Abstract
Recently, many studies have shown that pretreatment serum albumin can be closely linked to the prognosis of cancer patients, including those with renal cell carcinoma (RCC). However, not all studies have reached the same conclusion. We therefore conducted a systematic review and meta-analysis to evaluate the prognostic value of pretreatment serum albumin in RCC patients. A total of 17 studies involving 6,447 patients were included in our meta-analysis. Our results indicated that a lower pretreatment serum albumin level yielded a worse overall survival (hazard ratio [HR]=2.46, 95% confidence interval [CI] 1.92–3.13), cancer-specific survival (HR=2.22, 95% CI 1.87–2.64), and relapse-free survival/progression-free survival (HR=1.75, 95% CI 1.28–2.38). Generally, these findings were particularly pronounced when stratified by tumor type, analysis type, cut-off value, and HR-obtaining method. In conclusion, a decreased pretreatment serum albumin level implies a poor prognosis for RCC patients, and can be monitored for risk stratification and individualized treatment in RCC patients.
Introduction
Renal cell carcinoma (RCC) is one of the most fatal types of urologic oncology and accounts for 2%–3% of adult malignancies. According to an American investigation, 58,000 patients are newly diagnosed with RCC, and nearly 13,000 patients die from this disease every year.Citation1 Rapid progress has been made in treatment methods, but some RCC patients with local recurrence and distant metastasis do not survive for long.Citation2 An effective prognostic model could be used to ascertain the malignant degree of the tumor, as well as to carry out risk stratification and allow individualized treatment for cancer patients. The postoperative tumor–node–metastasis (TNM) staging system is the conventional prognostic model used for RCC patients in clinical practice, but its precision may be unsatisfactory, with outcomes of patients at the same stage being significantly different. Moreover, the TNM staging system can only be evaluated in patients who undergo surgery. Therefore, a new laboratory index to complement the current risk stratification system of RCC patients is urgently required for clinical decision-making.
Serum albumin is synthesized by the liver and is the main serum protein.Citation3 The concentration of normal serum albumin ranges from 3.5–5.0 g/dL for adults; when serum albumin is <3.5 g/dL, it is defined as hypoalbuminemia.Citation4 Serum albumin is closely related to the degree of malnutrition, so it is often used for evaluating nutritional status.Citation5,Citation6 Furthermore, many studies have indicated that the inflammatory response also affects the concentration of serum albumin, which can therefore be used as a reliable indicator of inflammation.Citation7,Citation8 Cancer is often accompanied by malnutrition and chronic inflammation, which often develop into tumor cachexia and speed up the deterioration of cancer patients.Citation9 In recent years, numerous studies have indicated that there is a close correlation between pretreatment serum albumin and tumor prognosis; specifically, the lower the concentration of pretreatment serum albumin, the worse the prognosis of cancer patients.Citation10–Citation13 Some of these studies have directly or indirectly elaborated the relationship between pretreatment serum albumin and the prognosis of RCC patients.Citation14–Citation28 However, owing to differences in research design, sampling protocols, and other factors, the prognostic value of pretreatment serum albumin in RCC patients is not consistent. Some investigations have shown that pretreatment serum albumin is related to the prognosis of RCC patients, but others have failed to draw similar conclusions. Therefore, a systematic review and meta-analysis are necessary to assess the prognostic value of pretreatment serum albumin in RCC patients.
Materials and methods
Search strategy and selection criteria
This meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analyses guidelines.Citation29
We searched PubMed, Embase, and Web of Science for published studies that analyzed the prognostic value of pretreatment serum albumin in RCC patients up to November 3, 2015. Search terms used were: “kidney cancer or renal cancer or renal carcinoma or renal cell carcinoma” (all fields) and “albumin or hypoalbuminemia” (all fields) and “prognosis or prognostic or survival or outcome”. We checked the titles and abstracts of the papers retrieved, excluded irrelevant studies, screened the full text for the remaining papers, included satisfactory studies, and extracted the required data. In addition, we manually screened the references of related papers, including all the identified studies, reviews, and editorials, in order to retrieve unpublished but relevant investigations. Considering overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), and progression-free survival (PFS) were the frequently used outcomes of RCC, we chose them as the primary outcomes of the studies that were selected for this meta-analysis. Inclusion criteria: 1) diagnosis of RCC was histopathologically confirmed; 2) treatment was limited to surgery, targeted therapy, or immunotherapy; 3) pretreatment albumin values were measured and their potential association with the prognosis of RCC patients was analyzed; 4) retrospective or prospective study design; and 5) studies that directly offered the hazard ratio (HR) and 95% confidence interval (CI) or cases in which the presented data were available for reconstruction of HR and 95% CI.
Exclusion criteria: 1) studies that were not written in English; 2) letters, review papers, meeting records, commentaries, case reports, or clinical guidelines; 3) lack of critical data, such as HR or 95% CI; 4) studies on cancer cells and experimental animal studies; 5) sample sizes smaller than 40; 6) in cases where data overlapped across several different articles, only the study with the largest sample size was reviewed.
Quality assessment
According to the Newcastle–Ottawa quality assessment scale, two researchers independently assessed the quality of each study.Citation30 For quality assessment, scores ranged from 0 (lowest) to 9 (highest), and studies with scores of 6 or more were rated as being of high quality.
Data extraction and conversion
All data were extracted from the literature by two independent reviewers. When divergence appeared in the data-extraction process, a consensus would be reached after discussion. We extracted the following data: 1) basic information on the study: first author’s last name, publication year, country; 2) basic features of the patients, ie, case number, age, TNM staging, pathological grading, follow-up time; 3) cut-off value of pretreatment serum albumin; and 4) HR of albumin for OS, CSS, PFS, or recurrence-free survival (RFS), as well as 95% CI. If both the results of univariate and multivariate analysis were reported, only the result of multivariate analysis was extracted because this is more accurate, as it accounts for confounding factors.
If an article provided HRs and 95% CIs, they were extracted directly. However, if a paper did not provide HR and 95% CI, they were calculated using the data provided in the paper. If only the Kaplan–Meier curves of pretreatment albumin were available, we reconstructed the HRs and 95% CIs from the data extracted from the survival plots. We also sent emails to the corresponding authors to request any additional data needed for our meta-analysis. All the calculation methods mentioned were provided by Parmar et alCitation31 and Tierney et al.Citation32
Statistical analysis
HRs with 95% CIs were used to describe the relationship between pretreatment serum albumin and survival of RCC patients. An HR >1 suggested a worse prognosis in patients with a low concentration of pretreatment serum albumin, and an HR <1 indicated a better prognosis. We used Cochran’s Q test and Higgins I-squared statistic to conduct the test of heterogeneity of the combined HRs. If the P-value was <0.1 and/or I2 was >50%, the heterogeneity of the combined HRs was considered statistically significant, and a random effects model (the DerSimonian–Laird method) was then applied; otherwise, a fixed effects model (the Mantel–Haenszel method) was applied. The factors contributing to heterogeneity were analyzed by subgroup analysis. By assessing the asymmetry of an inverted funnel plot, publication bias was evaluated. Furthermore, we performed Begg’s and Egger’s tests to provide quantitative evidence of publication bias. Data analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA). All statistical tests were two-sided, and when P<0.05, differences were considered statistically significant.
Results
Search results
The process of searching and screening the literature is shown in . We identified a total of 822 publications in accordance with the search method described. When scanning the titles, abstracts, publication types, and full texts of the 822 publications, it was found that only 35 articles mentioned the correlation between pretreatment serum albumin and the outcome of RCC patients. Among these, 18 articles were excluded (three lacked some important data, seven used continuous or two cutoffs, and eight only reported odds ratios or relative risks). Thus, a total of 17 studies comprising 6,447 cases were included in our meta-analysis.Citation14–Citation28
Characteristics of included studies
The basic information on the studies conforming to the inclusion criteria is collected in . The 17 included articles were published from 2000 to 2015, and most of them came from Asia (n=4), Europe (n=4), and North America (n=8). Our meta-analysis included a total of 6,447 patients, with a median number of 209 patients per study (range: 70–2,119). Of these studies, nine explored the effect of pretreatment serum albumin on the prognosis of metastatic renal cell carcinoma, three on localized RCC, and four on clear cell renal cell carcinoma. Meanwhile, ten studies were evaluated using multivariate analysis, and seven were evaluated using univariate analysis. OS was evaluated in eleven studies, CSS was reported in eight studies, PFS was reported in two studies, and RFS was reported in one study. Considering there are more studies about OS, we chose OS as the primary outcome of the studies selected for this meta-analysis. HRs were directly reported for 15 studies, and estimated indirectly for the remaining two studies. The cutoff value of pretreatment serum albumin used in most studies was 3.5 g/dL (the lower limit of normal).
Table 1 Main characteristics of all studies included in the meta-analysis
Quality assessment
According to the Newcastle–Ottawa scale, we assessed the quality of the 17 eligible studies included in our meta-analysis. The quality scores of the studies varied from 4 to 9, with a mean of 6.5. A higher score showed better methodology. None of the studies mentioned were excluded from subsequent analyses.
Meta-analysis results
Pretreatment serum albumin and survival of RCC patients
The main results of this meta-analysis are shown in . Considering the similarities between RFS and PFS, we merged them together to conduct this analysis. A decreased pretreatment serum albumin level yielded a worse OS (I2=54.7%; P=0.015, random-effects model; HR=2.46, 95% CI 1.92–3.13, P<0.001) (); CSS (I2=39.6%, P=0.128, fixed-effects model; HR=2.22, 95% CI 1.87–2.64, P<0.001); and RFS/PFS (I2=39.4%, P=0.192, fixed-effects model; HR=1.75, 95% CI 1.28–2.38, P<0.001) (). To explore the heterogeneity between these studies, the significance of pretreatment serum albumin was evaluated further via subgroup analysis based on the main features, including tumor type, analysis type, cutoff value, and HR-obtaining method. Considering that the number of studies that evaluated RFS and PFS was relatively small, we only conducted subgroup analysis for OS and CSS. In the tumor type subgroup, a decreased pretreatment serum albumin level was closely associated with the poor prognosis of RCC patients, except for OS in the all-stage RCC group (). As for the analysis type, cutoff value, and HR-obtaining method subgroups, previous findings were powerful, as shown in .
Figure 2 Forest plots of studies evaluating hazard ratios of decreased serum albumin levels for all renal cell carcinomas RCC.
Abbreviations: CSS, cancer-specific survival; HR, hazard ratio; OS, overall survival; RCC, renal cell carcinoma.
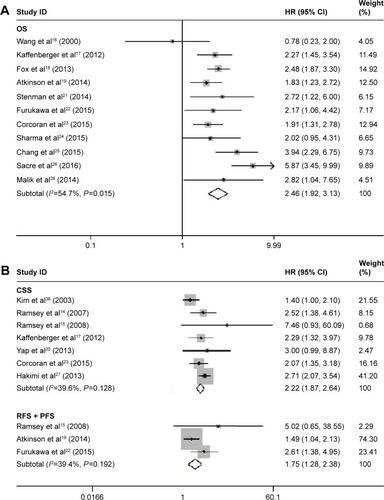
Figure 3 Forest plots of studies evaluating hazard ratios of decreased serum albumin levels for different tumor types.
Abbreviations: CSS, cancer-specific survival; HR, hazard ratio; OS, overall survival.
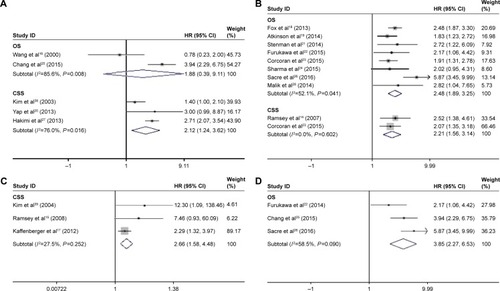
Table 2 The pooled associations between pretreatment serum albumin and the prognosis of RCC patients
Sensitivity analyses
Each single cohort included in our meta-analysis was deleted, in turn, to check if individual studies influenced the results. Results of sensitivity analyses indicated the strength of our findings (data not shown).
Publication bias
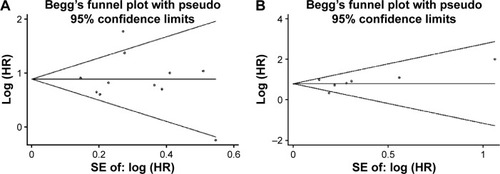
Publication bias was assessed for both OS and CSS. The publication bias of all enrolled studies was evaluated using funnel plots, and Egger’s and Begg’s tests. The funnel plots were almost symmetrical (). The P-values of the Egger’s and Begg’s tests were all greater than 0.05 (OS, P=0.938 for the Begg’s test, P=0.967 for the Egger’s test; CSS, P=0.230 for the Begg’s test, P=0.620 for the Egger’s test). Therefore, there was no significant publication bias in our meta-analysis.
Discussion
To the best of our knowledge, the present meta-analysis is the first and most comprehensive study to systematically analyze the prognostic value of pretreatment serum albumin in RCC patients. It is also the first meta-analysis to expound the relationship between serum albumin and the prognosis of patients with solid tumors. According to our final results, there was a significant correlation of low pretreatment serum albumin levels with poor survival of RCC patients, with a combined HR of 2.46 (95% CI 1.92–3.13) for OS, 2.22 (95% CI 1.87–2.64) for CSS, and 1.75 (95% CI 1.28–2.38) for RFS/PFS. Generally, in the subgroup analysis, decreased pretreatment serum albumin was an important prognostic marker in RCC patients, regardless of tumor type, analysis type, cutoff value, and HR-obtaining method, except for OS in all-stage RCC. Considering the lower sample size of the only subgroup with a different result (two studies involving 672 patients), we can ignore the inconsistent result to some extent.
Pretreatment serum albumin is closely related to the prognosis of RCC and other malignant tumors; the underlying reasons for this are still speculative, but explanations involving nutrition and inflammation have been widely recognized. The initiation and progression of cancer are often accompanied by malnutrition and chronic inflammation, which often develop into tumor cachexia and accelerate the deterioration of cancer patients. Malnutrition in cancer patients is usually caused by loss of appetite and malignant tumor depletion, which is reflected in hypoalbuminemia and pitting edema. The systematic inflammatory response in cancer patients also alters the concentration of serum albumin. Under inflammatory conditions, activated proinflammatory cytokines, including tumor necrosis factor, and interleukin −1, −6, and −8, inhibit the ability of liver cells to generate albumin.Citation8,Citation33,Citation34 In addition, the TNF generated by cancer cells increases the permeability of capillaries, which results in the direct loss of albumin from the circulatory system.Citation35 Furthermore, with the malignant progress of the tumor, development of micrometastases in the liver impairs liver function and reduces the synthesis of albumin.Citation8,Citation36 Therefore, the lower the level of serum albumin, the worse the prognosis of cancer patients, and consequently, pretreatment serum albumin is an effective prognostic indicator for cancer patients.
Serum albumin is a convenient and economic prognostic indicator for RCC patients; the concentration of serum albumin can be easily monitored by biochemical examination at any time. The reduction of serum albumin concentration in cancer patients not only demonstrates the deterioration of their nutritional condition, but also indicates a poor prognosis. Therefore, clinical personnel can conduct risk stratification on cancer patients on the basis of the concentration of pretreatment serum albumin, in order to individualize treatment. However, as a prognostic indicator in RCC patients, serum albumin does have some limitations. For example, in the overhydrated state or with other disease processes, serum albumin may not indicate the true nutritional status, so its prognostic value in RCC patients will be disturbed.Citation37 Meanwhile, the level of serum albumin is a blood index, which may be slightly affected by diet and other nontumor-related factors. Nevertheless, without the interference of these factors, pretreatment serum albumin is of great prognostic value in RCC patients.
There were several limitations in this study; first, only 17 studies and 6,447 patients were included in our meta-analysis, ie, there was a relatively small sample size. Second, there was heterogeneity between the studies that may have been caused by differences in research design, basic features of the patients, cutoff values for pretreatment serum albumin, therapeutic methods, and follow-up periods. Moreover, owing to the lack of sufficient data, the associations between pretreatment serum albumin and other important clinical characteristics were not explored.
Conclusion
In conclusion, this meta-analysis clarified that a decreased pretreatment serum albumin level implied a poor prognosis for RCC patients. The level of pretreatment serum albumin is a convenient and economic prognostic indicator, and can be monitored for risk stratification and treatment individualization in RCC patients. Considering the limitation of the present analysis, further prospective multicenter studies should be carried out to confirm our findings.
Acknowledgments
This work was supported by the National Science Foundation of Jiangsu Province (Grant no BK20150251).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- MargarsonMPSoniNSerum albumin: touchstone or totem?Anaesthesia19985387898039797524
- Di FioreFLecleireSPopDBaseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancerAm J Gastroenterol2007102112557256317680847
- BauerJCapraSComparison of a malnutrition screening tool with subjective global assessment in hospitalised patients with cancer – sensitivity and specificityAsia Pac J Clin Nutr200312325726014505986
- FuhrmanMPCharneyPMuellerCMHepatic proteins and nutrition assessmentJ Am Diet Assoc200410481258126415281044
- GabayCKushnerIAcute-phase proteins and other systemic responses to inflammationN Engl J Med199934064484549971870
- McMillanDCWatsonWSO’GormanPPrestonTScottHRMcArdleCSAlbumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight lossNutr Cancer200139221021311759282
- HolmesSDickersonJWMalignant disease: nutritional implications of disease and treatmentCancer Metastasis Rev1987633573813121201
- LaiCCYouJFYehCYLow preoperative serum albumin in colon cancer: a risk factor for poor outcomeInt J Colorectal Dis201126447348121190025
- SeebacherVHofstetterGPolterauerSDoes thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trialBr J Cancer2013109121521823764750
- TanriverdiOAvciNOktayEPretreatment serum albumin level is an independent prognostic factor in patients with stage IIIB non-small cell lung cancer: a study of the Turkish descriptive oncological researches groupAsian Pac J Cancer Prev201516145971597626320482
- HanSHuangYLiZHouHWuAThe prognostic role of preoperative serum albumin levels in glioblastoma patientsBMC Cancer20151510825880463
- RamseySLambGWAitchisonMGrahamJMcMillanDCEvaluation of an inflammation-based prognostic score in patients with metastatic renal cancerCancer2007109220521217149754
- RamseySLambGWAitchisonMMcMillanDCProspective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancerBJU Int2008101895996318190639
- WangYHuangCWuYMultivariate analysis of prognostic factors in renal cell carcinomaZhonghua Wai Ke Za Zhi200038644244411832079
- KaffenbergerSDMorganTMStrattonKLABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinomaBJU Int201211011 Pt BE641E64622958439
- FoxPHudsonMBrownCMarkers of systemic inflammation predict survival in patients with advanced renal cell cancerBr J Cancer2013109114715323778526
- AtkinsonBJKalraSWangXClinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedulesJ Urol2014191361161824018239
- YapNYNgKLOngTAClinical prognostic factors and survival outcome in renal cell carcinoma patients – a Malaysian single centre perspectiveAsian Pac J Cancer Prev201314127497750024460324
- StenmanMLaurellALindskogMPrognostic significance of serum albumin in patients with metastatic renal cell carcinomaMed Oncol201431384124477648
- FurukawaJMiyakeHKusudaYFujisawaMHyponatremia as a powerful prognostic predictor for Japanese patients with clear cell renal cell carcinoma treated with a tyrosine kinase inhibitorInt J Clin Oncol201520235135724894624
- CorcoranATKaffenbergerSDClarkPEHypoalbuminaemia is associated with mortality in patients undergoing cytoreductive nephrectomyBJU Int2015116335135725123843
- SharmaPZargar-ShoshtariKCaraccioloJTSarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinomaUrol Oncol2015338339 e317e323
- ChangYAnHXuLSystemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinomaBr J Cancer2015113462663326135896
- SacreABarthelemyPKorenbaumCPrognostic factors in second-line targeted therapy for metastatic clear-cell renal cell carcinoma after progression on an anti-vascular endothelial growth factor receptor tyrosine kinase inhibitorActa Oncol201655332934026494607
- HakimiAAFurbergHZaborECAn epidemiologic and genomic investigation into the obesity paradox in renal cell carcinomaJ Natl Cancer Inst2013105241862187024285872
- MalikLParsonsHMahalingamDClinical outcomes and survival of advanced renal cancer patients in phase I clinical trialsClin Genitourin Cancer201412535936524582088
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- ParmarMKTorriVStewartLExtracting summary statistics to perform meta-analyses of the published literature for survival endpointsStat Med19981724281528349921604
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- Onate-OcanaLFAiello-CrocifoglioVGallardo-RinconDSerum albumin as a significant prognostic factor for patients with gastric carcinomaAnn Surg Oncol200714238138917160496
- ChojkierMInhibition of albumin synthesis in chronic diseases: molecular mechanismsJ Clin Gastroenterol2005394 Suppl 2S143S14615758650
- GuptaDLisCGPretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literatureNutr J201096921176210
- BarberMDRossJAFearonKCChanges in nutritional, functional, and inflammatory markers in advanced pancreatic cancerNutr Cancer199935210611010693162
- DetskyASBakerJPMendelsonRAWolmanSLWessonDEJeejeebhoyKNEvaluating the accuracy of nutritional assessment techniques applied to hospitalized patients: methodology and comparisonsJPEN J Parenter Enteral Nutr1984821531596538911
- KimHLBelldegrunASFreitasDGParaneoplastic signs and symptoms of renal cell carcinoma: implications for prognosisJ Urol200317051742174614532767
- KimHLHanKRZismanAFiglinRABelldegrunASCachexia-like symptoms predict a worse prognosis in localized t1 renal cell carcinomaJ Urol200417151810181315076282