Abstract
Background
The purpose of this study was to retrospectively evaluate the therapeutic efficacy and safety of ultrasound-guided percutaneous microwave ablation (MWA) combined with synchronous transcatheter arterial chemoembolization (TACE) in patients with colorectal liver metastases (CRLM).
Patients and methods
A retrospective analysis was performed in 30 patients who were treated with ultrasound-guided percutaneous MWA combined with synchronous TACE for colorectal cancer liver metastases from November 2011 to December 2014 in Zhongshan Hospital, Fudan University. The response of the tumor to treatment was evaluated by follow-up computed tomography and/or magnetic resonance imaging. Local tumor control, procedure-related complications, and long-term survival data were analyzed.
Results
A total of 30 patients with 43 tumors ranging in size from 1.4 cm to 10.0 cm were analyzed. The patients’ mean age was 61.6±10.3 years (range, 44.0–78.0 years). The median follow-up time was 26.5±10.4 months (range, 13.3–50.6 months). The complete ablation rate was 81.4% (35/43 lesions) for CRLM. Complete response was achieved in eight cases (26.7%), and partial response was achieved in 17 cases (56.7%) 1 month after the procedure. The objective response rate (complete response + partial response) was 83.4%. Progression-free survival and overall survival were 5.0 months and 11.0 months, respectively. The 12-month and 24-month survival rates were 46.7% and 25.4%, respectively. A total of 22 patients succumbed during follow-up due to tumor progression. No major complications or perioperative mortalities were recorded.
Conclusion
Ultrasound-guided percutaneous MWA combined with synchronous TACE therapy is a safe and effective modality for patients with CRLM.
Introduction
Colorectal cancer is the third leading cause of cancer death in the world.Citation1 Approximately 50% of colorectal cancer patients will eventually develop metastatic disease in the liver during their natural course.Citation1,Citation2 Surgical resection is the only curative therapy for these patients. The reported 5-year survival rate after resection iŝ40%.Citation3 Unfortunately, only 10%–15% of patients with colorectal liver metastases (CRLM) are suitable for curative resection at initial diagnosis.Citation4,Citation5 The management of patients with untreated CRLM remains a common clinical challenge, as previous studies have reported a median survival time of 6.9 months.Citation6
In addition to systemic chemotherapy, alternative regional treatment strategies have been investigated, aiming to terminate the growth of the metastases and extend survival in patients who are not the candidates for resection.Citation7 Transcatheter arterial chemoembolization (TACE) is an available adjuvant therapy for unresectable CRLM.Citation8 Chemotherapeutic drugs combined with embolization agents deliver high doses of chemotherapy directly to the liver and result in selective ischemia on liver metastases. Meanwhile, local thermal ablation, including microwave ablation (MWA) and radio-frequency ablation (RFA), has been proven to be a safe and effective alternative treatment for nonsurgical candidates with liver metastases.Citation9 Compared to RFA, MWA has the advantages of a shorter procedure time, wider ablation area, higher ablation rate, and lower heat sink effect.Citation10 Studies have demonstrated that MWA combined with TACE may provide better tumor response and overall survival rates in patients with small hepatocellular carcinoma, without severe complications.Citation11 However, few clinical studies have focused on the feasibility, efficiency, and safety of CRLM under MWA combined with synchronous TACE. Therefore, we retrospectively reviewed our experience using MWA combined with synchronous TACE in the management of CRLM patients.
Patients and methods
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. All methods and procedures used were in accordance with the Declaration of Helsinki. Patients who visited the Zhongshan Hospital from November 2011 to December 2014 were recruited if they provided written informed consent and met the following criteria: complete resection of the primary tumor, Eastern Cooperative Oncology Group performance status <2, adequate liver function (serum alanine transaminase <60 U/L, serum aspartate transaminase <60 U/L, and total bilirubin concentrations <25.6 mmol/L), adequate renal function (creatinine level <2.0 mg/dL), and normal coagulative tests (platelet count >30×109/L and prothrombin activity >50%). Patients were deemed unresectable based on preoperative studies revealing bilobar diseases or metastases that were viewed as technically nonresectable by the local liver surgeon and radiologist on the basis of inadequate future liver remnant or because of patient choice. There were 18 patients with limited extrahepatic metastases, which affected the lungs in eight patients, lymph nodes in seven patients, and peritoneum in three patients. Patients with portal thrombosis or cardiovascular failure or respiratory failure were not included in this study.
MWA–TACE protocol
First, routine hepatic and superior mesenteric angiographies were obtained to evaluate the number, size, location, and arterial blood supply of the tumors. Next, ultrasound-guided MWA was performed by using an ECO-100C water-cooled microwave apparatus (ECO Medical Equipment Co., Ltd, Jiangsu, People’s Republic of China), with a frequency of 2,450 MHz and maximum power of 100 W. The whole process was guided and monitored in real time by ultrasound imaging. Multiple overlapping ablations were performed for tumors ≥3 cm in diameter, while single ablation was elected for lesions <3 cm. The electrode was inserted into the neoplasm to reach the farthest edge of the lesion to achieve an opposite margin of at least 1 cm. The microwave power was set at 60–100 W, and the procedure was administered for 10–20 minutes. After the first ablation, the electrode was gradually withdrawn by ~2 cm and the microwave emission was continued. The earlier procedure was repeated until the hyperechoic region overlapped with the area of the tumor. At the end of the procedure, the needle track was coagulated to prevent bleeding or surgery-induced metastasis. Multiple tumors were treated separately with the earlier procedure. Arteriography was performed again to evaluate the ablative results, residual tumor vascularity, contrast agent extravasation (which indicates hemorrhage), and portal venous system patency. Chemoembolization was administered via the targeted artery using a mixture of chemotherapeutic agents, which consisted of 50–150 mg oxaliplatin, 10–50 mg epirubicin, and 1.5–10 mL ethiodized oil. Additional gelatin sponge particles were used for insufficient embolization cases. If angiography showed hemorrhage or an arteriovenous fistula, the targeted artery was embolized with sponge particles or microcoils.
Postoperative care and follow-up
After the procedure, reduced glutathione injection (Chongqing Yaoyou Pharmaceutical Co., Ltd, Chongqin, People’s Republic of China) and ceftriaxone (Hoffmann-La Roche Ltd., Basel, Switzerland) were administered intravenously to all patients for 3 days. An omeprazole sodium injection (Changzhou Siyao Pharmaceutical Co., Ltd, Jiangsu, People’s Republic of China) and a sodium bicarbonate injection (ROMIT Pharmaceutical Corporation Jiangsu, Jiangsu, People’s Republic of China) were each administered once. Antiemetic therapy and analgesics were prescribed if necessary. After 3–7 days of the procedure, liver and kidney function, routine blood examination, and noncontrast computed tomography (CT) were reexamined to assess complications and the status of the ablation lesions. The total tumor load in relation to liver volume was estimated in three categories: ≤25%, 26%–50%, or >50%. Complications were defined as major or minor according to the clinical guidelines of the Society of Interventional Radiology. Local therapeutic efficacy was evaluated by contrast-enhanced CT and/or magnetic resonance imaging 1 month after the procedure and every 6–7 weeks thereafter. According to the modified Response Evaluation Criteria in Solid Tumors (m-RECIST),Citation12 the local tumor response was evaluated as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Local tumor progression was defined as increased size of a target lesion and/or the appearance of an enhanced area adjacent to the tumor zone on follow-up imaging >1 month after treatment. Intrahepatic recurrence was defined as the detection of new lesions. Follow-up laboratory tests, including liver and kidney function tests, tumor markers, and complete blood counts, were routinely performed at 1-month intervals. Patients with relapsing or progressive tumors were treated with appropriate protocols.
Statistical analyses
Qualitative data are expressed as frequency and rate. Continuous ordinal data are expressed as mean ± standard deviation. The estimated progression-free survival and overall survival rates were evaluated using the Kaplan–Meier method. Student’s t-test was used to compare quantitative variables. All two-sided P-values <0.05 were considered to be statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) program (Version 22.0; IBM Corporation, Armonk, NY, USA).
Results
General condition
Thirty consecutive patients (19 men and eleven women, mean age 61.6±10.3 years) who had undergone operative management at our institution were reviewed. Their general characteristics and demographic features are listed in . A total of 20 (66.7%) patients presented with colon cancer and ten (33.3%) with rectal cancer. Surgical resection of liver metastases, systemic chemotherapy, and TACE had been performed before the combined therapy in two (6.7%), 14 (46.7%), and seven (23.3%) of the patients, respectively. Three patients had both hepatectomy and systemic chemotherapy before the combined therapy. The main variable tumor marker was carcinoembryonic antigen, which was positive in 23 (76.7%) patients. The Child-Pugh class was A in eleven (36.7%) patients and B in 19 (63.3%) patients; there were no class C patients. The mean tumor number was 1.4±0.7 (range, 1–3) per patient. Twenty patients were treated for a single tumor, and ten patients were treated for more than one tumor, with 43 tumors in total. The mean tumor size was 4.4±2.6 cm (range, 1.4–10.0 cm) in diameter.
Table 1 General condition of CRLM patients
Local tumor response and survival
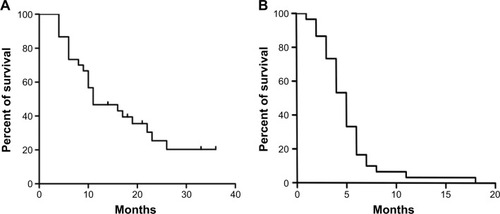
Complete ablation was achieved in 35 of 43 (81.4%) nodules (). Local recurrence occurred after treatment of 19 tumors in 17 patients. Tumor load in relation to liver volume was ≤25%, 26%–50%, and >50% in 12, nine, and nine patients, respectively. Median overall survival of patients with tumor load of ≤25%, 26%–50%, and >50% of liver volume was 33.1 months, 14.7 months, and 6.1 months, respectively. According to the m-RECIST criteria, CR and PR were observed in eight (26.7%) and 17 (56.7%) patients, respectively. The objective response rate (CR + PR) was 83.4%. SD and PD were observed in two and three patients, respectively. Twenty-two patients died during follow-up of 26.5±10.4 months (range, 13.3–50.6 months). The cause of death was tumor progression in 14 patients. Eight patients died from hepatic failure. Median progression-free survival and overall survival following the MWA–TACE procedure for the entire cohort were 5.0 months and 11.0 months, respectively (). The 12-month and 24-month overall survival rates after the initial ablation were 46.7% and 25.4%, respectively ().
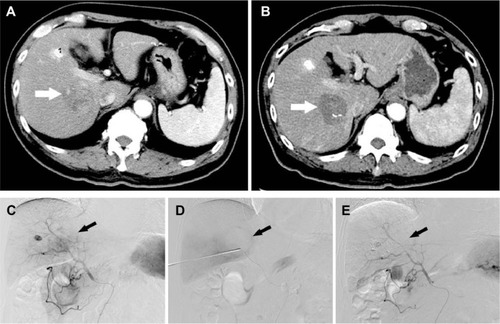
Figure 1 A 75-year-old man who presented with CRLM treated with MWA combined with synchronous TACE.
Abbreviations: CRLM, colorectal liver metastases; CT, computed tomography; DSA, digital subtraction angiography; MWA, microwave ablation; TACE, transcatheter arterial chemoembolization.
Complications
There was no treatment-related mortality. Following the MWA–TACE procedure, adverse reactions were mainly recorded as low-grade fever (n=26, 86.7%) and regional pain (n=25, 83.3%). Grade I–II elevations occurred in total bilirubin levels in seven patients (23.3%) and in serum transaminase levels in 22 patients (73.3%), which usually resolved within 7 days. Asymptomatic pleural effusion occurred in four patients (13.3%). Liver function test and complete blood counts in patients are listed in . No patient suffered intraperitoneal hemorrhage, hepatic abscess, peritonitis, or bile leakage due to the treatment.
Table 2 Comparison of laboratory test preprocedure and postprocedure after 1 month
Discussion
Currently, the optimal method used to treat CRLM is surgical resection. Unfortunately, due to poor baseline liver function, tumor burden, or hepatic vessel invasion of patients, it is rarely possible to perform surgical resection. During the past decade, imaging-guided minimally invasive therapies for liver cancer have been developed, such as TACE, RFA, and MWA. Thermal ablation therapy has become one of the most promising therapies for curing CRLM; furthermore, RFA, which is a safe, well-tolerated, highly repeatable, and less invasive approach, has been the most widely used version of the technique.Citation13–Citation15 Additionally, RFA is able to excise a single CRLM with a diameter <3 cm and has an effect equal to hepatectomy.Citation16 Moreover, RFA can treat CRLM better than direct excision when small tumors that hide in the depths of the liver are difficult to reach.Citation17 However, reliance on passive heating, increased impedance from tissue desiccation as temperatures reach 100°C, diminished effectiveness due to charring, and the heat sink effect all limit the effectiveness of RFA and will permit incomplete tumor destruction, which is associated with the risk of intrahepatic disease recurrence.Citation18,Citation19
Unlike RFA, the microwave in MWA produces active heating of water molecules via an electromagnetic field; therefore, the procedure is less affected by tissue desiccation and the heat sink effect. This creates larger, more homogeneous, and reliable tissue ablation volumes with complete tumor necrosis, resulting in less local recurrence in cases of large tumors.Citation20–Citation22 MWA can be used with multiple antennae, which can also provide a wider ablation zone range, and be performed more quickly.Citation21,Citation23 MWA remains effective in temperatures >100°C and does not require placement of grounding pads, which can result in skin burns. Research shows that thermal ablation therapy can improve the antineoplastic behavior of the immune system. After heat treatment, the tumor can synthesize HSP70, a heat shock protein, which can enhance tumor-specific antigen presentation and stimulate the body’s immune system.Citation24
Although thermal ablation is considered to be a curative option, its efficacy is severely limited by lesion size. It is difficult for RFA or MWA to achieve complete ablation in the treatment of medium- to large-sized hepatic tumors.
TACE, which is widely used for unresectable liver cancer due to its precision targeting and usually good results in tumor response, is not limited by lesion size.Citation19 However, the role of TACE for colorectal carcinoma is not as well documented in the literature as thermal ablation. TACE is most often used as a “salvage” procedure when tumor progression occurs after systemic chemotherapy.Citation25 TACE alone cannot achieve complete tumor necrosis in a single treatment due to incomplete embolization, which allows new tumor angiogenesis. Multiple TACE treatments can damage normal liver tissue, impair liver function, and even induce liver failure.Citation26 Therefore, both TACE and thermal ablation have their own limitations, and their long-term effects are not ideal when they are used as separate, single therapies.
The combination of TACE and thermal ablation can improve on the individual treatments and enhance their curative effect. The synergistic effect of combined TACE and thermal ablation, which may achieve an advantageous balance in successful tumor eradication and maximal preservation of liver function, has been well described.Citation27,Citation28
The advantages of combining TACE and thermal ablation may be explained through the following aspects: TACE can enlarge the thermal coagulation area of MWA or RFA by attenuating the cooling effect of the hepatic bloodstream, effectively reducing blood supply to the lesion.Citation2,Citation29,Citation30 Meanwhile, thermal ablation can sensitize tumor cells to chemotherapy; thus, TACE treatment times and the chemotherapy dose can both be effectively reduced, decreasing adverse reactions in the liver and improving patient prognosis.Citation31,Citation32 Comprehensive treatment can also reduce the number of TACE treatments while delivering an equivalent effect. In our research, the average dose of ethiodized oil was only 1.5–10 mL, compared to 5–20 mL described in the literature for TACE alone.Citation32
For larger tumors, although multiple thermal ablations can be applied, incomplete necrosis is a common outcome. TACE therapy can improve thermal ablation on minimizing tumor residue for single large tumors. For multiple carcinomas, TACE is also able to detect some tiny tumors earlier, while they are still invisible on routine contrast-enhanced CT or magnetic resonance imaging, and deal with them effectively.Citation33 TACE can identify and provide timely treatment to some patients with MWA-related comorbidities, such as hemorrhage or arteriovenous shunt.
Previous studies demonstrated that MWA combined with TACE therapy for hepatocellular carcinoma resulted in a high complete response rate.Citation14,Citation31,Citation33 We theorized that tumor destruction and overall survival would be improved with this comprehensive treatment, which has been used with success for CRLM. In our study, the 12-month and 24-month overall survival rates were 46.7% and 25.4%, respectively. The median overall survival was 11.0 months. However, in a retrospective study, Albert et alCitation25 recently reviewed outcomes in 121 patients who were treated with TACE alone for colorectal carcinoma, and the median overall survival was 9 months. In another study, Huppert et alCitation8 found a median survival of 8 months in 27 CRLM patients receiving TACE. Both of these results compared poorly with the median survival of 11.0 months seen in our study group of similar patients.
Tumor response is an important issue for all patients with cancer because of increasing patient survival and reducing the possibility of tumor recurrence. In this study, the objective response rate (CR + PR) was 83.4%. However, Aliberti et alCitation34 found an objective response rate of 70% in 10 CRLM patients receiving TACE after 1 month in their study.
In our study, median overall survival was not significantly different between patients with liver metastases only and those with extrahepatic metastases. Survival was significantly associated with tumor extent. In patients with ≤25% tumor load, overall survival was 33.1 months. However, it was only 6.1 months in patients with >50% tumor load. Thirteen patients died within 10 months. Most of these patients were found with >50% tumor load and an Eastern Cooperative Oncology Group performance status of two. Considering their salvage situation, these early deaths should not undermine the safety of the combined therapy. However, the combined therapy has to be investigated further to confirm its efficacy.
There were no major complications or treatment-related mortality in our study. Minor complications included low-grade local pain and fever, which were typically transient and relieved by the usual treatment. Inflammatory reactions, hepatic insufficiency, and renal insufficiency may have been caused by absorption of intrahepatic tumor necrosis; recovery was expected to occur within 2–7 days. These results indicated that MWA combined with synchronous TACE was a safe and well-tolerated procedure for CRLM patients.
The limitations of this study include its retrospective nonrandomized design, relatively insufficient cohort of patients, and nonuniform systemic chemotherapy regimens. In addition, our limited duration of patient follow-up prevented analysis of more long-term outcomes. Therefore, it is difficult to draw any real conclusion about the survival benefit when compared with ablation and resection alone. Therefore, prospective randomized controlled studies covering a large sample size and long follow-up period are required to confirm the efficacy of the combination treatment for patients with CRLM.
Conclusion
The data of our study indicated that ultrasound-guided percutaneous MWA combined with synchronous TACE therapy is a safe and effective method of achieving disease control for CRLM.
Acknowledgments
This study was supported by the National Science Fund for Young Scholars (grant number 81201170), the Natural Science Foundation of Shanghai City (grant number 15ZR1406700), the Science Project of Health and Family Planning Commission of Shanghai City (grant number 201440540), and the National Natural Sciences Foundation of China (grant number 81171432). The authors thank Zhi-Ping Yan (Department of Interventional Radiology, Zhongshan Hospital, Fudan University) for providing general support as well as Liang Zhu (Department of Interventional Radiology, Zhongshan Hospital, Fudan University), Guo-Wei Yang (Department of Interventional Radiology, Zhongshan Hospital, Fudan University), and Qing Zhao (Department of Interventional Radiology, Zhongshan Hospital, Fudan University) for providing some study patients for this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- AdamRDe GramontAFiguerasJJean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) groupThe oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensusOncologist201217101225123922962059
- FongZVPalazzoFNeedlemanLCombined hepatic arterial embolization and hepatic ablation for unresectable colorectal metastases to the liverAm Surg201278111243124823089443
- WongSLManguPBChotiMAAmerican Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancerJ Clin Oncol201028349350819841322
- WagnerJSAdsonMAVan HeerdenJAAdsonMHIlstrupDMThe natural history of hepatic metastases from colorectal cancer. A comparison with resective treatmentAnn Surg198419955025086721600
- JemalASiegelRWardEMurrayTXuJThunMJCancer statistics, 2007CA Cancer J Clin2007571436617237035
- ArruMAldrighettiLCastoldiRAnalysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancerWorld J Surg20083219310318027020
- ChangAESchneiderPDSugarbakerPHSimpsonCCulnaneMSteinbergSMA prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastasesAnn Surg198720666856932961314
- HuppertPWenzelTWietholtzHTranscatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient populationCardiovasc Intervent Radiol201437115416423670568
- LiuYLiSWanXEfficacy and safety of thermal ablation in patients with liver metastasesEur J Gastroenterol Hepatol201325444244623470267
- BoutrosCSomasundarPGarreanSSaiedAEspatNJMicrowave coagulation therapy for hepatic tumors: review of the literature and critical analysisSurg Oncol2010191e22e3219268571
- YangWZJiangNHuangNHuangJYZhengQBShenQCombined therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation for small hepatocellular carcinomaWorld J Gastroenterol200915674875219222102
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis2010301526020175033
- MayoSCPawlikTMThermal ablative therapies for secondary hepatic malignanciesCancer J201016211111720404607
- WuYZLiBWangTWangSJZhouYMRadiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysisWorld J Gastroenterol201117364143414822039331
- GillamsARLeesWRRadiofrequency ablation of colorectal liver metastasesAbdom Imaging200530441942615759208
- WangJLiangPYuJClinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastasesOncol Lett20148132332624959270
- PathakSJonesRTangJMAblative therapies for colorectal liver metastases: a systematic reviewColorectal Dis2011139e252e26521689362
- MartinRC2ndJaquesDPBrennanMFKarpehMAchieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection?J Am Coll Surg2002194556857712025834
- ZhouBFineJLatoucheALabopinMCompeting risks regression for clustered dataBiostatistics201213337138322045910
- YuNCRamanSSKimYJLassmanCChangXLuDSMicrowave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine modelJ Vasc Interv Radiol20081971087109218589324
- WrightASSampsonLAWarnerTFMahviDMLeeFTJrRadiofrequency versus microwave ablation in a hepatic porcine modelRadiology2005236113213915987969
- SimonCJDupuyDEMayo-SmithWWMicrowave ablation: principles and applicationsRadiographics200525Suppl 1S69S8316227498
- DupuyDEMicrowave ablation compared with radiofrequency ablation in lung tissue-is microwave not just for popcorn anymore?Radiology2009251361761819474368
- NobuokaDMotomuraYShirakawaHRadiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytesInt J Oncol2012401637021922136
- AlbertMKieferMVSunWChemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcoholCancer2011117234335220830766
- ChoiSBKimKSParkYNThe efficacy of hepatic resection after neoadjuvant transarterial chemoembolization (TACE) and radiation therapy in hepatocellular carcinoma greater than 5 cm in sizeJ Korean Med Sci200924224224719399265
- TakayasuKTransarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspectiveJpn J Clin Oncol201242424725522407946
- GeorgiadesCSHongKGeschwindJFRadiofrequency ablation and chemoembolization for hepatocellular carcinomaCancer J200814211712218391617
- LiuCLiangPLiuFMWA combined with TACE as a combined therapy for unresectable large-sized hepatocellular carcinomaInt J Hyperthermia201127765466221966941
- XuLFSunHLChenYTLarge primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapyJ Gastroenterol Hepatol201328345646323216261
- LiuHCShanEBZhouLCombination of percutaneous radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: observation of clinical effectsChin J Cancer Res201426447147725232222
- YangGWZhaoQQianSPercutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinomaOnco Targets Ther201581245125026060410
- LiSZhangLHuangZMWuPHTranscatheter arterial chemoembolization combined with CT-guided percutaneous thermal ablation versus hepatectomy in the treatment of hepatocellular carcinomaChin J Cancer201534625426326063407
- AlibertiCTilliMBeneaGFiorentiniGTrans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary resultsAnticancer Res2006263793379517094403