Abstract
Defects in the innate immune system, particularly in Toll-like receptors (TLRs), have been reported in several cigarette smoke-promoted diseases. The aim of this study was to examine the impact of tobacco smoke on allelic frequencies of TLR4 single-nucleotide polymorphisms (SNPs) and to compare the genotypic distribution of these SNPs in a Saudi Arabian population with that in previously studied populations. DNA was extracted from 303 saliva samples collected from smokers and nonsmokers. Two transitional SNPs in the promoter region of TLR4 were selected, rs2770150 (T/C) and rs10759931 (G/A). Genotype frequencies were determined using quantitative polymerase chain reaction. Our results showed a slight effect of smoking on the distribution of rs2770150 and rs10759931. However, the differences were not significant. Thus, we conclude that the SNPs selected for this study were independent of smoking and may not be related to smoking-induced diseases.
Introduction
Innate immunity, which is considered to be the first line of defense against diseases, is well studied, and its role has been clarified through study of mutations in innate immunity genes as well as of patients with various diseases such as cystic fibrosis.Citation1 In addition to induction of autoimmune diseases,Citation2 defects in the innate immune system have been reported in several other diseases such as types of cancer,Citation3 asthma,Citation4 psoriasis,Citation5 and Alzheimer’s and other neurodegenerative diseases.Citation6,Citation7 The innate immune system initially recognizes microorganisms through pattern recognition receptors, in particular Toll-like receptors (TLRs).Citation8 TLRs are a family of at least 13 transmembrane receptors that are expressed on immune cells as well as on gingival epithelial cells and are involved in the initiation of inflammatory processes.Citation9–Citation11 Upon induction by certain ligands, TLRs activate intracellular signaling pathways that promote the production of multiple immune mediators that contribute to host defense.Citation12,Citation13 Several polymorphisms have been reported at different positions in TLR genesCitation14,Citation15 and were found to be associated with inflammatory diseases.Citation16,Citation17 Single-nucleotide polymorphisms (SNPs) are a class of polymorphisms involving single-base substitutions.Citation18 SNPs are thought to constitute the majority of sequence variants in human beings,Citation19 and they occur approximately once every 300 bases.Citation20 Several reports have demonstrated the role of TLR SNPs in the development of cancer.Citation21,Citation22 TLR4, which is located on chromosome 9, encodes a protein that plays a critical role in the immune system through recognition of lipopolysaccharides found in Gram-negative bacteria.Citation23,Citation24 TLR4 polymorphisms have been reported to be involved in different infectious and noninfectious diseases.Citation25,Citation26 In particular, the TLR4 SNPs rs2770150 and rs10759931 have been detected in association with many health complications. We previously demonstrated that TLR4 polymorphisms, specifically the SNPs rs2770150 and rs10759931, are associated with colon cancer.Citation27 Additionally, variations in the rs2770150 SNP were found to affect antibody response to whole-cell pertussis vaccination.Citation28 Furthermore, these variations alter the level of susceptibility to pollution-induced asthma.Citation29 The rs10759931 SNP was demonstrated to be associated with latent tuberculosis infection and subsequent pulmonary tuberculosis.Citation30 Many of these diseases have been shown to be caused by tobacco smoke, indicating the importance of examining the effects of smoking on these SNPs.Citation31–Citation33 The huge number of health problems and risks associated with tobacco smoke are widely known, including COPD,Citation34 different types of cancer,Citation35,Citation36 and periodontal diseases.Citation37 Smoking was found to induce epigenetic and genetic alterations that, in turn, may lead to the initiation of different diseases, including those described earlier.Citation38 Smoking may cause either transition or transversion mutations.Citation39 However, transitions are reported to generate more radical amino acid changes than transversions.Citation40 The aim of this study was to examine the impact of tobacco smoke on allelic frequencies of TLR4 rs2770150 and rs10759931 transitional SNPs and to compare the genotypic distribution of these SNPs in a Saudi Arabian population with that in other previously studied populations.
Materials and methods
Saliva collection
Saliva samples were collected from a total of 126 nonsmokers and 177 smokers. Samples were collected from male students and staff at King Saud University (KSU) between January and April 2015. From each participant, 2 mL of saliva was collected in a 15-mL falcon tube. The clinical data for these samples are listed in . This study was reviewed by the College of Applied Medical Sciences’ research ethics committee at KSU and was granted the approval number CAMS 13/3536. Each participant provided informed consent and completed a written survey. Data included in the survey comprised age, number of cigarettes smoked per day, years of smoking, and body mass index (BMI).
Table 1 Clinical characteristics of study subjects
DNA extraction
Each saliva sample was diluted with two volumes of phosphate-buffered saline immediately after collection. DNA extraction was performed using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA). A NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the DNA concentration and quality. Then, the DNA samples were stored at −20°C for later application.
Genotyping
Each DNA sample was diluted to 10 ng/µL before use in the genotyping experiments. Two transitional TLR4 SNPs were selected: rs2770150 (T/C) and rs10759931 (G/A). Few data are available regarding the association of the selected SNPs with different diseases. However, these SNPs were selected because they occur in the promoter region () and thus regulate TLR4 expression. Reactions were performed using 20 ng of DNA mixed with 5.6 µL of TaqMan® Genotyping Master Mix (Applied Biosystems, Foster City, CA, USA) and 0.2 µL of 40× TaqMan® SNP Genotyping assay (Applied Biosystems), using a QuantStudio™ 7 Flex Real-Time PCR System thermal cycler (Applied Biosystems). The amplification protocol included 40 cycles as follows: a pre-read stage for 30 sec at 60°C, a hold for 10 min at 95°C, amplification for 15 sec at 95°C and 1 min at 60°C, and a post-read stage for 30 sec at 60°C.
Table 2 Description of the selected SNPs
Statistical analysis
Genotypic and allelic frequencies were calculated and checked for deviation from Hardy–Weinberg equilibrium, as described in our previous work.Citation41 Case–control and other genetic comparisons were performed using the chi-square test and allelic odds ratios (ORs), and 95% confidence intervals (CIs) were calculated with Fisher’s exact test (two-tailed). Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 22.0 software (SPSS, Chicago, IL, USA). P-values ≤0.05 were considered significant.
Results
Characteristics of the study population
As shown in , the smokers and nonsmokers did not differ significantly in BMI, because the selection criteria for this study were independent of patient weight. In addition, approximately two-thirds of the samples were collected from students rather than staff, and the smoker and nonsmoker groups did not differ significantly in age. Thus, no genotyping analysis was conducted regarding these parameters. Given that the average age at which participants started smoking was 18.3 years and that half of the smokers (85/176) were less than 24 years of age, we separated the smoker participants into the following two groups: those who had smoked for >5 years and those who had smoked for ≤5 years. Approximately half of the smokers (71/159) consume 20 cigarettes (ie, one pack) per day, and we classified the smokers into the following two categories: those who consume ≥20 cigarettes per day and those who daily consume <20 cigarettes. The characteristics of the subjects are summarized in .
Genotypic patterns of TLR4 SNPs among smokers and nonsmokers
A total of 303 saliva samples, 177 from smokers and 126 from nonsmokers, were included in this study to investigate the effects of tobacco smoke on the genotypic distribution of TLR4 rs2770150 and rs10759931 SNPs. The homozygous ancestral alleles, TT in rs2770150 and AA in rs10759931, were used as references for the genotyping analysis. The allelic frequencies in nonsmokers and smokers, ORs, 95% CIs, chi-square results, and P-values are listed in . Neither SNP was significantly associated with smoking behavior. The genotypic distribution of rs2770150 was 49% TT, 38% TC, and 13% CC in nonsmokers compared to 54% TT, 35% TC, and 11% CC in smokers. The allele frequencies for rs10759931 were 12% AA, 32% AG, and 56% GG in nonsmokers and 8% AA, 37% AG, and 55% GG in smokers.
Table 3 Genotype frequencies of TLR4 gene polymorphism in smoker and control patients
Long- and short-term smoking and their impacts on TLR4 polymorphisms
The smokers were divided into two groups based on years of smoking: group A (>5 years) and group B (≤5 years). lists the genotypic frequencies and subsequent analysis of the SNPs for each group compared to nonsmokers. No correlation was observed between the SNPs and either long-term or short-term smokers. The genotypic allocation of the rs2770150 SNP in group A was 49% TT, 38% TC, and 13% CC in nonsmokers and 57% TT, 34% TC, and 9% CC in smokers. In group B, however, 53% TT, 33% TC, and 15% CC were observed in smokers compared to 49% TT, 38% TC, and 13% CC in nonsmokers. For the rs10759931 SNP, the genotypic frequencies in group A were 12% AA, 32% AG, and 56% GG in nonsmokers and 8% AA, 36% AG, and 56% GG in smokers. Allele frequencies for this SNP in group B were 8% AA, 32% AG, and 59% GG in smokers and 12% AA, 32% AG, and 56% GG in nonsmokers.
Table 4 Comparison of genotype frequencies of TLR4 gene SNPs with overall controls depending on smoking duration
Association between individual SNPs and the intensity of smoking
Allele frequency data for the smokers were also analyzed with regard to the quantity of cigarettes consumed per day. Here, we identified two categories: category A (≥20 cigarettes/day) and category B (<20 cigarettes/day). The SNP genotypes of both categories of smokers were compared to those of nonsmokers (); no significant differences were observed. For the rs2770150 SNP, the genotypes of smokers in category A were 61% TT, 29% TC, and 10% CC and the genotypes of smokers in category B were 45% TT, 42% TC, and 13% CC. Both categories were compared to nonsmokers, in whom the allele frequencies were 49% TT, 38% TC, and 13% CC. For the rs10759931 SNP, nonsmokers showed allele frequencies of 12% AA, 32% AG, and 56% GG. The genotypic distribution of category A smokers for this SNP was 9% AA, 37% AG, and 54% GG. In category B smokers for the same SNP, the allele frequencies were 7% AA, 33% AG, and 60% GG.
Table 5 Genotype frequencies of TLR4 gene SNPs with overall controls according to the daily quantity of cigarettes
Differentiation between the Saudi Arabian population and others and linkage studies
The genotyping results for nonsmokers were used to compare the Riyadh region population in Saudi Arabia (CRS), from which we collected our samples, with other previously studied populations (). Of 126 samples, we determined the rs2770150 SNP genotypes for 112 samples and the rs10759931 SNP genotypes for 118 samples. The frequency of the various alleles of rs2770150 differed significantly between Chinese (Han Chinese in Beijing), Japanese (Japanese in Tokyo), Nigerian (Yoruba in Ibadan), Kenyan (Maasai in Kinyawa), and Italian (Toscans) populations, and the Saudi population (P<0.005 for most). For TLR4 rs10759931, the CRS population differed significantly from African Americans (D-0) and North Americans (coronary artery bypass graft; P<0.05). Additionally, we constructed linkage disequilibrium plots for both the TLR4 rs2770150 and rs10759931 SNPs using SNP Annotation and Proxy Search (SNAP; http://www.broadinstitute.org/mpg/snap/ldplot.php; and ). The maximum r2 values for rs2770150 and rs10759931 were 0.958 and 0.965, respectively.
Figure 1 Regional LD plot for the TLR4 rs2770150 SNP.
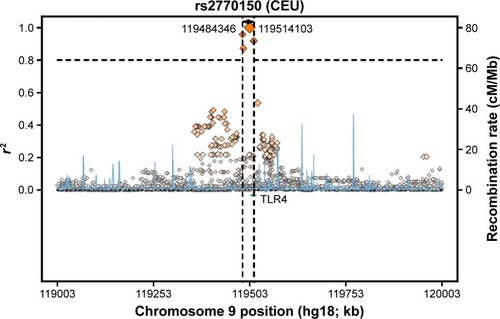
Figure 2 Regional LD plot for rs10759931 SNP in TLR4.
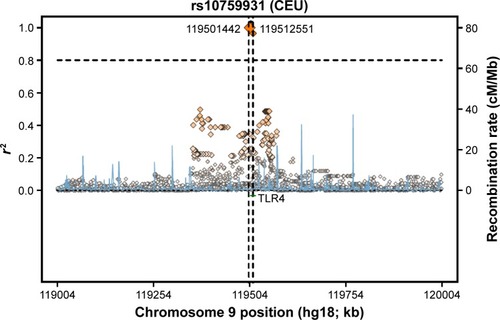
Table 6 Allele and genotype frequencies of TLR4 gene polymorphisms in the Riyadh region compared to other populations
Discussion
Several studies have assessed the genetic changes following cigarette smoke exposure, typically identifying changes in innate immunity genes. Others have evaluated changes in gene expression of gingival epithelial cells in response to cigarette smoke.Citation42 Previous studies showed a clear link between smoking cigarettes and the pathogenesis of many diseases, in particular, COPD, oral cancer, and periodontal disease. Additionally, we have previously shown that smoking tobacco affects TLR4 expression via different pathways.Citation42 Impairment of TLR4 signaling becomes evident through the presence of SNPs that are associated with cancer susceptibility, and we have recently described an association between TLR4 polymorphism and colon cancer development.Citation27 Although they could have either positive or negative effects, polymorphisms in TLR4 have been reported in various diseases.Citation25,Citation26,Citation43,Citation44 These diseases and others have been found to be caused by tobacco smoke.Citation31–Citation33
In the present study, we showed that smoking has a slight effect on the rs2770150 and rs10759931 SNPs of TLR4. However, no significant association was observed between cigarette smoking and the genetic distribution of the SNPs investigated. Our results are contradictory to those previously published that show that the TLR4 rs2770150 and rs10759931 SNPs are associated with different diseases.Citation27–Citation30 The lack of significant results may be explained by the lack of association between smoking and genetic variation in TLR4 rs2770150 and rs10759931 SNPs. Other SNPs in TLR4, especially those in regulatory regions or exons, may be associated with various diseases related to smoking. Thus, although these SNPs may not be related to smoking-induced diseases, we recommend performing other studies on SNPs located in the exons of TLR4.
Comparison of the data for the TLR4 rs2770150 SNP between the Riyadh population and other populations showed a pattern similar to that reported for SNPs located in other genes, such as the Thr241Met SNP in X-ray repair cross-complementing group 3 (XRCC3),Citation45 which reinforces the historical hypothesis of early human migration out of Africa.Citation46 The imbalance between the protective/affective effects of polymorphism is a key factor in the development of smoking-related diseases in human beings. Further investigations in larger populations of the same or mixed ethnicity could help to define the effects of smoking on different genes involved in the human innate immune system. Further insight into the genetic factors affected by smoking could lead to new approaches for cessation or prevention of smoking and treatment of many diseases caused by tobacco.
Acknowledgments
This study was supported by funding from the NSTIP Strategic Technologies Program (number 12 MED 2443) in the Kingdom of Saudi Arabia.
Disclosure
The authors report no conflicts of interest in this work.
References
- LevyJAThe importance of the innate immune system in controlling HIV infection and diseaseTrends Immunol200122631231611377290
- BachmannMFKopfMOn the role of the innate immunity in autoimmune diseaseJ Exp Med200119312F47F5011413199
- NowarskiRGaglianiNHuberSFlavellRAInnate immune cells in inflammation and cancerCancer Immunol Res201312778424777498
- SchroderNWArditiMThe role of innate immunity in the pathogenesis of asthma: evidence for the involvement of Toll-like receptor signalingJ Endotoxin Res200713530531217986489
- SweeneyCMTobinAMKirbyBInnate immunity in the pathogenesis of psoriasisArch Dermatol Res20113031069170521863252
- HenekaMTGolenbockDTLatzEInnate immunity in Alzheimer’s diseaseNat Immunol201516322923625689443
- HenekaMTKummerMPLatzEInnate immune activation in neurodegenerative diseaseNat Rev Immunol201414746347724962261
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell2006124478380116497588
- OldenburgMKrügerAFerstlRTLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modificationScience201233760981111111522821982
- SugawaraYUeharaAFujimotoYToll-like receptors, NOD1, and NOD2 in oral epithelial cellsJ Dent Res200685652452916723649
- RichAMHussainiHMParachuruVPSeymourGJToll-like receptors and cancer, particularly oral squamous cell carcinomaFront Immunol2014546425309546
- NarayananKBParkHHToll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathwaysApoptosis201520219620925563856
- MaruGBGandhiKRamchandaniAKumarGThe role of inflammation in skin cancerAdv Exp Med Biol201481643746924818733
- SunQZhangQXiaoHPBaiCToll-like receptor polymorphisms and tuberculosis susceptibility: a comprehensive meta-analysisJ Huazhong Univ Sci Technolog Med Sci201535215716825877346
- SkevakiCPararasMKostelidouKTsakrisARoutsiasJGSingle nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseasesClin Exp Immunol2015180216517725560985
- TakahashiMChenZWatanabeKToll-like receptor 2 gene polymorphisms associated with aggressive periodontitis in JapaneseOpen Dent J2011519019422235236
- ChanMJiSMLiawCSAssociation of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese populationBreast Cancer Res Treat2012136120922022965832
- ThomasPEKlingerRFurlongLIHofmann-ApitiusMFriedrichCMChallenges in the association of human single nucleotide polymorphism mentions with unique database identifiersBMC Bioinformatics201112suppl 4S4
- CollinsFSBrooksLDChakravartiAA DNA polymorphism discovery resource for research on human genetic variationGenome Res1998812122912319872978
- KeXTaylorMSCardonLRSingleton SNPs in the human genome and implications for genome-wide association studiesEur J Hum Genet200816450651518197193
- ZhengSLAugustsson-BälterKChangBSequence variants of Toll-like receptor 4 are associated with prostate cancer risk: results from the Cancer Prostate in Sweden StudyCancer Res20046482918292215087412
- KutikhinAGYuzhalinAEAre Toll-like receptor gene polymorphisms associated with prostate cancer?Cancer Manag Res20124232922359464
- KildingRAkilMTillSA biologically important single nucleotide polymorphism within the Toll-like receptor-4 gene is not associated with rheumatoid arthritisClin Exp Rheumatol200321334034212846053
- WengPHHuangYLPageJHPolymorphisms of an innate immune gene, Toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysisPLoS One2014910e11056925360682
- El-OmarEMNgMTHoldGLPolymorphisms in Toll-like receptor genes and risk of cancerOncogene200827224425218176606
- XuYJiangZHuangJMengQCohPTaoLThe association between Toll-like receptor 4 polymorphisms and diabetic retinopathy in Chinese patients with type 2 diabetesBr J Ophthalmol20159991301130525947554
- SemlaliAReddy ParineNArafahMExpression and polymorphism of Toll-like receptor 4 and effect on NF-kappaB mediated inflammation in colon cancer patientsPLoS One2016111e014633326771524
- BanusSBottemaRWSiezenCLToll-like receptor 4 polymorphism associated with the response to whole-cell pertussis vaccination in children from the KOALA studyClin Vaccine Immunol200714101377138017699831
- KerkhofMPostmaDSBrunekreefBToll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthmaThorax201065869069720685742
- WuLHuYLiDJiangWXuBScreening Toll-like receptor markers to predict latent tuberculosis infection and subsequent tuberculosis disease in a Chinese populationBMC Med Genet2015161925928077
- GillilandFDIslamTBerhaneKRegular smoking and asthma incidence in adolescentsAm J Respir Crit Care Med2006174101094110016973983
- HeGLiYZhaoFThe prevalence and incidence of latent tuberculosis infection and its associated factors among village doctors in ChinaPLoS One2015105e012409725996960
- FerraraGMurrayMWinthropKRisk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNF alpha drugsCurr Opin Pulm Med201218323324022388583
- BozinovskiSVlahosRAnthonyDCOPD and squamous cell lung cancer: aberrant inflammation and immunity is the common linkBr J Pharmacol2015173463564826013585
- GutierrezASuhRAbtinFGenshaftSBrownKLung cancer screeningSemin Intervent Radiol201330211412024436526
- CorralRLewingerJPVan Den BergDComprehensive analyses of DNA repair pathways, smoking and bladder cancer risk in Los Angeles and ShanghaiInt J Cancer2014135233534724382701
- El-ZaatariZMChamiHAZaatariGSHealth effects associated with waterpipe smokingTob Control201524suppl 1i31i4325661414
- SteenaardRVLigthartSStolkLTobacco smoking is associated with methylation of genes related to coronary artery diseaseClin Epigenetics2015715426015811
- AcevedoABrodskyLAndinoRMutational and fitness landscapes of an RNA virus revealed through population sequencingNature2014505748568669024284629
- FarrellAColemanBIBenenatiBWhole genome profiling of spontaneous and chemically induced mutations in Toxoplasma gondiiBMC Genomics20141535424885922
- AlanaziMPathanAAAbduljaleelZAssociation between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi populationPLoS One2013812e8554124392019
- SemlaliAWitoledCAlanaziMRouabhiaMWhole cigarette smoke increased the expression of TLRs, HBDs, and proinflammory cytokines by human gingival epithelial cells through different signaling pathwaysPLoS One2012712e5261423300722
- ZhuLYuanHJiangTWangRMaHZhangSAssociation of TLR2 and TLR4 polymorphisms with risk of cancer: a meta-analysisPLoS One2013812e8285824376595
- MinminSXiaoqianXHaoCSingle nucleotide polymorphisms of Toll-like receptor 4 decrease the risk of development of hepatocellular carcinomaPLoS One201164e1946621559380
- AlanaziMPathanAAAjajSADNA repair genes XRCC1, XRCC3, XPD, and OGG1 polymorphisms among the central region population of Saudi ArabiaBiol Res201346216116723959014
- AlsmadiOJohnSETharejaGGenome at juncture of early human migration: a systematic analysis of two whole genomes and thirteen exomes from Kuwaiti population subgroup of inferred Saudi Arabian tribe ancestryPLoS One201496e9906924896259
