Abstract
Glioblastoma is the most common form of malignant brain tumors and has a poor prognosis. Glioma stem cells (GSCs) are thought to be responsible for the aberrant proliferation and invasion. Targeting the signaling pathways that promote proliferation in GSCs is one of the strategies for glioma treatment. In this study, we found increased expression of contactin 2 (CNTN2) and amyloid β precursor protein (APP) in U87-derived GSCs (U87-GSCs). RNA interference (RNAi) for CNTN2 downregulated the expression of APP intracellular domain (AICD), which is the proteolytic product of APP. Treatment with CNTN2 RNAi inhibited the proliferation of U87-GSCs. CNTN2 RNAi decreased the expression of epidermal growth factor receptor and HES1, which are potential targets of AICD. In summary, inhibition of the CNTN2/APP signaling pathway may repress the proliferation in U87-GSCs via downregulating the expression of HES1 and epidermal growth factor receptor. CNTN2/APP/AICD signaling pathway plays an important role in U87 glial tumorigenesis. Further studies are warranted to elucidate the role of these signaling pathways in other sources of GSCs. Depending on their role in proliferation in other sources of GSCs, members of the CNTN2/APP/AICD signaling pathway may provide novel targets for the development of therapy for glioblastomas.
Introduction
Glioma is the most common primary tumor of the central nervous system. It accounts for 80% of malignant brain tumors in the US.Citation1 Glioblastoma multiforme (GBM) represents the most malignant form and accounts for 46% of malignant brain tumors in the US.Citation1 The standard treatment for glioma is surgical resection followed by radiotherapy, chemotherapy, or biotherapy. Despite improved therapies, the prognosis of patients remains poor. The ongoing research suggests that the genesis, development, and relapse of gliomas may originate from the aberrant proliferation of a subpopulation of cells, the glioma stem cells (GSCs). GSCs can proliferate indefinitely, self-renew, and initiate tumor, which make them different from differentiated tumor cells. They are becoming a main target for the treatment of gliomas.Citation2–Citation4
Though it is not clear whether the GSCs originate from neural stem cells (NSCs) or differentiated astrocytes, all glioma cells have the potential to develop stemness, depending on their microenvironment.Citation5 The U87 cell is an established cell line from a male patient with grade IV glioblastoma with known genome sequence.Citation6 Recently, a U87-derived GSC (U87-GSC) subline has been isolated and has become a broadly used model to study the pathological mechanisms and pharmacological therapy of gliomasCitation7–Citation14 due to its stemness characteristics of self-renewal, high proliferative potential, and tumor initiation capacity.Citation3
Targeting GSC signaling pathways or niche for proliferation is one of the strategies for glioma treatment.Citation15 Understanding the molecular mechanisms of GSC development is essential to identify novel molecular targets for the treatment of gliomas. Previous studies with U87-GSCs or other GSCs suggest that GSCs share many characteristics with NSCs, including expression of Nestin and CD133 and activation of Notch, epidermal growth factor receptor (EGFR), JAK/STAT, PI3K/AKT, and mGlu3 receptor signaling pathways.Citation7,Citation8,Citation10,Citation14,Citation16–Citation19 Activation of Notch pathway is correlated with the chemoresistance or radioresistance of GSCs.Citation17 A recent study in NSCs demonstrates that transient TAG1, or contactin 2 (CNTN2) as a functional ligand of amyloid β precursor protein (APP) can trigger the γ-secretase-dependent release of endogenous APP intracellular domain (AICD) and inhibit neurogenesis in a concentration-dependent manner.Citation20,Citation21 These findings raise the possibility that CNTN2–APP interaction may play a similar role in GSCs, which could be a potential signaling pathway target for glioma therapy.
CNTN2 is a glycophosphatidylinositol (GPI)-linked neuronal membrane protein, a member of the immunoglobulin superfamily. CNTN2 as a cell adhesion molecule has been implicated in the axon growth and myelination.Citation22–Citation24 Its mutation is associated with cortical myoclonic tremor and epilepsy. Its association to epilepsy is attributed to the interaction of CNTN2 with contactin-associated protein-like 2 (CNTNAP2), which is necessary to maintain voltage-gated potassium channels at the juxtaparanodal region.Citation25 Aberrant expression of CNTN2 also correlates with glial tumorigenesis.Citation26,Citation27 Blocking CNTN2 expression can inhibit the migration of glioma cells.Citation27 CNTN2 may also promote the proliferation of granule cells in the cerebellum by regulating the sonic hedgehog (SHH) pathway.Citation28 Since CNTN2 promotes proliferation in granule cells and inhibits neurogenesis in NSCs, we hypothesize that CNTN2 can stimulate proliferation of U87-GSCs, and CNTN2 RNA interference (RNAi) can inhibit the proliferation of U87-GSCs.
APP is an integral type I transmembrane protein widely distributed in the brain and involved in signal transduction, cell adhesion, and neuronal migration, differentiation, and regeneration. Its product β amyloid is associated with Alzheimer’s disease (AD).Citation29 APP is cleaved by γ-secretase to produce AICD, which could regulate phosphoinositide-mediated calcium signaling,Citation29 inhibit neurogenesis,Citation20,Citation21 and inhibit Wnt signaling.Citation30 APP expression is significantly increased in gliomas.Citation31 Therefore, we hypothesize that the anti-proliferation function of CNTN2 RNAi involves the APP/AICD pathway in U87-GSCs.
Analogous to APP, Notch is another membrane receptor that is cleaved by γ-secretase to produce the Notch intracellular domain (NICD).Citation32 The Notch/HES1 pro-proliferation pathway is activated in gliomas or GSCs, which may contribute to the radioresistance of GSCs. Inhibition of the Notch/HES1 pathway may induce cell autophagy.Citation33–Citation35 Cross talk between APP/AICD and Notch/NICD involves AICD overexpression, which activates Notch-targeting gene HES1. NICD overexpression activates APP-targeting gene KAI1.Citation36 CD133+GSCs have the characteristics of NSCs with neurosphere-like growth and asymmetrical division, and they express all three lineages of neural markers: glial fibrillary acidic protein (GFAP), β-tubulin III, and O1. CD133+ GSCs comprise 1%–20% of glioblastomas.Citation37 The CD133+ GSCs express more vascular endothelial growth factor (VEGF) than CD133− cells.Citation38 Furthermore, the activation of EGFR/PI3K/AKT pathway promotes the invasion of CD133+ GSCs;Citation8 cross talk between APP/AICD and EGFR involves AICD binding to EGFR promoter and regulates its expression.Citation39 Based on the earlier observations, we hypothesize that the anti-proliferation function of CNTN2 RNAi may also affect the Notch/HES1 and EGFR/PI3K/AKT pathways in U87-GSCs by reducing the expression of HES1 and EGFR as downstream targets of APP/AICD and ultimately inhibiting GSC proliferation.
In this study, we first isolated and characterized U87-GSCs and then showed the upregulation of CNTN2 and APP expressions in these cells. Next, we showed that CNTN2 RNAi could inhibit the proliferation of U87-GSCs and downregulate the AICD expression. Finally, we found that CNTN2 RNAi could also downregulate the expression of EGFR and HES1, which are downstream targets of AICD. This suggests that CNTN2 may stimulate the proliferation of U87-GSCs through activation of APP/AICD, Notch/HES1, and EGFR/PI3K/AKT pathways; CNTN2 could be an ideal upstream target for the development of glioma therapy.
Materials and methods
GSC culture and characterization
The human glioma cell line U87 (previously known as U87-MG)Citation6 was purchased from the Cell Library of the Chinese Academy of Sciences (Shanghai, China). U87 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; HyClone, Utah, USA) supplemented with 10% fetal bovine serum in a humidified incubator with 5% CO2 at 37°C and routinely passaged at 2- to 3-day intervals. U87-GSCs were obtained through stemness-associated tumor sphere formation by culturing U87 cells in serum-free DMEM/F12 medium (HyClone) supplemented with 2% B27 (Gibco, NY, USA), 20 ng/mL epidermal growth factor (EGF; PeproTech Inc., NJ, USA), and 20 ng/mL basic fibroblast growth factor (bFGF; PeproTech Inc.) with pH adjusted to 7.4. U87-GSCs were enriched by incubation in 1% O2 tension, as described by Liu et al.Citation40 The tumor spheres after the fifth passage (P5) were harvested for experiments.
Expression of GSC markers in tumor spheres was detected by immunocytochemistry and flow cytometry. Tumor spheres and differentiated cells were incubated in precoated 24-well culture plates. After washing with phosphate-buffered saline (PBS), the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 15 min. After blocking for 1 h with 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, USA), the attached cells were incubated with primary antibodies at 4°C overnight. The primary antibodies included CD133 (1:200; Santa Cruz, LA, USA) and nestin (1:500; Millipore, Massachusetts, USA) for tumor spheres and β-tubulin III (1:1000; Millipore), GFAP (1:250; Millipore), and O1 (1:500; Millipore) for the differentiated cells. After the primary antibody incubation, the cells were washed with PBS three times and incubated with the corresponding secondary antibodies (horseradish peroxide [HRP]-anti-mouse 1:500 or HRP-anti-rabbit 1:500) for 30 min at room temperature. After development with diaminobenzidine, the nuclei were counterstained with Mayer’s hematoxylin. Images were captured with a camera on the microscope (Olympus).
RNAi, lentivirus construction, and infection
To knockdown CNTN2 expression in U87-GSCs, we designed four RNAi target sequences for the human CNTN2 gene (NM_005076.3; ). The annealed oligo sequences were cloned into the empty plasmid pcDNA™ 6.2-GW/EmGFP-miR. Intact RNAi target sequences inserted into the expression vectors pcDNA 6.2-GW/EmGFP-miR-CNTN2-RNAi-1/-2/-3/-4 were confirmed by sequencing. The interference plasmids and the packaging plasmids (Packaging Mix) were co-transfected into 293T cells to make lentivirus carrying the CNTN2-RNAi or EGFP. The expression of EGFP was used to determine the titer and multiplicity of infection (MOI) to infect all cells, which was defined as an MOI of 100. An MOI of 100 was used in the next experiments.
Table 1 CNTN2 RNAi sequences
U87-GSCs (3×105) were dissociated into single cells and transferred to T25 Corning cell culture flasks (Corning Incorporated, Corning, NY, USA) with the sphere-growing medium. After 24 h, the cells were infected with lentivirus carrying RNAi or EGFP, and the medium was replaced 8 h after infection. After 48 h, the cells were harvested for the next experiments. The effects of CNTN2-RNAi in U87-GSCs were tested by Western blot with antibody specific for CNTN2 (1:800; Santa Cruz). The infection efficiency of lentivirus for both control group (EGFP) and experimental group (CNTN2-RNAi) was the same (100%), and the expression levels of EGFP normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were the same for both groups (data not shown).
Quantification of U87-GSC sphere diameters and numbers
Ten days after the RNAi lentiviral infection, we counted the number and measured the diameters of the U87-GSC spheres in three randomly chosen fields containing spheres. Images were captured with a Leica DMIRE2 inverted phase-contrast microscope with 10× objective lens and analyzed by ImageJ photo software.
Cell counting kit 8 (CCK-8) assay for cell proliferation
CCK-8 (Dojindo, Kumamoto, Japan) was used to detect cell proliferative activity in vitro. The U87-GSCs were dissociated into single cells, and they (200 cells/well) were plated into 96-well plates with 200 μL of medium. After 48, 72, and 96 h, CCK-8 assays were performed following the instructions of the manufacturer. The optical density (OD) was read by using a microplate reader (BIO-RAD 680) at 450 nm.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol (Invitrogen). After purification, the total RNA (1.0 μg) was reverse-transcribed into complementary DNA (cDNA) with a total volume of 20 μL per well in a reverse transcriptase reaction system (Takara, USA). The cDNA was subjected to real-time PCR amplification by using SYBR Premix Ex Taq™ (Takara) and the indicated primer sequences (). The relative expression level of messenger RNA (mRNA) of the target genes (CT [threshold cycle]) was determined by normalizing against the expression level of GAPDH.
Table 2 Primer sequences
Western blot
Cells were lysed with a lysis buffer supplemented with protease inhibitor mixture (KEYGEN Biochemicals, Nanjin, China). Proteins in samples were measured, and equal amounts of proteins (20 μg) were separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (10%) and were transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% BSA, Western blots were incubated with the primary antibodies: anti-CNTN2 antibody (1:800; Santa Cruz), anti-APP antibody (1:1000; Abcam, Cambridge, England), anti-EGFR antibody (1:200; Santa Cruz), and anti-HES1 antibody (1:200; Santa Cruz) at 4°C overnight. After rinsing, the blots were incubated with HRP-conjugated secondary antibodies (1:1000; Boster Biotechnology, Wuhan, China), and the enhanced chemiluminescence plus Western blotting detection system (Beyotime Biotechnology) was used to visualize the immune bands.
The detection of AICD by Western blot was as described by Ma et al.Citation20 In brief, the proteins were extracted with 5× tricine sample buffer containing protease inhibitor mixture (KEYGEN Biochemicals) and loaded on to 12% SDS-tricine polyacrylamide gel for electrophoresis after sonication and boiling. After transfer, the PVDF membranes were warmed by intermittent microwave irradiation (5×10 s irradiation at 5-min intervals) and processed for the subsequent procedures. The anti-APP C terminal antibody (1:2000, A8717; Sigma-Aldrich) was used as the primary antibody for AICD detection.
Statistical analysis
All experiments were repeated three times. The quantitative data were presented as mean ± standard deviation (SD) for each group. Student’s t-test was used to compare the mean in experiments with two groups. One-way analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) tests were used as post hoc for comparisons in experiments with more than two groups. If the homogeneity hypothesis failed, Dunnett’s t3 test was used to analyze the significant differences between groups. Two-way ANOVA was used in the CCK-8 experiments. The significance level of all tests was set at P≤0.05. Statistical analysis was performed by using IBM SPSS Version 20 (SPSS Statistics V20; IBM Corporation, Somers, NY, USA).
Results
Characterization of U87-GSCs
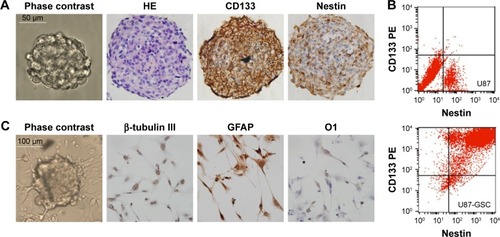
Glioblastomas are composed of heterogenous population of cells, including GSCs and differentiated astrocytes. To target the GSCs in glioblastoma therapy, we first need to understand the molecular pathology of these cells; thus, a GSC model with a pure population of cells, true stemness, and easy enrichment is necessary. For this purpose, we harvested the U87-GSCs (suspending sphere culture of U87 cells) and increased the stemness by hypoxia conditioning. After 1 month and five passages of culture in serum-free medium and 1% O2 tension, the U87-GSCs grew into suspending spheres with diameters ~120 μm (, phase contrast). These cells had healthy nuclei indicated by hematoxylin and eosin (HE) staining (, HE) and were mostly positive for CD133 and Nestin, the specific markers for GSCs or NSCs (, CD133, Nestin). The ratio of CD133+ and Nestin+ cells among total GSCs was 91.99%±0.24% for U87-GSCs and 0.54%±0.31% for U87 cells, as shown by the flow cytometry assay (). To identify the multilineage differentiation potential of the U87-GSCs, tumor spheres were cultured in serum-containing medium for 7 days and tested by immunocytochemistry with β-tubulin III, GFAP, and O1 antibodies, the markers for differentiated neurons, astrocytes, and oligodendrocytes, respectively. All three lineages of NSCs positively stained cells in the differentiated U87-GSCs population (), suggesting the capability of U87-GSCs can differentiate into three neural cell lineages.
Figure 1 Characterization of U87-GSCs.
Abbreviations: U87-GSCs, U87-derived glioma stem cells; HE, hematoxylin and eosin; GFAP, glial fibrillary acidic protein; PE, phycoerythrin.
Increased CNTN2 and APP expressions in GSCs-U87 cells
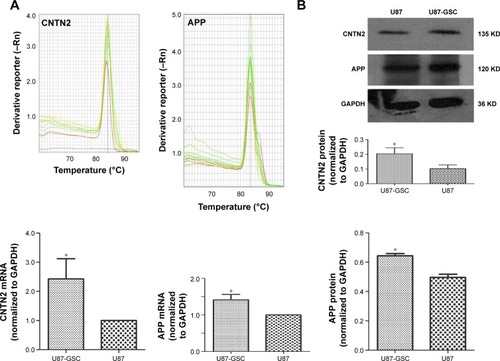
As mentioned earlier, CNTN2 and APP expressions have been observed in primary glioma cells, established glioma lines, and NSCs; CNTN2–APP interaction regulates proliferation of NSCs. To investigate the possible involvement of CNTN2 and APP in the growth regulation of U87-GSCs, we first examined their expression in U87-GSCs cells by qRT-PCR and Western blot. CNTN2 and APP mRNA levels in U87-GSCs cells were significantly increased (t=−3.581, P=0.023, and t=−4.913, P=0.039, respectively) than those in U87 cells. Protein levels of CNTN2 and APP were also significantly increased (t=−3.560, P=0.024, and t=−9.839, P=0.001) (). The change of CNTN2 (~1-fold) was higher than that of APP (~0.2-fold). These data showed that U87-GSCs supported higher CNTN2 and APP transcription and stable protein levels than U87 cells.
Figure 2 Expression of CNTN2 and APP in U87-GSCs and U87 cells.
Abbreviations: CNTN2, contactin 2; APP, amyloid β precursor protein; U87-GSCs, U87-derived glioma stem cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CNTN2 RNAi inhibited AICD expression and proliferation in U87-GSCs
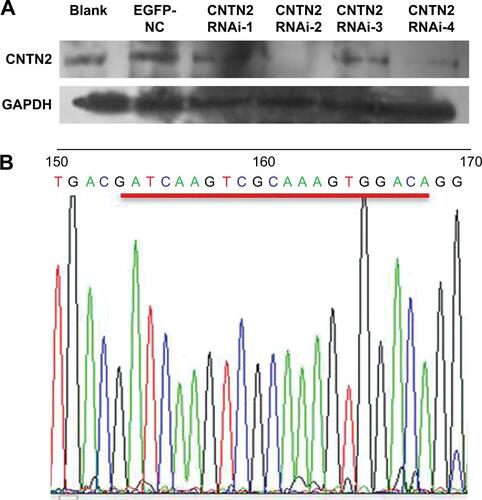
Since CNTN2 binds APP, activates AICD, and inhibits neurogenesis in NSCs, the CNTN2-APP/AICD pathway may also exist in GSCs. To test the hypotheses that CNTN2 can stimulate proliferation of U87-GSCs and that CNTN2 RNAi can inhibit the proliferation of U87-GSCs through APP/AICD, we designed and engineered vectors that express one of four CNTN2 RNAi sequences. We tested their effects on selectively silencing CNTN2 expression in U87-GSCs. After infecting the U87-GSCs with lentivirus carrying one of four different CNTN2 RNAi sequences, we screened the most effective RNAi by Western blot. Compared to other CNTN2 RNAi vectors, CNTN2 RNAi-2-containing vector had the strongest silencing effect (Figure S1).
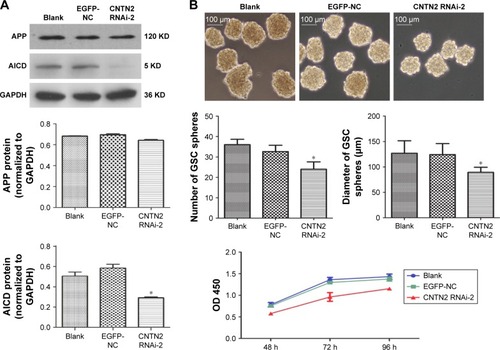
Thus, we used CNTN2 RNAi-2 to block the CNTN2 expression in U87-GSCs and observed its effects on AICD expression and sphere growth in these cells. As expected, the AICD expression in U87-GSCs was significantly downregulated by CNTN2 RNAi-2; however, the APP expression was not affected (). However, the growth of U87-GSC spheres was inhibited by CNTN2 RNAi-2, with reduced sphere number and size and a slower growth curve (). These results suggest that CNTN2 RNAi can inhibit the proliferation of GSCs and AICD expression, and CNTN2 may function through AICD to regulate proliferation in U87-GSCs.
Figure 3 CNTN2 RNAi inhibited AICD expression and U87-GSCs proliferation.
Abbreviations: CNTN2, contactin 2; RNAi, RNA interference; AICD, APP intracellular domain; U87-GSCs, U87-derived glioma stem cells; APP, amyloid β precursor protein; OD, optical density; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CNTN2 RNAi inhibited EGFR and HES1 expressions in U87-GSCs
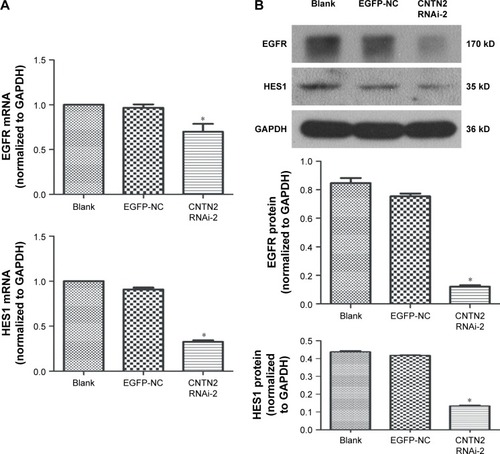
To further investigate the mechanisms of CNTN2/APP/AICD signaling in regulating the proliferation of U87-GSCs and the potential downstream targets of AICD, we tested the hypothesis that CNTN2 RNAi can inhibit EGFR and HES1 expressions in U87-GSCs. Expression levels of EGFR and HES1 were both significantly downregulated as shown by qRT-PCR () and Western blot (). These data suggest that CNTN2 may regulate proliferation of U87-GSCs through EGFR and HES1.
Figure 4 CNTN2 RNAi inhibited EGFR and HES1 expressions in U87-GSCs.
Abbreviations: CNTN2, contactin 2; RNAi, RNA interference; EGFR, epidermal growth factor receptor; U87-GSCs, U87-derived glioma stem cells; qPCR, quantitative polymerase chain reaction; mRNA, messenger RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
CD133 is the most commonly used cell surface marker for GSCs. CD133+ cells from gliomas are capable of multilineage differentiation, have a high capacity for sphere formation, express significantly higher levels of NSC genes, and can form tumor after intracranial transplantation into mice.Citation3 Increased expression of antiapoptotic genes in CD133+ GSCs may contribute to the resistance of GBM to chemotherapy,Citation40 and Nestin+ GSCs are a subpopulation resistant to chemotherapy.Citation16 In our model of U87-GSCs, the CD133+/Nestin+ cells comprised ~90% of total cells; thus, it is a relatively purified population with characteristics of NSCs.
In this study, we used the standard sphere-forming method to grow U87-GSCs. The tumor spheres were enriched in serum-free culture supplemented with growth factors. There might be different populations of GSCs in the beginning of the culture, but after continuous passages, the faster subpopulation should overgrow the slower sub-population in the microenvironment promoting growth. The O2 tensions in solid tumors can range from 0.1% to 5.3%, with the lowest levels in the center region.Citation41,Citation42 Severe hypoxia can inhibit the proliferation, whereas mild hypoxia may promote the proliferation of GSCs.Citation43 Mild hypoxia can increase VEGF and hypoxia-inducing factor (HIF) expression in GSCs, and it stimulates Notch signaling pathway: these three effects support the proliferation of GSCs.Citation4 Our culture with 1% oxygen tension at mild range helped to promote the sphere growth of U87-GSCs, and we can enrich the spheres over five passages in 1 month. However, sphere culture is limited by increasing differentiation and cell death in the larger sizes of spheres. Laminin-coated culture could be an alternative choice.Citation3
In the GSCs model, GSCs rather than differentiated cells are the origins of tumorigenic cells. The proliferation and apoptosis of GSCs thus play an important role in the development of malignancy. Several signaling pathways involved in cell proliferation and apoptosis, such as Notch/HES1 and EGFR/PI3K/AKT signaling pathways, have been proposed to be important in GSCs.Citation8,Citation33 Another closely related pathway, CNTN2/APP/AICD, has been found in NSCs, where CNTN2 as the functional ligand of APP can activate the release of downstream proteolytic signal AICD and inhibit neurogenesis.Citation20,Citation21 Therefore, we intended to examine these signaling pathways in our model of U87-GSCs.
Both CNTN2 and APP are expressed in gliomas. Rickman et alCitation27 have reported that increased expression of CNTN2 was found both in middle- and high-grade glioma cells from clinical patients and in glioma cell lines. Culicchia et alCitation31 demonstrated that APP expression levels were significantly higher in primary glioma cells and glioblastoma cell lines than in primary neuronal cells: the higher APP levels were considered to be related to abnormal proliferation and abnormal morphology of glioma cells. Consistent with these observations, we also found upregulation of CNTN2 and APP in U87-GSCs, with CNTN2 more significant. Xenaki et alCitation28 found that CNTN2 promoted the proliferation and suppressed the differentiation of granule neuron progenitors through SHH signaling pathway. Consistent with this observation and that of Ma et al,Citation20 we found that CNTN2 RNAi can inhibit the proliferation and growth of U87-GSCs. In contrast, overexpression of contactin did not affect the proliferation of glioblastoma cells.Citation26 One possibility is that these glioblastoma cells are different compared to our U87-GSCs, which are stem cells. Alternatively, contactin has a distinct function compared to CNTN2: CNTN1 and CNTN2 may have opposite functions and play antagonistic roles on proliferation.Citation28
AICD, as the downstream intracellular signaling CNTN2/APP pathway, is considered to regulate the transcription of its target genes associated with cell proliferation and apoptosis within the nucleus.Citation43 These nuclear targets may include EGFR promoter, or transcription factor HES1, as suggested by their involvement in Notch/HES1, and EGFR/PI3K/AKT signaling pathways in GSCs,Citation8,Citation33 and by HES1 expression induced by AICD overexpression.Citation36 Our results expanded these earlier observations, where EGFR and HES1 were downregulated in U87-GSCs after CNTN2 RNAi treatment. A seemingly contradictory result is that CNTN2 RNAi decreased AICD but not APP expression, while APP was increased in U87-GSCs. CNTN2 RNAi decreased the CNTN2 protein, which is the ligand of APP. The APP level was not affected, only less ligand led to less activation of APP and lower AICD production. These data indicate that CNTN2/APP/AICD/HES1 or CNTN2/APP/AICD/EGFR signaling pathway exists in U87-GSCs. However, the reduction of EGFR expression after CNTN2 RNAi treatment in U87-GSCs is not consistent with Zhang et al,Citation39 where AICD overexpression decreased EGFR expression.Citation39 Here are some possible explanations. 1) The study of Zhang et al investigated a mouse model that overexpressed AICD, and effects of AICD overexpression may be different in different cells. 2) EGFR signaling pathway, as an important signal of glioma genesis and proliferation, may be regulated by multiple signaling pathways, which may complicate the interpretation of observations in different models. For example, the cross talk between Notch/HES1 and APP/AICD pathways may also exist between Notch/HES1 and EGFR pathway. We need further experiments to confirm the effects of CNTN2 on EGFR signaling pathway.
Although our study on U87-GSC line elucidated that the CNTN2/APP/AICD/HES1 or CNTN2/APP/AICD/EGFR signaling pathway is involved in proliferation and can be inhibited by interfering RNA, a limitation of our study is that we investigated these pathways only in a single cell line. While we hypothesize that these pathways are activated in GSCs from other gliomas, these results may not be applicable to all GSCs from all gliomas. Our future studies will investigate the role of these and other pathways in additional cell lines.
Conclusion
Our results reveal for the first time that CNTN2/APP signaling can regulate the proliferation of U87-GSCs. CNTN2 RNAi inhibits the expression of AICD, HES1, and EGFR in U87-GSCs. At least partially, inhibition of CNTN2/APP signaling pathway may repress proliferation in U87-GSCs via downregulating the expression of HES1 and EGFR.
Acknowledgments
Yang Guo and Peidong Zhang are co-first authors.
Supplementary material
Figure S1 CNTN2 RNAi selection.
Notes: (A) The Western blot shows that CNTN2 RNAi-2 was the most effective among the four RNAi vectors to inhibit CNTN2 expression in U87-GSCs. (B) Gene sequencing results of CNTN2 RNAi-2 after lentiviral infection of U87-GSCs.
Abbreviations: CNTN2, contactin 2; RNAi, RNA interference; U87-GSCs, U87-derived glioma stem cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Disclosure
The authors report no conflicts of interest in this work.
References
- OstromQTGittlemanHFulopJCBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012Neuro Oncol201517suppl 4iv1iv6226511214
- GalliRBindaEOrfanelliUIsolation and characterization of tumorigenic, stem-like neural precursors from human glioblastomaCancer Res200464197011702115466194
- GilbertCARossAHCancer stem cells: cell culture, markers, and targets for new therapiesJ Cell Biochem200910851031103819760641
- SundarSJHsiehJKManjilaSLathiaJDSloanAThe role of cancer stem cells in glioblastomaNeurosurg Focus2014376E6
- CruzMHSidenACalafGMDelwarZMYakisichJSThe stemness phenotype modelISRN Oncol2012201239264722928120
- ClarkMJHomerNO’ConnorBDU87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell linePLoS Genet201061e100083220126413
- ChenLChenXRChenFFMicroRNA-107 inhibits U87 glioma stem cells growth and invasionCell Mol Neurobiol201333565165723572380
- ChenXChenLChenJADAM17 promotes U87 glioblastoma stem cell migration and invasionBrain Res2013153815115823470260
- ChenXChenLZhangRADAM17 regulates self-renewal and differentiation of U87 glioblastoma stem cellsNeurosci Lett2013537444923356982
- KannoHSatoHYokoyamaTAYoshizumiTYamadaSThe VHL tumor suppressor protein regulates tumorigenicity of U87-derived glioma stem-like cells by inhibiting the JAK/STAT signaling pathwayInt J Oncol201342388188623338840
- LiPLuXWangYMiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cellsJ Biomed Res201024643644323554660
- QiuBSunXZhangDWangYTaoJOuSTRAIL and paclitaxel synergize to kill U87 cells and U87-derived stem-like cells in vitroInt J Mol Sci20121379142915622942757
- Sassi FdeACaesarLJaegerMInhibitory activities of trichostatin A in U87 glioblastoma cells and tumorsphere-derived cellsJ Mol Neurosci2014541274024464841
- YuSCPingYFYiLIsolation and characterization of cancer stem cells from a human glioblastoma cell line U87Cancer Lett2008265112413418343028
- LamszusKGuntherHSGlioma stem cells as a target for treatmentTarget Oncol20105321121520737293
- ChenJLiYYuTSA restricted cell population propagates glioblastoma growth after chemotherapyNature2012488741252252622854781
- WangJWakemanTPLathiaJDNotch promotes radioresistance of glioma stem cellsStem Cells2010281172819921751
- WeiYJiangYZouFActivation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cellsProc Natl Acad Sci U S A2013110176829683423569237
- ZhouKSongYZhouWmGlu3 receptor blockade inhibits proliferation and promotes astrocytic phenotype in glioma stem cellsCell Biol Int201438442643424482010
- MaQHBagnardDXiaoZCDaweGSA TAG on to the neurogenic functions of APPCell Adh Migr2008212819262125
- MaQHFutagawaTYangWLA TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesisNat Cell Biol200810328329418278038
- BizzocaAVirgintinoDLorussoLTransgenic mice expressing F3/contactin from the TAG-1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neuronsDevelopment20031301294312441289
- FalkJBonnonCGiraultJAFaivre-SarrailhCF3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelinationBiol Cell200294632733412500940
- FujitaNSaitoRWatanabeKNagataSAn essential role of the neuronal cell adhesion molecule contactin in development of the Xenopus primary sensory systemDev Biol2000221230832010790328
- StogmannEReinthalerEEltawilSAutosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2Brain2013136pt 41155116023518707
- EckerichCZapfSUlbrichtUContactin is expressed in human astrocytic gliomas and mediates repulsive effectsGlia200653111216078236
- RickmanDSTyagiRZhuXXThe gene for the axonal cell adhesion molecule TAX-1 is amplified and aberrantly expressed in malignant gliomasCancer Res20016152162216811280781
- XenakiDMartinIBYoshidaLF3/contactin and TAG1 play antagonistic roles in the regulation of sonic hedgehog-induced cerebellar granule neuron progenitor proliferationDevelopment2011138351952921205796
- LeissringMAMurphyMPMeadTRA physiologic signaling role for the gamma-secretase-derived intracellular fragment of APPProc Natl Acad Sci U S A20029974697470211917117
- ZhouFGongKSongBThe APP intracellular domain (AICD) inhibits Wnt signalling and promotes neurite outgrowthBiochim Biophys Acta2012182381233124122613765
- CulicchiaFCuiJGLiYYLukiwWJUpregulation of beta-amyloid precursor protein expression in glioblastoma multiformeNeuroreport200819998198518521005
- ShimojoHOhtsukaTKageyamaRDynamic expression of notch signaling genes in neural stem/progenitor cellsFront Neurosci201157821716644
- KanamoriMKawaguchiTNigroJMContribution of Notch signaling activation to human glioblastoma multiformeJ Neurosurg2007106341742717367064
- ShenYChenHZhangJIncreased Notch signaling enhances radioresistance of malignant stromal cells induced by glioma stem/progenitor cellsPLoS One20151011e014259426599017
- YaoJZhengKLiCLiuHShanXInterference of Notch1 inhibits the growth of glioma cancer cells by inducing cell autophagy and down-regulation of Notch1-Hes-1 signaling pathwayMed Oncol201532661025920606
- FischerDFvan DijkRSluijsJAActivation of the Notch pathway in Down syndrome: cross-talk of Notch and APPFASEB J200519111451145816126912
- KahlertUDBenderNOMaciaczykDCD133/CD15 defines distinct cell subpopulations with differential in vitro clonogenic activity and stem cell-related gene expression profile in in vitro propagated glioblastoma multiforme-derived cell line with a PNET-like componentFolia Neuropathol201250435736823319191
- BaoSWuQSathornsumeteeSStem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factorCancer Res200666167843784816912155
- ZhangYWWangRLiuQZhangHLiaoFFXuHPresenilin/gamma-secretase-dependent processing of beta-amyloid precursor protein regulates EGF receptor expressionProc Natl Acad Sci U S A200710425106131061817556541
- LiuGYuanXZengZAnalysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastomaMol Cancer200656717140455
- LjungkvistASBussinkJKaandersJHvan der KogelAJDynamics of tumor hypoxia measured with bioreductive hypoxic cell markersRadiat Res2007167212714517390721
- PersanoLRampazzoEDella PuppaAPistollatoFBassoGThe three-layer concentric model of glioblastoma: cancer stem cells, microenvironmental regulation, and therapeutic implicationsScientific World Journal2011111829184122125441
- KonietzkoUAICD nuclear signaling and its possible contribution to Alzheimer’s diseaseCurr Alzheimer Res20129220021621605035
