Abstract
Background
Proteins in the cofilin pathway regulate actin dynamics and may be involved in cancer cell migration and invasion. However, there are no direct data that suggest that dephosphorylated cofilin can affect breast cancer prognosis.
Methods
We assessed the expressions of cofilin and phosphorylated cofilin (P-cofilin) in breast cancer tissue microarrays (290 patients, mean follow-up: 95.7±2.49 months) to evaluate dephosphorylated cofilin and its relationship with breast cancer prognosis. The associations of pathological characteristics with cumulative survival were evaluated using Kaplan–Meier analysis.
Results
Univariate analyses revealed that overall survival was associated with cofilin levels, N category, TNM stage, estrogen receptor status, progesterone receptor status, and molecular subtypes. Cofilin status and TNM stage independently affected overall survival, although P-cofilin expression was not associated with patient survival. In the P-cofilin-negative subgroup, cofilin expression was significantly associated with patient survival, although cofilin expression was not significantly associated with patient survival in the P-cofilin-positive subgroup. We further analyzed the P-cofilin-negative cases and found that Ki-67 expression was significantly elevated in the subgroup that was strongly positive for cofilin (P=0.002).
Conclusion
Among P-cofilin-negative patients with breast cancer, cofilin expression defines a population of patients with lower overall survival, which suggests that dephosphorylated cofilin expression might predict the prognosis in cases of P-cofilin-negative breast cancer. Furthermore, our results suggest that inhibitors of dephosphorylated cofilin expression may provide therapeutic benefits in patients with breast cancer.
Introduction
Breast cancer is the most common carcinoma among women and the second most common cause of cancer-related death among women. Unfortunately, the incidence of breast cancer has been increasing during recent decades.Citation1–Citation3 Nevertheless, considerable progress in breast cancer treatments (eg, surgery, chemotherapy, radiation, and hormone and targeted therapies) has recently led to decrease in breast cancer-related mortality rates.Citation4 However, not all cases benefit from these treatments. Therefore, it would be useful to develop additional biomarkers to predict the prognosis of patients with breast cancer.
Cancer cell migration and invasion lead to cancer metastasis, which accounts for a majority of cancer-related deaths. The increased motility of cancer cells helps drive migration and invasion and is an essential step in breast cancer metastasis.Citation5,Citation6 Therefore, targeting tumor cell motility is a potential antitumor strategy.Citation7 As proteins in the cofilin pathway are key regulators of actin dynamics at the leading edge of motile cells, these proteins are likely to be involved in cancer cell migration and invasion. Recent data also indicate that components of the cofilin pathway are frequently misregulated in cancer cells, although there are conflicting reports regarding how cofilin and the upstream regulators contribute to the malignant phenotype (via overexpression or suppression). These data suggest that the balance between the cofilin pathway regulators and the “output” of the cofilin pathway (cofilin activity, most contributed by dephosphorylated cofilin) determines the invasiveness of tumor cells.Citation8 However, there is currently no direct evidence that suggests that dephosphorylated cofilin expression can affect breast cancer prognosis. Our previous data revealed that cofilin expression was associated with poor outcomes, although cofilin expression itself is not a direct indicator of dephosphorylated cofilin expression. Therefore, this study used immunohistochemistry (IHC) to analyze the expressions of cofilin and phosphorylated cofilin (P-cofilin) in tissue microarrays of tumors from 290 patients with breast cancer (mean follow-up: 95.7±2.49 months). Our data provide insight regarding the role of cofilin levels in invasive breast cancer and highlight the correlations between cofilin levels and various clinical and pathological parameters.
Materials and methods
Tissue microarrays and immunostaining
We obtained breast cancer tissues from 300 patients who were diagnosed with primary breast cancer at the Institute of the National Engineering Center for BioChips in Shanghai, People’s Republic of China. This study and the study design were approved by the Human Research Ethics Committee of Taizhou Hospital (Zhejiang Province), and written informed consent to be included in the study was obtained from each patient before their original examination. All tissues had originally been fixed using formalin and embedded in paraffin. Due to lack of tumor tissues in some certain individual punches, we finally evaluated 290 cases of breast cancer solid tumors successfully. Clinicopathological data were obtained from the records and pathology reports of the Breast Cancer Surgery Department.
The expressions of cofilin, P-cofilin, estrogen receptor (ER), progesterone receptor (PR), Ki-67, and human epidermal growth factor receptor 2 (HER-2) were determined in the arrays and normal samples using IHC. To evaluate cofilin expression, we used a cofilin-specific antibody from Abcam (ab42824; Cambridge, UK) at a dilution of 1:1,500, based on the manufacturer’s protocol. To evaluate P-cofilin expression, we used a P-cofilin-specific antibody (CST, #3313) at a dilution of 1:100. We defined ER and PR negativity according to the current Swedish clinical guidelines (<5% positive nuclei). Ki-67 expression was defined as positive (>14% immunostained nuclei) or negative (≤14% immunostained nuclei). The patient’s HER-2 status was semiquantitatively assessed using a standard protocol (HercepTest, DakoCytomation Denmark A/S, Glostrup, Denmark), and fluorescence in situ hybridization (FISH) analysis was performed for HER-2-positive samples. HER-2 expression was defined as weak (IHC grade 0–1+ or FISH-) or strong (IHC grade 3+ or FISH+). The patient’s metastasis status was expressed using the American Joint Committee on Cancer TNM staging system.
Scoring for cofilin and P-cofilin
All IHC staining results were evaluated by two experienced pathologists, who were blinded to the patient’s clinical information. Cofilin staining intensity in the cytoplasm of tumor cells was graded using a scale of 0–3, and the percentage of cofilin-positive cells was graded using a scale of 0–4 (0: 0%–5%, 1: 6%–25%, 2: 26%–50%, 3: 51%–75%, and 4: 76%–100%). The final cofilin expression score ranged from 0 to 3 and was based on the sum of the intensity and percent-positive scores (0: total score 0–1, 1: total score 2–3, 2: total score 4–5, and 3: total score 6–7). For the Kaplan–Meier analysis, the final cofilin scores were analyzed as either weak (scores of 0–2) or strong (a score of 3) expression. We graded the percentage of P-cofilin-positive cells as 0 or 1 (0: 0%–10%, 1: >10%) and graded the intensity of P-cofilin staining using a scale of 0–3. The final P-cofilin expression (score 0 or 1) was calculated as the sum of the percent-positive and intensity scores (0: total score 0–2, 1: total score 3–4).
Survival and statistical analysis
Overall survival (OS) was defined as the time from the first operation to death because of any cause, and survivors were censored at the date of last contact. The chi-square test, Student’s t-test, and Pearson’s correlation test were used to analyze the patient and clinical characteristics that were associated with the expressions of cofilin and P-cofilin. The Kaplan–Meier method and log-rank test were used to analyze the correlation between OS and dephosphorylated cofilin expression. All statistical analyses were performed using SPSS software (Version 13.0; SPSS Inc., Chicago, IL, USA); all tests were two-sided, and a P-value of <0.05 was considered statistically significant.
Results
Pathological information from the invasive ductal cancer tissue microarrays
The tissue microarrays and examples of positive and negative P-cofilin expression are shown in . P-cofilin expression was not significantly associated with patient survival in the univariate analysis (P=0.232) (). When we performed subgroup analyses according to cofilin expression status, we found that patient survival was only significantly associated with negative P-cofilin expression (P=0.002) (). Positive P-cofilin expression was not significantly associated with patient survival (P=0.355) (; –).
Figure 1 Tissue microarray and immunohistochemistry staining of P-cofilin in invasive ductal breast cancer tumors.
Abbreviation: P-cofilin, phosphorylated cofilin.
Figure 2 Association of P-cofilin with overall survival among patients with invasive ductal breast cancer.
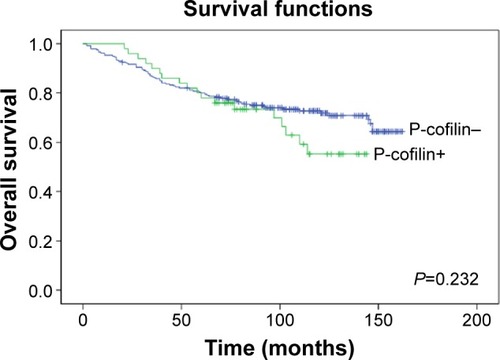
Figure 3 Association of cofilin with overall survival among P-cofilin-negative patients with invasive ductal breast cancer.
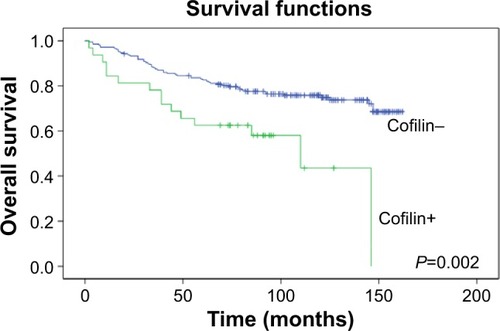
Figure 4 Association of cofilin with overall survival among P-cofilin-positive patients with invasive ductal breast cancer.
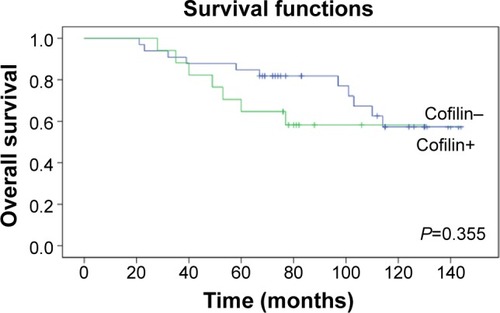
Table 1 Cofilin with or without phosphorylation for predicting overall survival in invasive ductal breast cancer
We excluded ten patients who had missing information and 50 patients with positive P-cofilin expression. Thus, 240 patients with invasive ductal cancer (IDC) were included in the subsequent analyses, and their clinicopathological characteristics are shown in . Among the 240 patients with IDC, the mean follow-up was 95.7±2.49 months (range: 2–160 months) and the mean age at diagnosis was 56.5 years (range: 29–88 years). Approximately 17.2% of these patients exhibited positive P-cofilin expression, and ~82.8% of these patients exhibited negative P-cofilin expression. We observed T1, T2, and T3/T4 stages in 27.9%, 60.8%, and 11.3% of the P-cofilin-negative patients, respectively. No lymph node metastasis (N0) was observed in 44.2% of the cases. The patients with IDC exhibited a TNM stage of 0/I (13.8%), II (53.8%), and III (32.5%), and positive ER expression (60.8%), positive PR expression (45.8%), positive HER-2 expression (25.0%), positive Ki-67 expression (30.0%), and the luminal A subtype (42.1%). When we excluded the interference from P-cofilin, we found that only Ki-67 expression was significantly higher in the cofilin-positive subgroup (P=0.002) ().
Table 2 Clinicopathological characteristics of breast cancer with different cofilin expression
Associations of P-cofilin, cofilin, and other clinicopathological features with IDC prognosis
The Kaplan–Meier method and log-rank test were used to evaluate the associations of the various factors with OS (; ). The univariate analyses of the patients with IDC revealed that cofilin status, N category, TNM stage, ER status, PR status, and molecular subtypes were associated with OS. Multivariate analyses revealed that cofilin status and TNM stage were independent predictors of OS ().
Table 3 Univariate and multivariate analysis of factors that predict overall survival in invasive ductal breast cancer
Discussion
Actin is the major component of the cytoskeleton, which plays an important role in tumor cell migration, invasion, and mitosis. Cofilin is a ubiquitously expressed actin-binding protein, which is responsible for the formation of the actin cytoskeleton and is indispensable for cell cycle control.Citation8 Furthermore, cofilin is essential for cell cycle progression and cancer cell migration, intravasation, and invasion.Citation9 Cofilin overexpression increases the rate of cell migration in human glioblastoma culturesCitation10 and is correlated with poor prognosis in other human malignancies.Citation11–Citation13 The overexpression of cofilin has also been detected in invasive subpopulations of mammary tumor cells and is directly related to the invasion, intravasation, and metastasis of mammary tumors.Citation9,Citation14
Our previous data revealed that cofilin expression was associated with poor outcomes, although we only measured total levels of cofilin, rather than dephosphorylated cofilin expression. However, recent studies have indicated that the overall activity of the cofilin pathway, and not that of any single gene within the pathway, determines the invasive and metastatic phenotype of tumor cells.Citation15 In this context, the cofilin pathway is composed of various kinases and phosphatases that regulate cofilin, and phosphorylation of serine 3 in cofilin is a critical step for cofilin recycling and actin polymerization.Citation10,Citation16 However, P-cofilin is inactive, and it is assumed that the amount of dephosphorylated cofilin in breast cancer cells is a direct measure of cofilin activity. Therefore, we analyzed the expressions of both cofilin and P-cofilin in the tissue microarrays in order to determine whether cofilin activity (dephosphorylated cofilin expression) was correlated with outcomes in cases of human breast cancer.
In this study, we found that elevated cofilin expression in P-cofilin-negative breast cancer tissues predicted poor clinical and survival outcomes. In this context, cofilin expression directly represents dephosphorylated cofilin expression when P-cofilin is not present. However, in the P-cofilin-positive subgroup, the strong and weak cofilin expressions were not significantly associated with OS. Therefore, the presence of P-cofilin may confound any correlation between dephosphorylated cofilin expression and breast cancer prognosis. These results indicate that dephosphorylated cofilin expression, rather than overall expression, might be a preferred biomarker for predicting prognosis in cases of P-cofilin-negative breast cancer.
Because of its effects on actin polymerization and depolymerization, active cofilin has been linked to mammary tumor invasion, intravasation, and metastasis, as well as lymph node metastasis and a higher nodal stage. Findings from studies of other human malignant tumor types also support these associations. However, in this study, cofilin expression was not correlated with nodal stage or the number of metastatic lymph nodes in the P-cofilin-negative subgroup. As the number of lymph nodes largely depends on the completeness of the axillary lymph node dissection, this approximation may not always be accurate. Moreover, the time interval between tumor diagnosis and surgery may affect the nodal stage, which may not accurately reflect any tendency toward lymphatic metastasis.Citation17,Citation18 Therefore, these factors may explain why dephosphorylated cofilin expression was not correlated with clinical nodal stage in the P-cofilin-negative subgroup. However, most breast cancer-related deaths result from metastasis and/or recurrence, and the correlation between dephosphorylated cofilin expression and poor prognosis may reflect an association with the potential for metastasis.
We also found that Ki-67 expression was significantly higher in the P-cofilin-negative samples that were strongly positive for cofilin. In this context, Ki-67 is a nuclear factor that is expressed in proliferating cells. As actin is the major component of the cytoskeleton, which also plays an important role in tumor cell mitosis, dephosphorylated cofilin expression may be associated with cellular proliferation (as indicated by Ki-67 expression). These results may indicate that dephosphorylated cofilin expression is correlated with Ki-67 expression and that cofilin levels may be a marker for rapid proliferation in patients who had negative P-cofilin expression.
This study included two important limitations. First, we used a retrospective single-center design. Second, we only evaluated associations with OS, as we did not have access to data regarding recurrence-free and disease-free survivals. Therefore, a prospective multicenter study with long-term follow-up is needed to confirm whether dephosphorylated cofilin expression is an independent prognostic biomarker for patients with P-cofilin-negative breast cancer.
Conclusion
Dephosphorylated cofilin expression might be a useful biomarker for predicting prognosis in patients with breast cancer. Furthermore, inhibitors of the cofilin pathway may provide therapeutic benefits in these patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- XiaoJZhouYZhuWAssociation of ultrasonographic features with NGX6 expression and prognosis in invasive ductal breast carcinomaInt J Clin Exp Pathol2015866458646526261522
- ZhangQMaBKangMA retrospective comparative study of clinicopathological features between young and elderly women with breast cancerInt J Clin Exp Med2015845869587526131178
- YangJLiXLiuXLiuYThe role of tumor-associated macrophages in breast carcinoma invasion and metastasisInt J Clin Exp Pathol2015866656666426261547
- RoussosETCondeelisJSPatsialouAChemotaxis in cancerNat Rev Cancer201111857358721779009
- SidaniMWesselsDMouneimneGCofilin determines the migration behavior and turning frequency of metastatic cancer cellsJ Cell Biol2007179477779118025308
- MorettiRMMontagnani MarelliMMaiSLimontaPGonadotropin-releasing hormone agonists suppress melanoma cell motility and invasiveness through the inhibition of alpha3 integrin and MMP-2 expression and activityInt J Oncol200833240541318636163
- WangWEddyRCondeelisJThe cofilin pathway in breast cancer invasion and metastasisNat Rev Cancer20077642944017522712
- WangWMouneimneGSidaniMThe activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumorsJ Cell Biol2006173339540416651380
- YapCTSimpsonTIPrattTPriceDJMaciverSKThe motility of glioblastoma tumour cells is modulated by intracellular cofilin expression in a concentration-dependent mannerCell Motil Cytoskeleton200560315316515662725
- YangZLMiaoXXiongLCFL1 and Arp3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinomas of gallbladderCancer Invest201331213213923320827
- LiDZhangYLiZWangXQuXLiuYActivated Pak4 expression correlates with poor prognosis in human gastric cancer patientsTumour Biol201536129431943626124003
- LuLIFuNILuoXULiXYLiXPOverexpression of cofilin 1 in prostate cancer and the corresponding clinical implicationsOncol Lett2015962757276126137141
- WangWGoswamiSLapidusKIdentification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumorsCancer Res200464238585859415574765
- TsaiCHChiuSJLiuCCRegulated expression of cofilin and the consequent regulation of p27(kip1) are essential for G(1) phase progressionCell Cycle20098152365237419556892
- YinCLiHZhangBRAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transitionBreast Cancer Res Treat2013142229730924177755
- OlivottoIAGomiABancejCInfluence of delay to diagnosis on prognostic indicators of screen-detected breast carcinomaCancer20029482143215012001110
- ChenLJianWLuLZhengLYuZZhouDElevated expression of E-cadherin in primary breast cancer and its corresponding metastatic lymph nodeInt J Clin Exp Med201587117521175826380015
