Abstract
The aim of this study was to investigate the association of the common polymorphisms of Toll-like receptor 4 (TLR-4) with breast cancer development in the Saudi Arabian population. Four TLR-4 polymorphisms (rs2770150, rs10759931, rs10759932, and rs4986790) were studied using 127 breast cancer patients and 117 controls. Relative expression of TLR-4 protein in the breast tumor and the matched normal breast tissues was determined in a large cohort of 70 clinical breast samples in a tissue micro-array format by immunohistochemistry using a specific anti-TLR-4 antibody. Our results demonstrated an increase in TLR-4 expression in estrogen receptor (ER)−, postmenopausal breast cancer patients compared to normal. We also demonstrated that the G allele of single-nucleotide polymorphism rs10759931 was found to be significantly higher in frequency among patients (36.3%) compared to the control group (26.7%), suggesting that this polymorphism is strongly associated with the development of breast cancer in this ethnic population. In addition, the TLR-4 polymorphism rs2770150 was shown to be highly correlated with breast cancer in patients over 48 years of age. The TLR-4 polymorphism rs4986790 was also found to be associated with this malignancy in the ER− patient groups. Our results suggested firstly that the variation in TLR-4 gene expression may influence breast cancer development and secondly a closely linked association between TLR-4 gene polymorphism and ER status. Our study provides support for a better understanding of the implication of TLR-4 polymorphism in breast tumorigenesis and for its eventual use as a cancer biomarker.
Introduction
Breast cancer is one of the most common cancers that threaten women’s lives around the world, with approximately a 3% chance that a woman will die of breast cancer.Citation1 Dynamic interplay between tumors and the immune system is essential for tumor survival, growth, and metastasis.Citation2 Development of an invasive cancer is the result of genetic and epigenetic changes within the host. Studies provide clear evidence that genetic variants could be implicated in increasing breast cancer risk.Citation3,Citation4 The immune system is known to play a major role in preventing tumor progression by recognizing tumor antigens, and dysregulation of immune system activity could contribute to tumorigenesis.Citation5 A dynamic association between immune response and breast cancer is important for its progression and incidence.Citation6–Citation8 Recent studies have shown that dysregulation of innate immunity receptors such as Toll-like receptors (TLRs) can promote cancer progression.Citation9–Citation12 To date, ten functional TLRs have been identified in humans as playing an active role in innate immunity through inflammatory cytokines such as interleukin (IL)-1 and IL-8. TLRs are also expressed in many cancer cells and are known to play a major role in tumor progression through regulating cell proliferation and survival.Citation13,Citation14 Of this TLR family, TLR-4 is a major TLR that plays an important role in regulating inflammation and immune responses through activating nuclear factor kappa-light-chain enhancer of activated B cells.Citation15 This Toll protein is a key receptor for lipopolysaccharide (LPS) from Gram-negative bacteria and is crucial for upregulation of mechanisms of defense against bacterial infections. Several other pathogen-associated molecular patterns, including fusion protein from respiratory syncytial virus and envelope protein from mouse mammary tumor virus, can also stimulate TLR-4 signaling.Citation16,Citation17 In addition to activating the MyD88-dependent pathway, TLR-4 is the only TLR that can activate the MyD88-independent pathway via its TLR domain-containing adaptor.Citation18 TLR-4 has received special attention in cancer research due to its role in tumor progression. It has been previously shown that TLR-4 is expressed in many cancer cells such as human melanoma cells,Citation19 human gastric cancer cells,Citation20 and human head and neck squamous cell carcinoma.Citation21 In addition, TLR-4 has also been linked to breast cancer. Previous studies have demonstrated that TLR-4 depletion leads to increased tumor progression and metastasis in a murine model of breast cancer.Citation9 There is evidence that genetic single-nucleotide polymorphisms (SNPs) may cause the impairment of immune responses, and consequently, the development of infectious diseases. The association between the development of infections and chronic inflammation could increase cancer risk. Few associations were observed between TLR genes and risk of developing breast cancer. Several studies highlighted the implication of TLR polymorphisms in pathogenesis of tumors. It was demonstrated that polymorphisms in TLR-2 and TLR-4 were associated with gastric cancer.Citation22,Citation23 Increasing bodies of evidence have suggested that TLR polymorphism is closely related to inflammation and may increase the risk of developing gastric malignancy.Citation22,Citation24,Citation25 TLR-4 polymorphism was also connected with colon cancerCitation26 and hepatitis C virus-induced hepatocellular carcinoma.Citation27 In addition, a polymorphism in TLR-10, TLR-6, TLR-2, and TLR-1 was shown to be associated with increasing susceptibility of prostate cancer.Citation28,Citation29 Other studies have also presented the correlation between TLR-2 polymorphism and papillary thyroid cancer in the Korean population.Citation30 Recently, several studies have demonstrated that the presence of the TLR-4 Asp299Gly polymorphism is associated with a higher frequency of prostate cancer in the North Indian population.Citation31 It has also been shown that TLRs are implicated in the progression of polyps to tumors and that increased metastatic potential of colorectal cancers was correlated with reduced TLR expression.Citation31,Citation32 TLR-4 polymorphism rs11536898 has also been shown to be connected with colon cancer.Citation26 The TLR-4 Asp299Gly polymorphism and TLR-2 Thr399Ile polymorphism were also observed to be associated with an increased risk of breast cancer.Citation33,Citation34 In addition, TLR-1 and TLR-6 polymorphisms have been linked with increased susceptibility of breast cancer in the African-American population.Citation35,Citation36 The present study aims to elucidate the potential correlation of the TLR-4 SNP (rs2770150, rs10759931, rs10759932, and rs4986790) with the risk of breast cancer in the Saudi Arabian population.
Materials and methods
Sample collection
Blood samples were collected from 127 subjects with histologically confirmed breast cancer, recruited among patients from King Faisal Medical City (KFMC) in Riyadh, Kingdom of Saudi Arabia (KSA). A detailed description of the breast cancer population is shown in . All subjects provided written informed consent to participate in the study and to approve the use of their biological samples for genetic analyses. The design of the study was approved by the local Ethics Committee (number 15-089E) from KFMC, Riyadh. Study subjects provided information on their lifestyle habits, diabetes, and family/personal history of cancer via a structured questionnaire in accordance with the guidelines of the Ethics Committee at KFMC in Riyadh, KSA, to determine demographic characteristics and potential risk factors for breast cancer development. The immunohistochemical (IHC) assay was performed on the fresh tissue samples. The genomic DNA was extracted from blood samples for genotyping.
Table 1 Clinical characteristics of study subjects
IHC array
IHC was performed on 3- to 5-mm thick sections obtained from formalin-fixed tissue embedded in paraffin. Histopathological representative tumor areas were defined on hematoxylin/eosin-stained sections. The array blocks were obtained as described elsewhere.Citation37 About 5-μm sections from each tumor tissue block were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany). Once the slides were ready, they were incubated for 15–20 minutes in a hot air oven at 60°C. Anti-TLR-4 antibody (H-80, sc-10741; Santa Cruz Biotechnology, CA, USA) was used at 1/100 dilution. Different steps and analyses of IHC were then performed as previously described by Semlali et al.Citation38
DNA extraction
Three hundred microliters of whole blood was used to extract genomic DNA by using DNA Blood Mini Kit (Qiagen, Qilden, Germany) according to the manufacturer’s instructions. After equilibration of blood at room temperature with protease, the blood samples were incubated at 56°C for 10 minutes. Appropriate volume of absolute ethanol was added, and the mixture was spun through the column. The column membrane was washed, and the DNA was eluted with 100 μL elution buffer. The concentration and the purity of DNA were determined by Nano-Drop 800 spectrophotometer (Thermo Fisher Scientific).
Genotyping
TLR-4 SNPs (rs2770150, rs10759931, rs10759932, and rs4986790) were assessed using TaqMan assay as previously described.Citation39 The positional information of the SNPs is described in . For this purpose, about 10 ng of DNA from each sample was used per reaction with 5.6 μL of 2X Universal Master Mix and 200 nM primers. All genotypes were determined by using an ABI 7500 real-time PCR machine.
Statistical analysis
As described previously by Semlali et al,Citation38 genotype and allelic frequencies were compared using Fisher’s exact test (two-tailed) to calculate the χ2 value with Yates corrections, and allelic odds ratios (ORs) with 95% confidence intervals (CIs). Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) 16.0 software for Windows.
Results
Analysis of clinical data parameters
A total of 127 breast cancer cases and 117 healthy controls were enrolled in the study. The clinical characteristics of the patients, including age, nationality, estrogen receptor (ER) status, family history, and stage of breast cancer, were recorded and compared to the control patients. The study population was divided into two age groups: under 48 years and over 48 years ().
Evaluation of expression of TLR-4 protein in breast cancer tissues
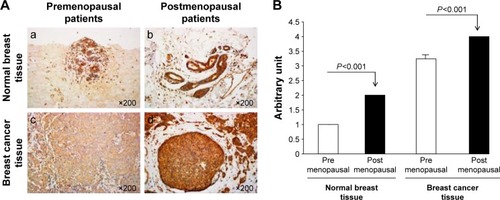
TLR-4 has been reported as an indicator of various types of cancers. In the present study, we investigated whether the expression of TLR-4 in breast cancer has any prognostic value in the Saudi population and whether TLR-4 expression is related to the TLR-4 polymorphism specially located in the regulatory regions (promoter and 5′ upstream). The expression of TLR-4 protein in breast cancer and normal tissues was determined in a large cohort of 70 clinical breast samples in a tissue micro-array format by IHC. As shown in , the levels of TLR-4 protein were very low in normal breast tissues. The strength of positive staining gradually increased from ER− premenopausal patients in situ to postmenopausal patients (). The expression of TLR-4 was found to be significantly associated with clinical parameters such as ER− pre- or postmenopausal age.
Figure 1 Immunohistochemical determination of TLR-4 protein expression in normal and breast cancer tissues. (A) Representative sections (×200) of positive immunostaining of TLR-4 protein expression in normal breast tissues (a and b) and ER− breast cancer tissues (c and d). The staining of samples from ER− premenopausal patients is illustrated in (a and c) and from ER− postmenopausal patients in (b and d). Tissues were immunostained using specific TLR-4 antibodies. (B) TLR-4 positive staining was estimated as follows: 0 point, no positive staining; 1 point, <20% positive staining; 2 points, 21%–50% positive staining; 3 points, 51%–75% positive staining; and 4 points, >75% positive staining.
Interaction between TLR-4 polymorphism (rs2770150, rs10759931, rs10759932, and rs4986790) and breast cancer risk in the study populations
DNA samples obtained from the blood of 127 breast cancer patients and 117 healthy controls were included in this study. Distribution of TLR-4 SNPs genotype and allele frequencies in breast cancer cases and control population are given in . Four TLR-4 SNPs (rs2770150 T/C, rs10759931 A/G, rs10759932 T/C, and rs4986790 A/G) were genotyped and tested for the Hardy–Weinberg equilibrium. The distribution of alleles and genotypes and the association analysis are reported in . The data for three TLR-4 SNPs, rs2770150, rs10759932, and rs4986790, located in the promoter, did not present any correlation with breast cancer in the Saudi population (). However, the frequencies of rs10759931 SNP (Asp299Gly) in breast cancer cases were significantly different from the healthy controls. The G allele was found to be significantly higher in frequency among the patients (36%) compared to the control group (27%) (). These data suggested that TLR-4 Asp299Gly SNP is strongly associated with increased susceptibility to breast cancer in the Saudi Arabian population.
Table 2 Frequencies of TLR-4 gene polymorphism in breast cancer
Link between pre- and postmenopause status and the genotype frequencies of TLR-4 gene polymorphism in breast cancer patients
The analysis of the TLR-4 genotype distribution after correlation with age indicated that the median age of onset of breast cancer patients included in the study was 48 years. To investigate the association of TLR-4 SNPs with a younger age at diagnosis of breast cancer, we classified the patients as aged ≤48 or >48 years. The genotype distribution for the individual SNP along with the statistical analysis is given in and . Interestingly, patients over 48 years of age showed higher numbers of the C allele of SNP rs2770150 than healthy subjects (43% versus 28%, respectively) (OR 1.985; 95% CI 1.177–3.346 and P=0.00956). The prevalence of the homozygous CC of the same SNP was also significantly increased in the patient group compared to the control group (24% versus 2%, respectively) (). However, we did not find any association between this SNP and the subpopulation of patients <48 years of age (). Additionally, no significant difference in the distribution of genotypes between patients and healthy controls for both subpopulations was observed (P>0.05) for the other three SNPs (rs10759931, rs10759932, and rs4986790; and ).
Table 3 Genotype frequencies of TLR-4 in breast cancer patients aged <48 years versus control
Table 4 Genotype frequencies of TLR-4 in breast cancer patients aged >48 years versus control
Association between the genotype frequencies of TLR-4 gene polymorphism and ER status
We next wanted to evaluate the association of breast cancer risk with the individual SNPs based on the patient’s ER status. The genotype distributions in the ER+ and the ER− breast cancer patient groups were compared with those of the ER+ and ER− control subjects, respectively ( and ). Interestingly, in the ER− group, the AA genotype of SNP rs4986790 presented a frequency that was significantly higher in the patients than in the controls (98% versus 88%, respectively). Similarly, the genotype AG was significantly less frequent in the cases compared to the controls (2% versus 12%, respectively) (OR 0.16; 95% CI 0.020–1.257 and P=0.04860) (). However, this SNP was not associated with breast cancer in the ER+ group (). For SNPs rs2770150, rs10759931, and rs10759932, no significant difference in the distribution of genotypes between affected and non-affected ER+ and ER− status was observed (P>0.05) ( and ).
Table 5 Genotype frequencies of TLR-4 in ER− breast cancer patients versus control
Table 6 Genotype frequencies of TLR-4 in ER+ breast cancer patients versus control
Discussion
TLR-4 activation has been previously shown to promote tumor progression and chemoresistance in breast cancer,Citation40,Citation41 whereas impairment of the TLR-4 signaling pathway can reduce breast cancer growth and prolong survival.Citation9 Our hypothesis is that TLR-4 plays an important role in activating innate immune system and that dysregulation of this receptor compromises the immune surveillance leading to breast cancer progression and metastasis. Using a Saudi population, we demonstrated that TLR-4 is overexpressed in ER−, postmenopausal breast cancer patients compared to normal controls. Thus, an increase in TLR-4 could be seen as a biological indicator of potential cancer in ER−, postmenopausal patients. Interestingly, the presence of a higher level of TLR-4 protein in breast cancer tissue supports previous studies reporting an overexpression of TLR-4 in various types of cancers, including gastric cancer,Citation42 breast cancer,Citation40,Citation41,Citation43 colon cancer,Citation38,Citation43 and ovarian cancer.Citation44 Higher TLR-4 expression was also shown to be significantly associated with invasive ductal carcinoma, one of the most common types of breast cancer.Citation45 Evidence has demonstrated that TLR-4 activation promotes tumor cell adhesion and metastasis.Citation46,Citation47 Injection of 4T1 tumor cells into TLR-4−/− mice leads to increased tumor volume and lung metastasis.Citation9 In agreement with these studies, we suggest that high activation of TLR-4 in breast cancerous tissues may promote tumor growth and resistance to apoptosis.
Genetic alterations in the TLR pathway are also known to be associated with an increased risk of developing breast cancer.Citation48 This is the first study investigating the role of several common TLR-4 polymorphisms in susceptibility to breast cancer in Saudi Arabia. We identified that the TLR-4 polymorphism rs10759931 could be a risk factor for this disease in the Saudi Arabian population. This polymorphism affects the population regardless of ER status or age group. In contrast, the TLR-4 polymorphism rs2770150 is correlated with breast cancer in patients 48 years and older, which is in agreement with the decreased levels of female sex hormones during the postmenopausal period. However, MarinoCitation49 has linked the crucial role of estrogen and progesterone in protecting against colon cancer particularly in women patients.Citation49–Citation52 Our previous study showed that TLR-4 rs2770150 is associated with colon cancer in postmenopausal Saudi women.Citation38 The TLR-4 polymorphism rs4986790 is associated with this malignancy in the ER− patient groups. These two SNPs were shown to be strongly associated with prostate cancerCitation53 and gastric cancerCitation54 in other studies and in other populations. TLR-4 rs4986790 is the most common polymorphism, which causes an amino acid exchange (aspartate to glycine) at position 299 (Asp200Gly) located within the extracellular domain of the receptor. This polymorphism causes a significant local conformational change at the D299G site,Citation55 which may abolish ligand binding, and consequently, impair the TLR-4 signaling response to LPS.Citation56 This polymorphism was also shown to be associated with an increased risk of breast cancer.Citation33 Recently, we have demonstrated that these same TLR-4 SNPs were associated with TLR-4 expression and colon cancer susceptibility in Saudi patients.Citation38 Because TLR-4 plays an active role in the innate immune system, the loss of function of this protein may lead to a depressed immune response, thus promoting cancer development.
In conclusion, our study shows for the first time that TLR-4 polymorphism rs10759931 is strongly associated with risk of breast cancer in the Saudi population. Our data suggest that genetic variation in TLR-4 may influence the development of this disease. We believe that our study provides support for a better understanding of the implication of TLR-4 polymorphism in breast tumorigenesis and for its eventual use as a cancer biomarker.
Abbreviations
| CI | = | confidence interval |
| ER | = | estrogen receptor |
| IHC | = | immunohistochemistry |
| IL | = | interleukin |
| KFMC | = | King Faisal Medical City |
| KSA | = | Kingdom of Saudi Arabia |
| LPS | = | lipopolysaccharide |
| OR | = | odds ratio |
| SNP | = | single-nucleotide polymorphism |
| TLR | = | Toll-like receptor. |
Acknowledgments
This project was supported by the Research Group Program (number RGP-VPP-260) in the KSA.
Supplementary material
Table S1 Characteristics of selected TLR-4 polymorphisms
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts & Figures 2013 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2013.htmlAccessed February 8, 2017
- YuHKortylewskiMPardollDCrosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironmentNat Rev Immunol200771415117186030
- MavaddatNAntoniouACEastonDFGarcia-ClosasMGenetic susceptibility to breast cancerMol Oncol20104317419120542480
- YuJCDingSLChangCHGenetic susceptibility to the development and progression of breast cancer associated with polymorphism of cell cycle and ubiquitin ligase genesCarcinogenesis20093091562157019587092
- de VisserKEEichtenACoussensLMParadoxical roles of the immune system during cancer developmentNat Rev Cancer200661243716397525
- YangCXLiCYFengWToll-like receptor 4 genetic variants and prognosis of breast cancerTissue Antigens201381422122623510418
- MaFJLiuZBHuXPrognostic value of myeloid differentiation primary response 88 and Toll-like receptor 4 in breast cancer patientsPLoS One2014910e11163925360699
- YangHWangBWangTToll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasisPLoS One2014910e10998025299052
- AhmedAWangJHRedmondHPSilencing of TLR4 increases tumor progression and lung metastasis in a murine model of breast cancerAnn Surg Oncol201320Suppl 3S389S39622890596
- BergmannCBachmannHSBankfalviAToll-like receptor 4 single-nucleotide polymorphisms Asp299Gly and Thr399Ile in head and neck squamous cell carcinomasJ Transl Med2011913921854645
- BhateliaKSinghKSinghRTLRs: linking inflammation and breast cancerCell Signal201426112350235725093807
- RodríguezNCKaakoushNKhean-LeeGMingFKMitchellHPattern recognition receptors in helicobacter pylori-related gastric cancerHelicobacter20141985
- BasithSManavalanBYooTHKimSGChoiSRoles of toll-like receptors in cancer: a double-edged sword for defense and offenseArch Pharm Res20123581297131622941474
- DrexlerSKFoxwellBMThe role of Toll-like receptors in chronic inflammationInt J Biochem Cell Biol201042450651819837184
- SchreibeltGTelJSliepenKHToll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapyCancer Immunol Immunother201059101573158220204387
- Kurt-JonesEAPopovaLKwinnLPattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virusNat Immunol20001539840111062499
- RassaJCMeyersJLZhangYKudaravalliRRossSRMurine retroviruses activate B cells via interaction with toll-like receptor 4Proc Natl Acad Sci U S A20029942281228611854525
- HuangBZhaoJLiHToll-like receptors on tumor cells facilitate evasion of immune surveillanceCancer Res200565125009501415958541
- MolteniMMarabellaDOrlandiCRossettiCMelanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4Cancer Lett20062351758315922507
- RenTWenZKLiuZMLiangYJGuoZLXuLFunctional expression of TLR9 is associated to the metastatic potential of human lung cancer cell: functional active role of TLR9 on tumor metastasisCancer Biol Ther20076111704170917986857
- SzczepanskiMJCzystowskaMSzajnikMTriggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attackCancer Res20096973105311319318560
- de OliveiraJGSilvaAEPolymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian populationWorld J Gastroenterol201218111235124222468087
- MilneANCarneiroFO’MorainCOfferhausGJNature meets nurture: molecular genetics of gastric cancerHum Genet2009126561562819657673
- Garza-GonzalezEBosques-PadillaFJMendoza-IbarraSIFlores-GutierrezJPMaldonado-GarzaHJPerez-PerezGIAssessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8-25 I polymorphisms in the risk for the development of distal gastric cancerBMC Cancer200777017462092
- TaharaTArisawaTWangFToll-like receptor 2-196 to 174del polymorphism influences the susceptibility of Japanese people to gastric cancerCancer Sci200798111790179417711514
- SlatteryMLHerrickJSBondurantKLWolffRKToll-like receptor genes and their association with colon and rectal cancer development and prognosisInt J Cancer2012130122974298021792899
- AgúndezJAGarcía-MartínEDevesaMJPolymorphism of the TLR4 gene reduces the risk of hepatitis C virus-induced hepatocellular carcinomaOncology2012821354022286521
- StevensVLHsingAWTalbotJTGenetic variation in the toll-like receptor gene cluster (TLR10-TLR1-TLR6) and prostate cancer riskInt J Cancer2008123112644265018752252
- MandalRKGeorgeGPMittalRDAssociation of Toll-like receptor (TLR) 2, 3 and 9 genes polymorphism with prostate cancer risk in North Indian populationMol Biol Rep20123977263726922311043
- KimMKParkSWKimSKAssociation of Toll-like receptor 2 polymorphisms with papillary thyroid cancer and clinicopathologic features in a Korean populationJ Korean Med Sci201227111333133823166414
- PriyadarshiniAChakrabortiAMandalAKSinghSKAsp299Gly and Thr399Ile polymorphism of TLR-4 gene in patients with prostate cancer from North IndiaIndian J Urol2013291374123671363
- NiedzielskaINiedzielskiZTkaczMToll-like receptors and the tendency of normal mucous membrane to transform to polyp or colorectal cancerJ Physiol Pharmacol200960Suppl 16571
- AhmedARedmondHPWangJHLinks between Toll-like receptor 4 and breast cancerOncoimmunology201322e2294523526132
- ApetohLTesniereAGhiringhelliFKroemerGZitvogelLMolecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapiesCancer Res200868114026403018519658
- El-OmarEMNgMTHoldGLPolymorphisms in Toll-like receptor genes and risk of cancerOncogene200827224425218176606
- Barnholtz-SloanJSShettyPBGuanXFGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger womenCarcinogenesis20103181417142320554749
- ParkerRLHuntsmanDGLesackDWAssessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarrayAm J Clin Pathol2002117572372812090420
- SemlaliAReddy ParineNArafahMExpression and polymorphism of Toll-like receptor 4 and effect on NF-κB mediated inflammation in colon cancer patientsPLoS One2016111e014633326771524
- AlanaziMPathanAAAbduljaleelZAssociation between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi populationPLoS One2013812e8554124392019
- YangHZhouHFengPReduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretionJ Exp Clin Cancer Res2010299220618976
- RajputSVolk-DraperLDRanSTLR4 is a novel determinant of the response to paclitaxel in breast cancerMol Cancer Ther20131281676168723720768
- YuanXZhouYWangWActivation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS productionCell Death Dis20134e79424030146
- TangXZhuYTLR4 signaling promotes immune escape of human colon cancer cells by inducing immunosuppressive cytokines and apoptosis resistanceOncol Res2012201152423035361
- KellyMGAlveroABChenRTLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancerCancer Res20066673859386816585214
- EhsanNMuradSAshiqTSignificant correlation of TLR4 expression with the clinicopathological features of invasive ductal carcinoma of the breastTumour Biol20133421053105923338716
- WangJHManningBJWuQDBlanksonSBouchier-HayesDRedmondHPEndotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanismJ Immunol2003170279580412517943
- LiaoSJZhouYHYuanYTriggering of Toll-like receptor 4 on metastatic breast cancer cells promotes αvβ3-mediated adhesion and invasive migrationBreast Cancer Res Treat2012133385386322042369
- ReslerAJMaloneKEJohnsonLGGenetic variation in TLR or NFkappaB pathways and the risk of breast cancer: a case-control studyBMC Cancer20131321923634849
- MarinoMXenoestrogens challenge 17β-estradiol protective effects in colon cancerWorld J Gastrointest Oncol201463677324653796
- BarziALenzAMLabonteMJLenzHJMolecular pathways: estrogen pathway in colorectal cancerClin Cancer Res201319215842584823965904
- al-AzzawiFWahabMEstrogen and colon cancer: current issuesClimacteric20025131411974557
- HendrickseCWJonesCEDonovanIANeoptolemosJPBakerPROestrogen and progesterone receptors in colorectal cancer and human colonic cancer cell linesBr J Surg19938056366408518910
- ChenYCGiovannucciELazarusRKraftPKetkarSHunterDJSequence variants of Toll-like receptor 4 and susceptibility to prostate cancerCancer Res20056524117711177816357190
- SantiniDAngelettiSRuzzoAToll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypesClin Exp Immunol2008154336036418826495
- OhtoUYamakawaNAkashi-TakamuraSMiyakeKShimizuTStructural analyses of human Toll-like receptor 4 polymorphisms D299G and T399IJ Biol Chem201228748406114061723055527
- FigueroaLXiongYSongCPiaoWVogelSNMedvedevAThe Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of adapter proteins MyD88 and TRIFJ Immunol201218894506451522474023
