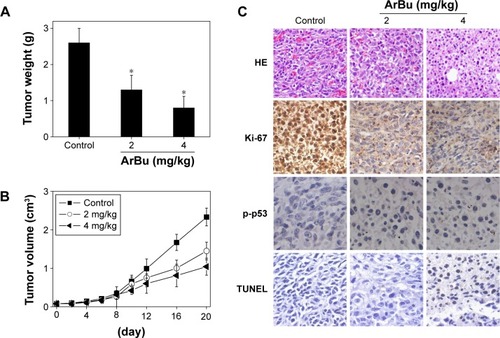
In vivo anticancer activities of ArBu with involvement of p53 phosphorylation
Furthermore, the in vivo anti-ESCC activity of ArBu was evaluated in Eca-109 xenografts in a nude mice model. After being given ArBu intraperitoneally (2 mg/kg and 4 mg/kg body weight every other day) for 20 days, the nude mice were sacrificed and the tumors were collected for further analysis. The results showed that the tumor weight in nude mice was significantly reduced from 2.3 g to 1.4 g and 1 g, respectively. The tumor volume was dramatically decreased to 68% and 46% of control group (). Moreover, the body weight of the mice that had received ArBu treatment maintained constant throughout the treatment process. These results demonstrate the novel in vivo anticancer efficacy of ArBu against ESCC. Besides, immunostaining of histological sections was also conducted to examine the in vivo anticancer mechanisms. As shown in , significant decrease in blood vessel and cancer cell density was observed in tumors treated with 2 mg/kg and 4 mg/kg ArBu. The expression of Ki-67, a biomarker of proliferation, was significantly inhibited by ArBu. Moreover, the expression of phosphorylated p53 was also greatly enhanced after treatment. Furthermore, we have also used TUNEL analysis to confirm the induction of cell apoptosis in the tumor in vivo by ArBu. Therefore, these in vivo data support that ArBu could inhibit tumor growth through activation of p53-mediated cell apoptosis.
Figure 6 In vivo antitumor efficacy and action mechanisms of ArBu.
Abbreviations: ArBu, arenobufagin; HE, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Conclusion
In this study, the in vitro and in vivo anticancer activities of two bufadienolides (including ArBu and Bu) were examined, and the action mechanisms were also elucidated. The results demonstrated that ArBu and Bu could effectively inhibit the cell growth of a panel of five ESCC cells through induction of cell apoptosis through activation of p53 signaling pathway. Besides, ArBu also demonstrated novel in vivo anticancer efficacy in nude mice model, where ArBu remarkably suppressed the tumor growth through activation of p53 pathway. Considering all, these results illustrate the therapeutic potential of bufadienolides against ESCC by regulating p53 pathway.
Acknowledgments
This work was completed with the support of The Construction of Scientific Medicine Research Project of Guangdong Province (2013 20131105).
Disclosure
The authors report no conflicts of interest in this work.
References
- LuCXNanKJLeiYAgents from amphibians with anticancer propertiesAnticancer Drugs2008191093193918827558
- OelkrugCHartkeMSchubertAMode of action of anticancer peptides (ACPs) from amphibian originAnticancer Res201535263564325667440
- QiFHLiAYLvHApoptosis-inducing effect of cinobufacini, Bufo bufo gargarizans Cantor skin extract, on human hepatoma cell line BEL-7402Drug Discov Ther20082633934322504743
- LiuCCaoWChenYQuDZhouJComparison of toad skins Bufo bufo gargarizans Cantor from different regions for their active constituents content and cytotoxic activity on lung carcinoma cell linesPharmacogn Mag2014103920721225210305
- HuangHYangYLvCPharmacokinetics and tissue distribution of five bufadienolides from the Shexiang Baoxin Pill following oral administration to miceJ Ethnopharmacol201516117518525196822
- WangZWenJZhangJYeMGuoDSimultaneous determination of four bufadienolides in human liver by high-performance liquid chromatographyBiomed Chromatogr200418531832215236440
- WenLHuangYXieXAnti-inflammatory and antinociceptive activities of bufalin in rodentsMediators Inflamm2014201417183924719521
- ZhaiXFFangFFLiuQMengYBGuoYYChenZMiR-181a contributes to bufalin-induced apoptosis in PC-3 prostate cancer cellsBMC Complement Altern Med20131332524267199
- QiFInagakiYGaoBBufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathwaysCancer Sci2011102595195821288283
- ChenYYLuHFHsuSCBufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-kB and matrix metalloproteinase-2/-9-signaling pathwaysEnviron Toxicol2015301748223949904
- WangWShiAFanZApoptosis of T-47D cells induced by cinobufacini via a caspase-3-dependent mannerChem Res Chin Univ2014301108
- LiMYuXGuoHBufalin exerts antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cellsTumour Biol20143532461247124218335
- ZhangDMLiuJSDengLJArenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathwayCarcinogenesis20133461331134223393227
- XuGXWangTTApoptosis of lens epithelial cells induced by cinobufagin in vitroInt J Ophthalmol20103212813122553535
- LiBJTianHYZhangDMBufadienolides with cytotoxic activity from the skins of Bufo bufo gargarizansFitoterapia201510571526022446
- LiMWuSLiuZArenobufagin, a bufadienolide compound from toad venom, inhibits VEGF-mediated angiogenesis through suppression of VEGFR-2 signaling pathwayBiochem Pharmacol20128391251126022305746
- LuoHWangFBaiYChenTZhengWSelenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrestColloids Surf B Biointerfaces20129430430822377217
- LiuSCaoWYuLZinc(II) complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers that trigger DNA damage-mediated p53 phosphorylation in cancer cellsDalton Trans201342165932594023463103
- LiuWLiXLWongYSSelenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergismACS Nano2012686578659122823110
- LiXLChenTWongYSInvolvement of mitochondrial dysfunction in human islet amyloid polypeptide-induced apoptosis in INS-1E pancreatic beta cells: an effect attenuated by phycocyaninInt J Biochem Cell Biol201143452553421163363
- ZhangYLiXHuangZZhengWFanCChenTEnhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decorationNanomedicine201391748422542821
- MaieseKChongZZShangYCWangSTargeting disease through novel pathways of apoptosis and autophagyExpert Opin Ther Targets201216121203121422924465
- ChenTWongYSSelenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylationCell Mol Life Sci200865172763277518661100
- ChenTWongYSSelenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generationInt J Biochem Cell Biol200941366667618718551
- RiedlSJShiYMolecular mechanisms of caspase regulation during apoptosisNat Rev Mol Cell Biol200451189790715520809
- FridmanJSLoweSWControl of apoptosis by p53Oncogene200322569030904014663481