Abstract
Metastatic urothelial carcinoma of the bladder is an aggressive malignancy with poor prognosis, reflecting a lack of effective systemic therapies. The current standard of care includes multiagent platinum-based chemotherapy; however a majority of patients do not respond to treatment and most eventually succumb to disease. Recently, renewed interest in immunotherapy in the form of immune-checkpoint inhibition has gained widespread attention for a number of malignancies. Atezolizumab, an anti-PDL1 antibody, has been shown to be effective in a subset of patients previously treated with or unfit for platinum-based chemotherapy, and has shown durable responses with a good tolerability profile. We review the mechanism of action and clinical evidence of atezolizumab for metastatic urothelial bladder cancer, and discuss this drug within the context of ongoing developments in this dynamic field of immunooncology.
Introduction
Bladder cancer is the ninth-commonest malignancy worldwide, with differences in prevalence explained largely by differences in exposure to certain risk factors.Citation1,Citation2 In the developed world, where the main risk factor is tobacco exposure, the vast majority of bladder cancer is of urothelial histology.Citation1,Citation3 In other regions, where Schistosoma haematobium infection is common, squamous cell carcinoma histology is predominantly present.Citation4 While most urothelial carcinoma of the bladder (UCB) presents as non-muscle-invasive disease, approximately 30% of bladder cancers are already muscle-invasive at diagnosis.Citation5 When amenable to surgical therapy, approximately 25% of patients with muscle-invasive disease are found to harbor lymph-node metastasis, with associated decreased survival due to the frequent development of distant metastases. Additionally, about 10% of patients present with systemic metastatic disease at the time of diagnosis.Citation6
Since the 1970s, the cornerstone of therapy for advanced and metastatic UCB (mUCB) has been platinum-based chemotherapy (PBCT), first as a single agent and subsequently as part of combination therapy.Citation7 Since the 1990s, the standard first-line therapy has been methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) after demonstrating a superior response rate and overall survival (OS) compared to cisplatin alone.Citation8 A number of chemotherapeutic regimens have been evaluated to improve the response rate and significant toxicity profile of MVAC, with gemcitabine and cisplatin showing similar overall response rate and OS with a better tolerability profile.Citation9 As such, both MVAC and gemcitabine–cisplatin are considered first-line therapies for mUCB. Numerous permutations of these platinum-containing regimens have been studied, including high-intensity MVAC, coadministration of growth-factor support, and substitution of other platinum agents (eg, carboplatin) for those unable to receive cisplatin.Citation7 While advances in systemic chemotherapies have led to modest improvements in outcomes, mUCB remains a deadly disease in the majority of cases, with median progression-free survival (PFS) and OS at approximately 8 and 14 months, respectively.Citation7
Options for patients who have progressed after first-line therapy have historically been limited. Only vinflunine monotherapy has been proven to be superior to best supportive care (BSC) alone, demonstrating a modest survival benefit (6.9 vs 4.3 months, hazard ratio 0.78, 95% confidence interval [CI] 0.61–0.96; P=0.0227) in the eligible population, which was defined as patients without major protocol violations.Citation10 In the intention-to-treat population, the survival benefit was not statistically significant.Citation10 While this drug was approved as a second-line agent in Europe, until recently there has not been a US Food and Drug Administration (FDA)-approved regimen for second-line use in mUCB.Citation11
Recently, a Phase II clinical trial was conducted to evaluate an anti-PDL1 monoclonal antibody – atezolizumab (MPDL3280A; Hoffman-La Roche, Basel, Switzerland) – for treatment of mUCB after failure of PBCT or in platinum-ineligible patients. The findings demonstrated an overall response rate of 15% in the entire study population, a favorable tolerability profile, and long lasting responses not seen in mUCB to that point.Citation12 These encouraging findings led to the granting of guaranteed breakthrough status and further early FDA approval in 2016; the first new drug approved for mUCB in over 20 years. In this manuscript, we review the mechanism of action and clinical evidence of efficacy of atezolizumab for mUCB, and discuss this drug within the context of ongoing developments in this dynamic field of immunooncology.
Mechanism of action
Immunooncology
In the last few years, we have seen a great deal of excitement about co-opting the immune system to fight malignancies through approaches with monoclonal antibodies, cancer vaccines, and cytokine therapies.Citation13 One of the most attractive modalities is the activation of antitumor activity by blocking immune checkpoints (ICPs).Citation14 The numerous genetic and epigenetic alterations that characterize malignant cells are thought to result in antigens that could reasonably be used to distinguish these cells from benign counterparts. The concept of immunosurveillance is based on this premise: circulating immune cells can attack and destroy premalignant or malignant cells, in a similar way to how these cells might act against infectious pathogens. However, immunoactivation is a complex phenomenon, requiring an intricate balance between stimulatory and inhibitory signals.Citation15 To prevent autoimmunity, ICPs (inhibitory signals) are crucial, and their expression can be altered by tumors; an important mechanism of immune resistance (). Studies indicate that blocking these ICPs results in an increase in effector T cells and inflammatory cytokines within the tumor, as well as a decrease in immunoregulatory cells.Citation16
Figure 1 Mechanism of anti-PD1 and anti-PDL1 checkpoint blockades.
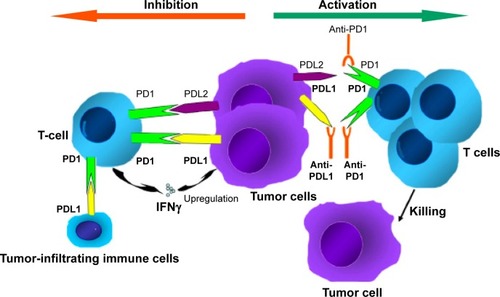
Immune-checkpoint inhibition
Inhibition of these ICPs to alter the tumor microenvironment is one of the most promising areas of active cancer research. T-cell-receptor activation requires costimulatory molecules and the absence of inhibitory molecules. The first ICP inhibitor (ICPI) clinically investigated was ipilimumab (MDX-010; Bristol-Meyers Squibb, New York, NY, USA), a monoclonal anti-CTLA4 antibody that blocks the interaction of B7 (a costimulatory molecule on antigen-presenting cells) with the inhibitory CTLA4 receptor expressed on the surface of T cells.Citation17 Ipilimumab has been shown to be effective in metastatic melanoma, and gained FDA approval in 2011.Citation18 One drawback of this approach appears to be a lack of selectivity of CTLA4 blockade in T-cell expansion, and this may underlie the significant immunorelated toxicities associated with ipilimumab.Citation19
Another ICP is the interaction between PD1 and its ligands PDL1 and PDL2. A more exhaustive review of this ICP is available by Ohaegbulam et al.Citation20 Briefly, PD1 is expressed on a number of immune cells, including activated T cells, regulatory T cells, B cells, monocytes, natural killer cells, and dendritic cells. For activated T cells, binding of PD1 by its ligands results in inhibition of the T-cell receptor and subsequent termination of the immune response. This is an important physiological mechanism to avoid autoimmunity; however, when expressed by tumor cells (TCs) or tumor-infiltrating immune cells (ICs) within the tumor microenvironment, this leads to termination of the antitumor activity of T cells in an analog fashion.Citation20,Citation21 Tumors themselves can express these inhibitory ligands, as well as induce their expression by immune-infiltrating cells, resulting in an effective checkpoint against immunosurveillance.Citation20,Citation22 Cancer therapeutics in the form of monoclonal antibodies that block the interaction between the PD1 receptor and its ligands have been a very active area of research, with promising results in a number of malignancies.
A number of monoclonal antibodies have been developed to target PD1, including pidilizumab (CT-011; CureTech, Yavne, Israel),Citation23 pembrolizumab (MK-3475; Merck, Kenilworth, NJ, USA),Citation24 and nivolumab (BMS-936558; Bristol-Meyers Squibb).Citation25 The most extensively studied of these is nivolumab, which has been FDA-approved in non-small-cell lung cancer, Hodgkin lymphoma, metastatic melanoma, and metastatic renal cell carcinoma.Citation26 Of the two ligands that are known to activate PD1, PDL1 appears to play a more prominent role in lymphocyte regulation,Citation27 and as such a number of agents have been developed to block PDL1, including atezolizumab,Citation28 BMS-936559 (Bristol-Meyers Squibb),Citation29 durvalumab (MEDI4736; AstraZeneca, London, UK),Citation30 and avelumab (MSB0010718C; Pfizer, New York, NY, USA).Citation31 These agents are the subjects of ongoing clinical trials.
Immunotherapy in bladder cancer
While there is a growing interest in immunotherapies for systemic malignancies, immunotherapy actually has an extensive history in bladder cancer, albeit for non-muscle-invasive disease. Forty years ago, Morales et al demonstrated that intravesical instillation of bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, was effective at treating noninvasive bladder cancer,Citation32 and this form of immunotherapy remains the standard of care today for high-risk non-muscle-invasive disease.Citation33 The mechanism underlying the efficacy of BCG remains incompletely elucidated, but it is broadly believed that BCG activates the immune system and induces an inflammatory response, ultimately leading to immunomediated cytotoxicity through CD8+ T lymphocytes, natural killer cells, and granulocytes.Citation34
This evidence of effective immunomanipulation in the treatment of noninvasive disease and growing evidence of efficacy of immunotherapies, in particular ICP inhibition, in the treatment of other nonlocalized malignancies led to the logical investigation of ICP inhibition as a potential therapy for mUCB. Inman et al described PDL1 TC expression by immunohistochemistry (IHC) in 280 high-risk bladder patients, and found that PDL1 expression increased significantly with higher disease stage and grade, hypothesizing that PDL1 expression may be one mechanism of BCG resistance.Citation35 Additionally, a hallmark of UCB is the presence of a high somatic mutational load,Citation36 which may result in increased neoantigen expression and may make the tumors more susceptible to immunosurveillance.Citation13
Atezolizumab
Pharmacokinetics and pharmacodynamics
Atezolizumab (MPDL3280A) was developed as a human monoclonal IgG1 antibody with a high affinity for PDL1 (binding affinity dissociation constant =0.4 nM).Citation37 Atezolizumab blocks the interaction of PDL1 (also called B7-H1 or CD274) with PD1 and B7.1 (also called CD80).Citation37,Citation38 Both PD1 and B7.1 are receptors for PDL1 binding, which results in T-cell tolerance and restriction of cellular killing. Therefore, the inhibition of the host immune response against the tumor can be prevented by interaction of the anti-PDL1 antibody with its ligand. Of note, atezolizumab has an engineered fragment (Fc) domain that prevents active T-cell depletion via antibody-dependent cellular toxicity.
It has a half-life of 27 days, and steady-state concentration is reached in two to three cycles (6–9 weeks) of repeated intravenous (IV) doses.Citation39 Its volume of distribution is 6.9 L and clearance is 0.2 L/day. Systemic accumulation area under the curve (AUC), maximum concentration (Cmax), and minimum concentration (Cmin) are 1.91-, 1.46-, and 2.75-fold, respectively. Atezolizumab follows a biphasic distribution until day 7 after IV bolus administration.Citation40 It shows nonlinear (dose-dependent) and linear (dose-independent) pharmacokinetics in doses of 0.5–5 mg/kg and 5–20 mg/kg, respectively. Herbst et al detected antitumor activity in doses of 1–20 mg/kg following IV administration once every 3 weeks.Citation37
Available data for atezolizumab in mUCB
Most initial studies evaluating the efficacy and safety of anti-PDL1 treatments were based on biomarker-enriched cohorts, including only patients with positive immunoreactivity for PDL1. However, Powles et al expanded the initial cohort in their Phase I multicenter dose-escalation expansion study (NCT01375842/PCD4989g) to mUCB patients without immunoreactivity against PDL1.Citation28 Patients were scored according to IHC status within a range between 0 and 3. Among a total of 67 enrolled patients, 18% had no expression of PDL1 (IHC score 0), 34% low expression (IHC score 1), 30% intermediate expression (IHC score 2), and 15% high expression (IHC score 3). Most patients (93%) had received previous PBCT, and 72% had received two or more lines of treatment. Patients with an Eastern Cooperative Oncology Group (ECOG) score ≥2 were excluded. Patients received atezolizumab at a dose of 15 mg/kg IV every 3 weeks. The first infusion of atezolizumab lasted 60 minutes, which was subsequently reduced to 30 minutes if well tolerated. The total number of planned cycles was 16, comprising a total treatment time of 1 year. The reasons for termination of treatment were progression of disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1Citation41 or immunorelated response criteria,Citation42 intolerable toxicity, and incompliance with the study protocol. After a minimum 6 weeks of follow-up, 43% of patients with IHC score 2/3 tumors and 11% of patients with IHC score 0/1 tumors showed an objective response (OR; complete remission and partial remission). Of those with IHC score 2/3 tumors, 7% had a complete response. Following the outcomes of this study, atezolizumab was granted breakthrough status for mUCB by the FDA.Citation28
A subsequent Phase II study with two subgroups (group 1, patients with mUCB ineligible for PBCT for first-line treatment; group 2, patients with mUCB after progression on PBCT for second-line treatment) was performed (NCT02108652).Citation43 In 2016, Rosenberg et al reported on group 2 of the Phase II trial (NCT02108652/IMvigor210), in which 310 patients with mUC whose disease had progressed despite previous treatment with PBCT were enrolled.Citation12 The urinary bladder was the primary tumor site in 74% of patients, while the renal pelvis, ureter, urethra, and other sites were the primary site in 14%, 7%, 2%, and 3% of patients, respectively.
Patients were grouped according to PDL1-expression status, which was based on percentage of PDL1-positive ICs within the tumor environment determined by the SP142 assay (Hoffman-La Roche): IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%). Of the 310 patients included 103, 107, and 100 were in the IC0, IC1, and IC2/3 groups, respectively. A standard fixed dose of 1,200 mg was administered every 3 weeks, with a median duration of 12 (range 0–66) weeks. OR rates (ORRs) were 26%, 18%, 11%, and 8% in IC groups 2/3, 1/2/3, 1, and 0, respectively. Therefore, a combined ORR of 15% was reported for all patients in the study. In comparison to the previously published Phase I expansion cohort, the response rate of IC2/3 patients was lower (26% vs 43%); however, in the Phase I cohort, ICs and TCs were evaluated for PDL1 expression, whereas in the Phase II cohort only ICs were considered.
The median time to response was about 2 months. After a median follow-up of 11.7 months, 84% of the initially responding patients showed ongoing response to therapy (median time of response not reached), including those with poor risk factors and upper-tract disease. Interestingly, many responses were ongoing, despite discontinuation of treatment for various reasons. For the entire cohort, median PFS and OS were 2.1 and 7.9 months, respectively, while for IC2/3 patients median PFS and OS were 2.1 and 11.4 months, respectively. An updated report of the same trial after approximately 1.5 years of median follow-up revealed 1-year OS rates of 50% for patients with IC2/3 and 37% for the overall cohort.Citation44 Complete responses occurred in 7% of the overall cohort, as well as 15%, 6%, and 2% of patients with IC2/3, IC1, and IC0, respectively, with median duration of response not reached. For mUC of the upper urinary tract, OS was 7.6 and 10.9 months, whereas for metastatic bladder cancer OS was 7.9 and 12.8 months, for the overall cohort and IHC2/3 patients, respectively.
Due to these favorable results, after which atezolizumab was FDA-approved for second-line therapy, it is currently being evaluated as part of a Phase III randomized clinical trial (IMvigor211) that is comparing atezolizumab to other chemotherapeutic agents (docetaxel, paclitaxel, vinflunine) in patients with locally advanced and mUCB who have progressed during or following PBCT. The results of this ongoing study are expected in mid-2017.
Further, regarding first-line use of atezolizumab, the data for group 1 of the phase II IMvigor trial have not been finally published, but excerpts were presented at 2016 annual cancer meetings.Citation45,Citation46 Overall, 119 patients were included in group 1 of IMvigor210, and the definition of platinum ineligibility was one or more of the following: glomerular filtration rate (GFR) 30–60 mL/min (70%), ≥ grade 2 hearing loss (14%)/neuropathy (6%), or ECOG performance status of 2 (20%). The primary end point was ORR, and the treatment regimen was the same as in group 2. Of the whole cohort, 92% had metastatic disease (66% visceral metastases), and the bladder was the primary tumor site in 71% of patients. Again, the patients were grouped by PDL1-expression status on ICs as described earlier, and 33% were IC0, 40% were IC1, and 27% were IC2/3.
The ORR for the whole cohort was 24% (7% complete response and 17% partial response). Here as well, some correlation with the IC group and response to therapy was seen; however, it was not as pronounced as in group 2. ORRs in groups IC0, IC1, and IC2/3 were 21%, 23%, and 28%, respectively, with all groups showing 6%–8% complete response. Also in this trial, responses were durable, and after a median follow-up of 14.4 months, median OS was 14.8 months (95% CI 10.1 months to not reached) and 57% alive at 12 months. Interestingly, patients in the IC0/1 groups fared similarly to patients in the IC2/3 group (15.3 vs 12.3 months, respectively). These favorable data might lead to approval in the first-line space for non-platinum-eligible patients in the near future.
Additionally, there are other ongoing clinical trials of atezolizumab for different stages of urothelial carcinoma (UC), especially in the adjuvant setting, in which PBCT is the current standard of care, as well as for high-risk non-muscle-invasive UCB, in which intravesical treatment, foremost with BCG, is currently utilized.Citation1 A summary of currently listed clinical trials on ClinicalTrials.gov of atezolizumab for UC is presented in .Citation12,Citation28,Citation37,Citation43,Citation45–Citation58 More information regarding efficacy and safety will become available through these trials, and new indications for atezolizumab and treatment-responsive patient subgroups may be discovered.
Table 1 Clinical trials of atezolizumab for urothelial carcinoma
Biomarkers are vital in oncology to determine patients who might or might not be responsive to certain treatments. With regard to immunobiomarkers for atezolizumab, the SP142 assay was developed using rabbit monoclonal anti-PDL1 clone SP142 for assessment of PDL1 expression in UC and non-small-cell lung carcinoma.Citation37 The IHC assay was performed in formalin-fixed, paraffin-embedded urothelial tissue and based on the percentage of TCs or ICs with PDL1 expression, regardless of intensity. Both tumors and ICs showed membranous and cytoplasmic staining; however, detection of PDL1 expression was difficult in ICs, which had scant cytoplasms and were small. Distribution of PDL1-expressing TCs was typically focal, and mostly located at the interface between malignant cells and stroma. However, distribution and location of PDL1-expressing ICs varied within tumors; they were located at either the periphery of the tumor, at stromal bands dissecting the tumor, within IC aggregates, or scattered as single cells throughout. IHC status of specimens was categorized into four groups: IHC0, -1, -2, or -3 if the percentage of PDL1-positive cells per area was <1%, ≥1% but <5%, ≥5% but <10%, or ≥10%; respectively. Patients with multiple specimens were scored according to the specimen with the highest score.
Rosenberg et al determined a cutoff value of ≥5% PDL1 expression in ICs determined by SP142 assay as high, which was associated with response.Citation12 However, although PDL1 positivity was beneficial for response to treatment, ORRs of approximately 10% were also detected in PDL1-negative patients with mUCB. In addition to PDL1-expression status, mutation load and Cancer Genome Atlas gene expression were analyzed with respect to PDL1 expression, as well as response. Despite significantly higher prevalence of PDL1 positivity in the basal vs the luminal subtypes of UC (60% vs 23%), the highest response rate was seen in cluster II of the luminal subtype (34%). The authors concluded that future multibiomarker systems might accurately predict response to atezolizumab. Bellesoeur et al evaluated 346 patients with various malignancies eligible for treatment with ICPI, and found UC was associated with higher PDL1 expression than other tumor types.Citation59 However, depending on the assay used for PDL1 evaluation, as well as the cell type studied, PDL1 expression can vary.Citation28
In many solid cancers, OR is better correlated with PDL1 expression on TCs than on ICs,Citation60 contrary to mUCB, in which PDL1 expression on ICs is favorable. PDL1 expression is a dynamic process, requires recognition of antigens by ICs, and might differ during course of treatment,Citation61 and thus subsequent examinations of IHC status may be necessary after initial evaluation prior to treatment. In malignant melanoma and renal cell carcinoma, heterogeneity of PDL1 expression has been reported between primary tumor and metastatic sites.Citation62,Citation63
There are still limitations with such biomarkers as PDL1 expression for predicting response to treatment. These limitations are due to the multitude of PDL1 antibodies, assays, scoring systems, and thresholds for positivity. However, there is still value in knowing the PDL1 expression, since in the Rosenberg et al study ORRs were 26% (95% CI 18%–36%) in the combined IC2 and IC3 group and only 10% and 8% in the IC1 and IC0 groups, respectively.Citation12 At this time, due to limitations in understanding the role of a biomarker in selecting patients for therapy, the FDA does not require PDL1 positivity for initiation of atezolizumab treatment for mUCB patients.
Blood-based immunobiomarkers, such as IL-6, IL-18, and IFNγ, have not been found to be associated with response to atezolizumab treatment.Citation37 Anantharaman et al evaluated the value of circulating TCs (CTCs) with PDL1 expression as a noninvasive and safe biomarker in 25 patients with muscle-invasive bladder cancer or mUCB.Citation64 While CTCs were found in 80% of patients overall, 35% of CTCs showed PDL1 positivity. Although statistically insignificant as the study was not powered to detect signals for survival, patients with a high PDL1-positive CTC burden showed decreased median OS (194 days) compared to those with low PDL1-positive CTC burden (303 days). Of note, most PDL1-positive CTCs showed no cytokeratin expression in the analysis, which might be a sign of epithelial–mesenchymal transition during metastasis development and consistent with escape from immunosurveillance by PDL1 expression. However, only five patients had anti-PDL1 therapy after analysis of CTCs, thus limiting the value of the study regarding predictive information. Moreover, in non-small-cell lung cancers, certain types of blood cells, which showed PDL1 expression without expression of common myeloid specific markers, resulted in decreased specificity and interfered with identification of true TCs in CTC analysis, thus indicating possible problems with this approach.Citation65
Theoretically, a multitude of possibilities regarding biomarkers are possible, including CTCs, cell-free DNA, micro-RNA, specific tumor mutations, or number of mutations, as well as markers of immunoactivity; however, reliable data in this regard is still lacking. Finding and improving biomarkers, as well as assays and cutoff values, may enable better selection of patients for more accurate prediction of treatment outcomes, while reducing toxicity and cost.
Furthermore, pseudoprogression or atypical response patterns have been described in patients receiving immunotherapy for solid tumors.Citation42 Patients may have favorable response, despite an initial increase in tumor size or even appearance of new lesions during immunotherapy, possibly due to increased IC localization and inflammation in tumor sites. Therefore, contrary to cytotoxic agents, RECIST 1.1 is not always an appropriate tool for evaluation of response to immunotherapeutic agents. Loriot et al reported that among mUCB patients who continued atezolizumab despite progression of disease, 19% had a subsequent ≥30% decrease in target lesions from their baseline scans, thus warranting reliable biomarkers to determine treatment success, as well as innovative methods to differentiate late response and primary failure.Citation44
As late favorable response to atezolizumab treatment occurs in some patients, when to initiate systemic therapy after failed salvage immunotherapy remains an open question. Furthermore, management of patients with mUCB who have failed or had to discontinue salvage anti-PD1/PDL1 immunotherapy following failed first-line systemic therapy is not yet defined. Nevertheless, Sonpavde et al reported that the median survival rate of these patients was comparable (189 days after start of anti-PD1/PDL1 therapy) to historically observed patients who received second-line systemic therapy only after PBCT.Citation66,Citation67 The only significant prognostic factor for survival was patient performance status. Response to and duration of prior immunotherapy was not correlated with survival. Therefore, delivery of third-line and beyond therapies for patients who have failed salvage anti-PD1/PDL1 immunotherapy seems possible.
Alternative agents to atezolizumab for second-line therapy for mUCB
The prognosis of patients with mUCB who fail PBCT is very poor, and prior to the approval of atezolizumab, no second-line therapy for mUCB was available in the US, whereas vinflunine was available only in Europe and showed limited efficacy. So far, very few randomized clinical trials have been performed in the second-line setting for mUCB, as trials have been limited by poor performance status, impaired renal function, and comorbidities that restricted trial design and contributed to poor patient accrual.Citation47 Furthermore, significant disease heterogeneity exists, which limited the interpretation and applicability of Phase II trials.
While no data comparing atezolizumab with other treatments are available yet, the published results for atezolizumab exceed the ones of previously evaluated second-line agents. Studies evaluating the impact of rechallenging with MVAC reported an ORR of approximately 20% in mUCB patients.Citation68,Citation69 Several monotherapies and combinations have been utilized in patients who progressed after first-line platinum-based regimens. The highest ORRs reported for docetaxel, gemcitabine, ifosfamide, fluorouracil/recombinant human IFNα2a, paclitaxel, and pemetrexed monotherapy as second-line therapy were 6% (44% for patients with subsequent platinum-containing chemotherapy), 11%, 20%, 30%, 10%, and 27.7%, respectively.Citation70–Citation75 However, these Phase II trials were usually hampered by a low number of patients (generally ten to 30), methodological issues (no randomization or no blinding), poor study designs (selection bias and no prior stratification of risks), and associated with serious adverse events (AEs), such as grade 3/4 myelosuppression and neuropathy. Research evaluating oxaliplatin, piritrexim, and irinotecan as second-line therapy for this setting also reported poor outcomes and was hampered by similar limiting factors.Citation76 Sorafenib, aflibercept, and pazopanib as second-line targeted therapy in UC demonstrated ORRs of 0, 5%, and 17%, respectively, with a high rate of AEs.Citation77–Citation79 The trial in which the combination of everolimus with paclitaxel was studied was negative as well.Citation80 None of these agents received approval for second-line therapy, due to their limited efficacy and high toxicity.
To our knowledge, other than atezolizumab, there are only two completed Phase III trials for second-line therapy of mUCB. The first was a report in 2009 from the Phase III trial of vinflunine, a second-generation vinca-alkaloid, which inhibits microtubules (NCT00315237).Citation67 The study was designed to compare OS of UC patients receiving second-line vinflunine and BSC to patients receiving BSC only (palliative radiotherapy, antibiotics, analgesics, corticosteroids, and transfusions) after failure of PBCT. A total of 253 patients were randomly assigned to vinflunine plus BSC, while 117 patients received BSC only. Of note, some patients received vinflunine with dose reductions, due to serious hematologic AEs observed during the trial. Overall response rate (8.6% vs 0%) and median PFS (3 vs 1.5 months) were all statistically significant in favoring the treatment arm. In the vinflunine-treatment arm, approximately half of patients had grade 3/4 neutropenia, whereas grade 3/4 levels of febrile neutropenia, anemia, fatigue, and constipation were observed in 6%, 19%, 19%, and 16% of patients, respectively. The updated study with a median follow-up of 45.4 months reported that vinflunine increased survival by 2.3 months and provided a partial response rate of 10%.Citation10 After these results, it was approved in Europe and European Association of Urology guidelines recommended vinflunine as a second-line agent in mUCB patients who failed PBCT; however, as mentioned earlier, statistical significance was lacking in the intention-to-treat cohort, which probably precluded approval by the FDA.Citation81
The other Phase III trial for advanced UC evaluated the combination of gemcitabine and paclitaxel as second-line therapy (German Cancer Society/Deutsche Krebsgesellschaft Studien ID 213, Studienkennung 01-09).Citation82 All patients received 3-week cycles of gemcitabine and paclitaxel until either a maximum of six cycles (48 patients, short-term treatment arm) or until documented disease progression (48 patients, prolonged-treatment arm). Both OS (7.8 vs 8 months) and PFS rates (4 vs 3.1 months) were comparable. Furthermore, patients experienced severe anemia, two patients died due to treatment-related AEs (TrAEs; pulmonary fibrosis and neutropenic septicemia), and six patients were withdrawn from the prolonged treatment due to disease progression and toxicity. Therefore, it was concluded that the prolonged combined regimen of gemcitabine and paclitaxel for mUCB in second-line settings was not feasible.
Atezolizumab as second-line/salvage therapy for patients who progressed during or after PBCT had comparable OR and OS rates to other agents. Moreover, as described earlier, atezolizumab treatment is associated with durable response, has a favorable safety profile with low incidence of grade 3–5 AEs, and does not necessitate any dose adjustments for the majority of comorbidities, as it allows for treatment in spheres that cytotoxic agents do not; therefore, it seems the best choice for second-line therapy of mUC in the light of currently available literature.
In addition to atezolizumab, other ICPIs are currently of interest in cancer treatment, and their role has also been investigated for UC. A Phase I trial with avelumab, an anti-PDL1 antibody, was carried out in 129 patients with mUC who had progressed after PBCT or were platinum-ineligible.Citation83 The ORR was 16%, the incidence rate of grade 3/4 AEs 7%, and only one death (or grade 5 AE) occurred, due to pneumonitis. A Phase III trial with avelumab is currently recruiting patients to evaluate its role for maintenance therapy in mUCB patients who have not progressed during or following first-line systemic therapy (NCT02603432); primary outcomes of the study are expected in July 2019.Citation84 Durvalumab, another PDL1 antibody, was studied in a Phase I/II trial, which included 61 patients with mUC.Citation85 The ORR was 31%, with median time of response not reached. Interestingly, 46.4% of the patients with positive PDL1 expression showed response, while none of the PDL1-negative patients responded. Grade 3 AEs occurred only in 4.9% of patients, and grade 4 or 5 AEs were not seen. A Phase III trial of durvalumab is currently recruiting patients with stage IV UC to evaluate it as a monotherapy and a combined therapy with tremelimumab (CTLA4 inhibitor) versus standard-of-care chemotherapy (gemcitabine + cisplatin or gemcitabine + carboplatin) in first-line treatment, and might lead to the first first-line approval for immunotherapeutics in bladder cancer (NCT02516241).Citation86
Nivolumab, an anti-PD1 antibody, was evaluated in 270 mUC patients who progressed or recurred following first-line systemic therapy (CheckMate-275 study).Citation87 The ORR and median OS were 19.6% (15%–24.9%) and 8.7 months, respectively. While response rates were associated with numerically higher ORRs and OS in patients with high PDL1 expression (PDL1 ≥5%: 28.4% and 11.3 months vs 16.1% and 5.95 months, respectively), patients with little or no PDL1 expression still showed ORRs of over 10% as well as durable responses. Grade 3/4 TrAEs occurred in 17.8% of patients, and three patients died from treatment-related pneumonitis, acute respiratory failure, and acute cardiovascular failure in this trial. As of February 2017, Nivolumab is the second FDA approved agent for treatment of mUCB after failure of PBCT.
Pembrolizumab, an anti-PD1 antibody and an approved agent for treatment of metastatic melanoma and metastatic non-small-cell lung cancer, has been tested in mUC patients resistant to, or who have progressed during, PBCT (NCT02256436/KEYNOTE-045).Citation88 This Phase III clinical trial started on October 2014, and aimed to compare efficacy and safety of pembrolizumab 3 mg/kg every 3 weeks with investigator choice of second-line paclitaxel, docetaxel, or vinflunine, thus offering the first comparative data in this space. Recently, the trial was stopped early, since it had demonstrated superiority of pembrolizumab over chemotherapeutics and met the primary end point (improved OS) already at interim analyses. Overall, 542 patients were randomized in a 1:1 fashion to either treatment group, and the ORR for the total cohort was 21.1% without difference with respect to PDL1 expression.Citation89 The rate of complete remissions was 7%. While PFS was not significantly different between pembrolizumab and chemotherapy, OS was significantly different, with a hazard ratio of 0.73 (0.59–0.91) and a median OS of 10.3 (8–11.8) vs 7.4 (6.1–8.3) months. One-year survival probability was 43.9% vs 30.7%. Grade 3–5 AEs were observed in 15% of patients for pembrolizumab vs 49.4% for the chemotherapy arm. The current velocity in the field of immunooncology in UC is highlighted by the designation of breakthrough status of durvalumab as well as subsequent fast track approval of atezolizumab and nivolumab by the FDA.
Safety profile and side effects
According to the results reported thus far in clinical trials, atezolizumab appears to be safe and highly tolerable; however, some rare immunomediated AEs (ImAEs) have been reported. A Phase I trial in metastatic renal cell carcinoma offered patients 10–20 mg/kg IV atezolizumab every 3 weeks, comprising a total of 16 cycles.Citation90 Neither maximum-tolerated dose was reached nor dose-limiting toxicity detected. Powles et al evaluated the safety of atezolizumab in Phase Ia expansion study of 68 patients with mUCB, of whom 93% had received previous PBCT.Citation28 Approximately a third of patients had liver metastasis and impaired renal function, defined as a creatinine-clearance rate of less than 60 mL/min/1.73 m2. Atezolizumab was administered IV 15 mg/kg every 3 weeks for a median of 65 (range 1–259) days. Overall, 91.2% of patients reported AEs of any grade; nevertheless 19.1% were grade 3 or 4. Among grade 3/4 AEs, dehydration (grade 3/4, 4.4%), cerebrovascular accident (grade 3/4, 2.9%), urinary tract infection (grade 3/4, 2.9%), and anemia (grade 3/4, 2.9%) were the most common, whereas asthenia (1.5%), blood phosphorus decrease (1.5%), and thrombocytopenia (1.5%) were grade 3 AEs only. None of the patients experienced a grade 5 TrAE (or death).
Further conclusions about the safety profile of atezolizumab can be drawn from the Phase II trial.Citation12 It evaluated the safety of atezolizumab with a fixed dose of IV 1,200 mg every 3 weeks. Due to the expectation of ImAEs, the authors reported AEs in three categories (all-cause, treatment-related, immunomediated). Any-grade all-cause AEs were reported in 97% of patients, of whom 55% had grade 3–4 AEs. TrAEs of any grade and grade 3/4 were observed in 69% and 16% of patients, respectively. Among them, fatigue (any grade, 30%; grade 3/4, 2%), nausea (any grade, 14%; grade 3/4, 0), decreased appetite (any grade, 12%; grade 3/4, 2%), and pruritus (any grade, 10%; grade 3/4, <1%) were the most common. Pyrexia, diarrhea, arthralgia, vomiting, anemia, hypotension, hypertension, and colitis were less common TrAEs, with incidence of <10% for any grade and <1% for grade 3/4. No grade 5 TrAE was observed. ImAEs of any grade and grade 3/4 were observed in 7% and 5% of patients, respectively. Rash (any grade, 7%; grade 3/4, <1%) was the most common ImAE; pneumonitis, dyspnea, and elevated liver enzymes (ALT and AST) were the other ImAEs, with incidence rates of 1%–3% for both any grade and grade 3/4. Neither immunomediated nephrotoxicity nor febrile neutropenia was reported. Overall, temporary dose interruptions due to AEs were necessary for 30% of patients, and 4% of patients had to discontinue atezolizumab permanently. Up to 22% of patients were treated with systemic steroids, due to ImAEs and other AEs.
Despite overall favorable tolerability and limited follow-up, rare severe ImAEs and other AEs have been described for immunotherapies, including several fatalities. The analysis of group 1 of the IMvigor210 study, utilizing atezolizumab as a first-line therapy for mUCB patients ineligible for PBCT due to impaired renal function or ECOG score ≥2 (n=119), reported one grade 5 TrAE, due to sepsis.Citation45 In a Phase IB study of durvalumab (an anti-PDL1 antibody) in combination with tremelimumab (an anti-CTLA4 antibody) for non-small-cell lung cancer treatment, three treatment-related deaths were reported, due to complications arising from myasthenia gravis, pericardial effusion, and a neuromuscular disorder.Citation91 It is important to recognize that ImAEs appear to be a class effect of these drugs, and not dose-related. Therefore, they need to be managed in a timely manner, and will not necessarily be adequately managed by dose reduction or interruption of treatment alone, but might require systemic immunosuppression.
Dose reductions are generally not recommended for atezolizumab; however, permanent discontinuation is warranted under certain circumstancesCitation39 (). No significant drug interactions have been reported. Dose adjustments are not necessary for patients with moderate renal impairment or mild hepatic impairment or for geriatric patients. Patients should be offered liver- and thyroid-function tests periodically during treatment. Monitoring patients for signs and symptoms of infections and inflammations, such as pneumonitis, meningitis, and colitis, is also recommended. Pregnant and lactating women should be warned about potential risks to fetus and infants.
Table 2 Adverse events that warrant permanent discontinuation of atezolizumab treatment
The low incidence of AEs seen in anti-PDL1 agents like atezolizumab may be due to their unique mechanism of action. It leaves PDL2, the other ligand that interacts with PD1, uninhibited, thus decreasing the incidence of severe inflammatory reactions.Citation29,Citation92 Ipilimumab (antibody against CTLA4), on the other hand, led to such ImAEs as hypophysitis, colitis, and vitiligo in 60% of patients overall. Grade 3/4 ImAEs were as high as 10%–15%, with a need for high-dose corticosteroids and infliximab in refractory cases.Citation93 More information about the safety profile of this novel antitumoral agent may be obtained from forthcoming Phase III trials.
Perspective
While PBCT remains the standard of care for advanced or mUCB, the advent of ICPIs has ushered in a new era of immunotherapy for systemic bladder cancer. With a long history of immunotherapy in the form of BCG instillations to treat non-muscle-invasive bladder cancer and a large somatic mutational burden associated with UC, mUCB seems to be an ideal candidate for treatment with ICP inhibition. Atezolizumab, an anti-PDL1 antibody, has shown efficacy as a second-line agent for patients with mUCB who progressed after PBCT, leading to FDA approval in this setting. Ongoing trials are being performed with atezolizumab in a number of different settings for UC, as are other competing ICPIs. One potential benefit of immunotherapies is that they work by a different mechanism than chemotherapy and have different toxicities. As such, there may be an opportunity for combining therapies to enhance efficacy. Also, the combination of different immunomanipulations, as seen for example in malignant melanoma, with the combination of different ICPIs to enhance immunosensitizing and outcomes is possible in bladder cancer and presents an interesting approach.
The landscape of treatment of UCB has already shifted significantly, and based on the number of active trials with different ICPIs and combination treatments with ICPIs, further manipulations of the immunooncology axis are awaited. Further elucidation of biomarkers predictive of response to systemic treatment will guide a more personalized approach to therapies, and immunotherapies will be no different from already-existing paradigms for precision medicine.
Conclusion
Atezolizumab appears to be a safe and well-tolerated second-line agent for mUCB. In a Phase II study, atezolizumab was reported to have higher response rates than historical comparisons to other second-line agents, and in some cases patients were noted to experience durable responses. These exciting findings have led to early FDA approval of atezolizumab in this setting. While large, comparative trials are lacking to date, these are under way and will further inform us about the efficacy and safety of atezolizumab for UCB. From numerous ongoing clinical trials of this agent and other ICPIs in various clinical settings, it is likely that many other options will exist for the management of UC.
Disclosure
MB has received honoraria for advisory boards from Genentech/Roche, Bristol-Myers Squibb, and MSD, speaking fees from Bristol-Myers Squibb, and travel compensation from Bristol-Myers Squibb. LMK has received speaking fees and travel compensation from MSD. The other authors report no conflicts of interest in this work.
References
- StenzlACowanNCDe SantisMThe updated EAU guidelines on muscle-invasive and metastatic bladder cancerEur Urol200955481582519157687
- AntoniSFerlayJSoerjomataramIZnaorAJemalABrayFBladder cancer incidence and mortality: a global overview and recent trendsEur Urol20177119610827370177
- PloegMAbenKKHulsbergen-van de KaaCASchoenbergMPWitjesJAKiemeneyLAClinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer RegistryJ Urol2010183391592020083267
- MostafaMHSheweitaSAO’ConnorPJRelationship between schistosomiasis and bladder cancerClin Microbiol Rev1999121971119880476
- NielsenMESmithABMeyerAMTrends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006Cancer20141201869524122346
- AndreassenBKAagnesBGislefossRAndreassenMWahlqvistRIncidence and survival of urothelial carcinoma of the urinary bladder in Norway 1981–2014BMC Cancer20161679927737647
- AbidaWBajorinDFRosenbergJEFirst-line treatment and prognostic factors of metastatic bladder cancer for platinum-eligible patientsHematol Oncol Clin North Am2015292319328ixx25836937
- SaxmanSBPropertKJEinhornLHLong-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group studyJ Clin Oncol1997157256425699215826
- von der MaaseHHansenSWRobertsJTGemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III studyJ Clin Oncol200018173068307711001674
- BellmuntJFougerayRRosenbergJELong-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapyAnn Oncol20132461466147223419284
- OingCRinkMOechsleKSeidelCvon AmsbergGBokemeyerCSecond line chemotherapy for advanced and metastatic urothelial carcinoma – vinflunine and beyond: a comprehensive review of the current literatureJ Urol2016195225426326410730
- RosenbergJEHoffman-CensitsJPowlesTAtezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trialLancet2016387100311909192026952546
- ChenDSMellmanIOncology meets immunology: the cancer-immunity cycleImmunity201339111023890059
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- ZouWChenLInhibitory B7-family molecules in the tumour microenvironmentNat Rev Immunol20088646747718500231
- ZangXAllisonJPThe B7 family and cancer therapy: costimulation and coinhibitionClin Cancer Res20071318 Pt 15271527917875755
- KrummelMFAllisonJPCD28 and CTLA-4 have opposing effects on the response of T cells to stimulationJ Exp Med199518224594657543139
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- BertrandAKostineMBarnetcheTTruchetetMESchaeverbekeTImmune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysisBMC Med20151321126337719
- OhaegbulamKCAssalALazar-MolnarEYaoYZangXHuman cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathwayTrends Mol Med2015211243325440090
- DongHStromeSESalomaoDRTumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasionNat Med20028879380012091876
- FliesDBChenLThe new B7s: playing a pivotal role in tumor immunityJ Immunother200730325126017414316
- BergerRRotem-YehudarRSlamaGPhase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignanciesClin Cancer Res200814103044305118483370
- PatnaikAKangSPRascoDPhase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumorsClin Cancer Res201521194286429325977344
- WongRMScotlandRRLauRLProgrammed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLsInt Immunol200719101223123417898045
- MotzerRJEscudierBMcDermottDFNivolumab versus everolimus in advanced renal-cell carcinomaN Engl J Med2015373191803181326406148
- BidnurSSavdieRBlackPCInhibiting immune checkpoints for the treatment of bladder cancerBladder Cancer201621152527376121
- PowlesTEderJPFineGDMPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancerNature2014515752855856225428503
- BrahmerJRTykodiSSChowLQSafety and activity of anti-PD-L1 antibody in patients with advanced cancerN Engl J Med2012366262455246522658128
- BeckAWurchTReichertJM6th Annual European Antibody Congress 2010: November 29–December 1, 2010, Geneva, SwitzerlandmAbs20113211113221441785
- BoyerinasBJochemsCFantiniMAntibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cellsCancer Immunol Res20153101148115726014098
- MoralesAEidingerDBruceAWIntracavitary bacillus Calmette-Guérin in the treatment of superficial bladder tumorsJ Urol19761162180183820877
- DoninNMLenisATHoldenSImmunotherapy in the treatment of urothelial carcinomaJ Urol20171971142227460757
- Redelman-SidiGGlickmanMSBochnerBHThe mechanism of action of BCG therapy for bladder cancer: a current perspectiveNat Rev Urol201411315316224492433
- InmanBASeboTJFrigolaXPD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progressionCancer200710981499150517340590
- Cancer Genome Atlas Research NetworkComprehensive molecular characterization of urothelial bladder carcinomaNature2014507749231532224476821
- HerbstRSSoriaJCKowanetzMPredictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patientsNature2014515752856356725428504
- ParkJJOmiyaRMatsumuraYB7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell toleranceBlood201011681291129820472828
- Tecentriq (atezolizumab) injection for intravenous use [prescribing information]San FranciscoGenentech2016
- DengRBumbacaDPastuskovasCVPreclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitormAbs20168359360326918260
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- WolchokJDHoosAO’DaySGuidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteriaClin Cancer Res200915237412742019934295
- Hoffmann-La RocheA study of atezolizumab in patients with locally advanced or metastatic urothelial bladder cancer [IMvigor210] Available from: https://clinicaltrials.gov/ct2/show/NCT02108652. NLM identifier: NCT02108652Accessed December 6, 2016
- LoriotYRosenbergJEPowlesTBAtezolizumab (atezo) in platinum (plat)-treated locally advanced/metastatic urothelial carcinoma (mUC): updated OS, safety and biomarkers from the Ph II IMvigor210 studyAnn Oncol2016276266295
- BellmuntJBalarAGalskyMDIMvigor210: updated analyses of first-line (1L) atezolizumab (atezo) in cisplatin (cis)-ineligible locally advanced/metastatic urothelial carcinoma (mUC)Ann Oncol2016276266295
- Hoffmann-La RocheA study of atezolizumab in participants with locally advanced or metastatic urothelial bladder cancer (cohort 1) Available from: https://clinicaltrials.gov/ct2/show/NCT02951767. NLM identifier: NCT02951767Accessed December 6, 2016
- BellmuntJChoueiriTKSchutzFARosenbergJERandomized phase III trials of second-line chemotherapy in patients with advanced bladder cancer: progress and pitfallsAnn Oncol201122224524721278220
- Hoffmann-La RocheA study of atezolizumab compared with chemotherapy in participants with locally advanced or metastatic urothelial bladder cancer [IMvigor211] Available from: https://clinicaltrials.gov/ct2/show/record/NCT02302807. NLM identifier: NCT02302807Accessed November 10, 2016
- Hoffmann-La RocheThe effect of atezolizumab in combination with gemcitabine/carboplatin and gemcitabine/carboplatin alone in participants with untreated locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-based therapy [IMvigor130] Available from: https://clinicaltrials.gov/ct2/show/NCT02807636. NLM identifier: NCT02807636Accessed November 10, 2016
- Hoffmann-La RocheA phase III study of atezolizumab treatment versus observation as adjuvant therapy in patients with PD-L1 positive, high risk muscle invasive bladder cancer after cystectomy [IMvigor010] Available from: https://clinicaltrials.gov/ct2/show/NCT02450331. NLM identifier: NCT02450331Accessed November 10, 2016
- Hoffmann-La RocheSafety and pharmacology study of atezolizumab alone and in combination with bacille Calmette-Guérin (BCG) in high-risk non-muscle-invasive bladder cancer (NMIBC) participants Available from: https://clinicaltrials.gov/ct2/show/record/NCT02792192. NLM identifier: NCT02792192Accessed November 15, 2016
- Queen Mary University of LondonPreoperative MPDL3280A in transitional cell carcinoma of the bladder (ABACUS) Available from: https://clinicaltrials.gov/ct2/show/record/NCT02662309. NLM identifier: NCT02662309Accessed November 15, 2016
- University of California San FranciscoStudy of MPDL3280A in bladder cancer Available from: https://clinicaltrials.gov/ct2/show/NCT02451423. NLM identifier: NCT02451423Accessed November 15, 2016
- National Cancer Institute (NCI)Atezolizumab in treating patients with recurrent BCG-unresponsive non-muscle invasive bladder cancer Available from: https://clinicaltrials.gov/ct2/show/NCT02844816. NLM identifier: NCT02844816Accessed November 15, 2016
- Corvus PharmaceuticalsPhase 1/1B study to evaluate the safety and tolerability of CPI-444 alone and in combination with atezolizumab in advanced cancers Available from: https://clinicaltrials.gov/ct2/show/record/NCT02655822. NLM identifier: NCT02655822Accessed November 15, 2016
- Celldex TherapeuticsA study of varlilumab and atezolizumab in patients with advanced cancer Available from: https://clinicaltrials.gov/ct2/show/record/NCT02543645. NLM identifier: NCT02543645Accessed November 15, 2016
- GenentechA study of atezolizumab administered in combination with bevacizumab and/or with chemotherapy in participants with locally advanced or metastatic solid tumors Available from: https://clinicaltrials.gov/ct2/show/NCT01633970. NLM identifier: NCT01633970Accessed November 15, 2016
- Incyte CorporationA study of atezolizumab (MPDL3280A) in combination with epacadostat (INCB024360) in subjects with previously treated stage IIIB or stage IV non-small cell lung cancer and previously treated stage IV urothelial carcinoma (ECHO-110) Available from: https://clinicaltrials.gov/ct2/show/NCT02298153. NLM identifier: NCT02298153Accessed November 20, 2016
- BellesoeurAMassardCSoriaJCBiomarkers in cancer immunotherapy: analysis of clinical, histological and immunohistochemical factors associated with PD-L1 statusAnn Oncol201627Suppl 682P
- TaubeJMKleinABrahmerJRAssociation of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapyClin Cancer Res201420195064507424714771
- GainorJFShawATSequistLVEGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysisClin Cancer Res201622184585459327225694
- MadoreJVilainREMenziesAMPD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trialsPigment Cell Melanoma Res201528324525325477049
- CalleaMAlbigesLGuptaMDifferential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinomaCancer Immunol Res20153101158116426014095
- AnantharamanAFriedlanderTLuDProgrammed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patientsBMC Cancer20161674427658492
- SchehrJLSchultzZDWarrickJWHigh specificity in circulating tumor cell identification is required for accurate evaluation of programmed death-ligand 1PLoS One2016117e015939727459545
- SonpavdeGPondGRMullaneSOutcomes in patients with advanced urothelial carcinoma after discontinuation of programmed death (PD)-1 or PD ligand 1 inhibitor therapyBJU Int Epub2016104
- BellmuntJTheodoreCDemkovTPhase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tractJ Clin Oncol200927274454446119687335
- LogothetisCJDexeusFHSellaAEscalated therapy for refractory urothelial tumors: methotrexate-vinblastine-doxorubicin-cisplatin plus unglycosylated recombinant human granulocyte-macrophage colony-stimulating factorJ Natl Cancer Inst19908286676722181151
- KattanJCulineSTheodoreCDrozJPSecond-line M-VAC therapy in patients previously treated with the M-VAC regimen for metastatic urothelial cancerAnn Oncol1993497937948280662
- KimYSLeeSIParkSHA phase II study of weekly docetaxel as second-line chemotherapy in patients with metastatic urothelial carcinomaClin Genitourin Cancer2016141768126454620
- AlbersPSienerRHärtleinMGemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma: prognostic factors for response and improvement of quality of lifeOnkologie2002251475211893883
- WitteRSElsonPBonoBEastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinomaJ Clin Oncol19971525895939053481
- LogothetisCJHossanESellaADexeusFHAmatoRJFluorouracil and recombinant human interferon alfa-2a in the treatment of metastatic chemotherapy-refractory urothelial tumorsJ Natl Cancer Inst19918342852881994058
- VaughnDJBroomeCMHussainMGutheilJCMarkowitzABPhase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancerJ Clin Oncol200220493794011844814
- SweeneyCJRothBJKabbinavarFFPhase II study of pemetrexed for second-line treatment of transitional cell cancer of the urotheliumJ Clin Oncol200624213451345716849761
- OrtmannCAMazharDSecond-line systemic therapy for metastatic urothelial carcinoma of the bladderFuture Oncol20139111637165124156324
- DreicerRLiHSteinMPhase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology GroupCancer2009115184090409519536901
- TwardowskiPStadlerWMFrankelPPhase II study of aflibercept (VEGF-Trap) in patients with recurrent or metastatic urothelial cancer, a California Cancer Consortium trialUrology201076492392620646741
- NecchiAMarianiLZaffaroniNPazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trialLancet Oncol201213881081622819172
- NiegischGRetzMThalgottMSecond-line treatment of advanced urothelial cancer with paclitaxel and everolimus in a German phase II trial (AUO trial AB 35/09)Oncology2015892707825765871
- WitjesJAComperatECowanNCEAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelinesEur Urol201465477879224373477
- AlbersPParkSINiegischGRandomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment (German Association of Urological Oncology [AUO] trial AB 20/99)Ann Oncol201122228829420682548
- PatelMREllertonJAgrawalMAvelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma progressed after platinum-based therapy or platinum ineligibleAnn Oncol201627Suppl 6777PD
- PfizerA Study of avelumab in patients with locally advanced or metastatic urothelial cancer (JAVELIN Bladder 100) Available from: https://clinicaltrials.gov/ct2/show/record/NCT02603432. NLM identifier: NCT02603432Accessed November 19, 2016
- MassardCGordonMSSharmaSSafety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancerJ Clin Oncol201634263119312527269937
- AstraZenecaStudy of MEDI4736 with or without tremelimumab versus standard of care chemotherapy in urothelial cancer Available from: https://clinicaltrials.gov/ct2/show/NCT02516241. NLM identifier: NCT02516241Accessed December 6, 2016
- GalskyMDRetzMSiefker-RadtkeAOEfficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: results from the phase II CheckMate 275 studyAnn Oncol201627Suppl 6LBA31
- MerckA study of pembrolizumab (MK-3475) versus paclitaxel, doc-etaxel, or vinflunine for participants with advanced urothelial cancer (MK-3475-045/KEYNOTE-045) Available from: https://clinicaltrials.gov/ct2/show/study/NCT02256436. NLM identifier: NCT02256436Accessed November 19, 2016
- BellmuntJde WitRVaughnDJOpen-label, phase III study of pembrolizumab versus investigator’s choice of paclitaxel, docetaxel, or vinflunine for previously treated advanced urothelial cancerPoster presented at: 2016 SITC Annual MeetingNovember 9–13, 2016National Harbor, MD
- McDermottDFSosmanJASznolMAtezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase IA studyJ Clin Oncol201634883384226755520
- AntoniaSGoldbergSBBalmanoukianASafety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1B studyLancet Oncol201617329930826858122
- ChenDSIrvingBAHodiFSMolecular pathways – next-generation immunotherapy: inhibiting programmed death-ligand 1 and programmed death-1Clin Cancer Res201218246580658723087408
- FongLSmallEJAnti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatmentJ Clin Oncol200826325275528318838703
- MengXHuangZTengFXingLYuJPredictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapyCancer Treat Rev2015411086887626589760
