Abstract
Purpose
The therapeutic efficacy of targeted therapy for adenosquamous carcinoma (ASC) of the lung remains unclear and the role of epidermal growth factor receptor (EGFR) testing in patients with ASC also remains controversial. We aimed to analyze the efficacy of EGFR tyrosine kinase inhibitors (EGFR-TKIs) in ASC.
Methods
Clinical records of patients with ASC who received treatment with EGFR-TKIs between January 2006 and December 2014 at two institutions were retrospectively reviewed.
Results
A total of 27 EGFR mutation-positive patients with ASC who received TKI therapy were enrolled in this study. EGFR mutations included a deletion in exon 19 in 15 cases and a point mutation at codon 858 (L858R) in exon 21 in 12 cases. Among the 27 ASC patients who received treatment with EGFR-TKIs, nine had a partial response and 11 achieved stable disease, accounting for a disease control rate of 74.1% (20/27). The median postoperative overall survival (OS) of the EGFR-mutant patients who received TKI therapy was 39 months (95% confidence interval [CI]: 25.6–52.4). The median progression-free survival for EGFR mutation-positive patients was 15 months (95% CI: 12.9–17.1), and the median relapse OS was 19 months (95% CI: 0.9–37.1). In addition, the 3- and 5-year postoperative survival rate was 51.9% and 15.3%, respectively.
Conclusion
ASC patients harboring EGFR mutations had a good response to TKI therapy. Routine EGFR testing for ASCs was recommended. Further studies on TKI therapy versus chemotherapy alone for EGFR-mutant ASCs are required.
Introduction
Adenosquamous carcinoma (ASC) of the lung is a rare subtype of non-small cell lung cancer (NSCLC), comprising 0.4%–4% of all lung cancers.Citation1–Citation3 ASC has the feature of mixed histology, defined as “a carcinoma showing components of both squamous cell carcinoma (SCC) and adenocarcinoma (ADC), with each comprising at least 10% of the tumor”, based on the 2015 World Health Organization (WHO) classification of tumors of the lung.Citation4 In addition, ASC is more aggressive and has a worse prognosis than ADC or SCC,Citation2,Citation3,Citation5 suggesting that there are biological differences between these three histologic types of NSCLC.Citation1,Citation6
Currently, there is no standard therapy for ASC due to the lack of a deep understanding about the molecular characteristics of this disease. It has been reported that epidermal growth factor receptor (EGFR) mutations have been observed frequently in ASC.Citation7,Citation8 Unlike lung ADC, research progress in the treatment of EGFR-targeted therapy for ASC has not improved over the past decade, and clinical studies focusing on the efficacy of EGFR tyrosine kinase inhibitors (EGFR-TKIs) are limited, owing to the low incidence of ASC. There is one case report of a Japanese ASC patient harboring EGFR-activating mutations 3 years following treatment with gefitinib.Citation9 A retrospective study demonstrated that treatment with TKIs was effective in ASC patients with EGFR mutations.Citation10 However, a pooled analysis showed that gefitinib is less effective in EGFR-mutant non-ADC NSCLC compared with EGFR-mutant ADC.Citation11 The therapeutic efficacy of targeted therapy for ASC remains unclear, and the role of EGFR testing in patients with ASC also remains controversial.
We clinically characterized EGFR-mutant patients with ASC who received EGFR-TKI therapy at two institutions, Shanghai Chest Hospital and Zhongshan Hospital, and analyzed the efficacy of TKIs in EGFR-mutant patients.
Methods
Patient demographics
A retrospective review of patients from Shanghai Chest Hospital and Zhongshan Hospital, two institutions located in the eastern districts of People’s Republic of China, was performed between January 2006 and December 2014. Overall, 21,445 patients underwent surgical resection and were diagnosed with primary NSCLC in our thoracic surgery departments. Of these, 205 primary ASC patients were retrospectively reviewed, 30 of whom harbored the EGFR mutation and received TKI therapy. Informed consent was not required because the review of the patient data was all anonymous. The study was approved by the Committee for Ethical Review of Research at Shanghai Cancer Hospital and the Institutional Ethics Committee of Zhongshan Hospital. Baseline characteristics included gender, age at diagnosis, smoking history, and performance status. All data were abstracted from the electronic medical records by professional staff. NSCLC staging was performed according to the seventh tumor, node, and metastases classification.Citation12 The inclusion criteria were as follows: 1) all patients underwent surgical treatment with pathologically confirmed primary ASC postoperatively; 2) all patients were proven to harbor EGFR mutation; 3) all patients were supplied with TKIs during the treatment course; 4) disease recurrence was confirmed using chest and abdomen computed tomography, brain magnetic resonance imaging, whole body bone scan, and abdominal ultrasound; 5) at least one measurable lesion; and 6) a Zubrod–Eastern Cooperative Oncology Group– WHO performance status of 0–3.
Pathology and EGFR mutation examination method
To confirm the histology of ASC, each slide was examined independently by two pathologists. Immunohistochemistry staining was used for modification of the classification of adenomatous and squamous carcinomatous components within ASC. The DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) was used to analyze DNA extracted from five serial slices of a 5 μm paraffin section. The molecular analysis of EGFR was performed using an amplification refractory mutation system following the manufacturer’s protocol of a DxS EGFR mutation test kit (DxS, Manchester, UK).
Clinical assessment
Tumor responses were assessed by physical examination, routine laboratory tests, and imaging examination at 4- to 8-week intervals until the lesions were determined to represent progressive disease (PD). Tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors 1.1. Objective tumor responses were assessed in terms of complete response (CR), partial response (PR), stable disease (SD), and PD. An objective response rate (ORR) included both CR and PR. Disease control rate (DCR) was defined as the addition of objective response and stabilization rates (CR + PR + SD). Progression-free survival (PFS) was measured from the date of initiation of EGFR-TKI therapy until the date of the first documented progression or the last follow-up visit. The postoperative OS was recorded from the date of surgery to the date of death or the last follow-up visit, and the relapse OS was defined as the period from the first documented tumor relapse to death or the final follow-up visit.
Follow-up
Generally, all patients were instructed to undergo routine imaging, laboratory tests, and physical examinations at 4- to 8-week intervals. The indication for adjuvant therapy after surgery in our department was stage IB in high-risk patients or stage II or higher disease. High-risk patients included those with poorly differentiated tumors, vascular invasion, tumors >4 cm, and visceral pleural involvement. All patients in the present study received 4–6 cycles of adjuvant platinum-based chemotherapy. The primary end points of the study were OS and PFS. Follow-up data were obtained through routine clinical follow-up and via telephone by our team members. All patients evaluated for TKI tumor response had a PFS and three patients were lost to follow-up. The median follow-up period was 39.4 months (range: 7–68 months); the last follow-up visit was on May 1, 2016.
Statistical methods
Demographic and clinical data were summarized as medians with a range of continuous variables; categorical variables were expressed and summarized by means of absolute numbers and percentages. Categorical variables were compared using the chi-squared test. Survival curves were calculated according to the method of Kaplan–Meier. Analyses were conducted using the SPSS software (version 22.0; IBM Corporation, Chicago, IL, USA).
Results
Patient characteristics
Overall, 27 patients with ASC and harboring EGFR mutations were enrolled. EGFR mutations included: deletion in exon 19 in 15 cases (55.6%) and point mutation at codon 858 (L858R) in exon 21 in 12 cases (44.4%), including one with an additional substitution of threonine 790 with methionine (T790M) and one with an exon 20 insertion mutation. The median patient age was 54 (range: 34–72) years. Seven patients (25%) had a history of smoking. Twenty-four patients (88.9%) received radical surgeries after diagnosis while others underwent palliative surgery. Most patients were pathologically staged as IIIA after surgery (n=19, 70.4%) while the remainder included one patient each with stage IA, IB, IIA, IIB, IIIB, and three with stage IV disease. TKI therapy was administered to all these patients using gefitinib, erlotinib, or icotinib. Eight patients (29.6%) received TKI therapy as first-line treatment and 19 as second-line treatment. Patients who received first-line treatment underwent chemotherapy after the recurrence of TKI, and patients who recurred after initial chemotherapy received TKI as second-line treatment.
All patients were divided into two groups: seven presented with local recurrence before receiving EGFR-TKI therapy (group 1), and 20 presented with metastasis before taking the EGFR-TKI therapy (group 2). Baseline characteristics of patients are listed in .
Table 1 Clinical characteristics of patients with adenosquamous carcinoma of the lung
Efficacy and survival
Among the 27 ASC patients who received EGFR-TKI treatment, nine had PR and 11 patients achieved SD, accounting for a DCR of 74.1% (20/27; group 1 vs 2, 85.7% vs 70%, P=0.414), while the ORR was 33.3% (9/27; group 1 vs 2, 42.9% vs 30%, P=0.535). Twenty-one patients died during the follow-up period.
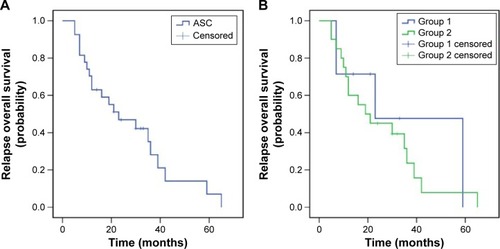
The median PFS during TKI treatment was 12 months (95% confidence interval [CI]: 9.1–14.9; group 1 vs 2, 17 months, 95% CI: 13.7–20.3 vs 10 months, 95% CI: 8.9–11.1, P=0.037). The median postoperative OS was 39 months (95% CI: 25.6–52.4, group 1 vs 2, 49 months, 95% CI: 34.4–63.6 vs 31 months, 95% CI: 13.5–48.5, P=0.191), and the median relapse OS was 23 months (95% CI: 6.6–39.4; group 1 vs 2, 23 months, 95% CI: 0.0–55.1 vs 19 months, 95% CI: 8.0–30.0, P=0.500). Additionally, the 3-year postoperative survival rate for patients was 51.9% (group 1 vs 2, 85.7% vs 40%, P=0.037), while the 5-year survival rate was 15.3% (group 1 vs 2, 35.7% vs 9.5%, P=0.064; –).
Figure 1 Progression-free survival of patients with ASC of the lung (A), and subgroup analysis for patients from groups 1 and 2 (B). Note: Group 1, patients with local recurrence; group 2, patients with metastasis.
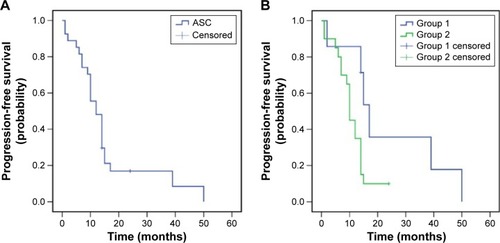
Figure 2 Postoperative overall survival of patients with ASC of the lung (A), and subgroup analysis for patients from groups 1 and 2 (B). Note: Group 1, patients with local recurrence; group 2, patients with metastasis.
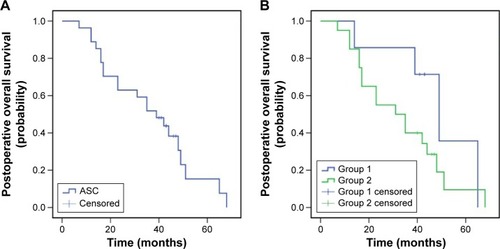
Figure 3 Relapse overall survival of patients with ASC of the lung (A), and subgroup analysis for patients from groups 1 and 2 (B). Note: Group 1, patients with local recurrence; group 2, patients with metastasis.
Because the majority of the patients were stage III (20/74.1%), it is necessary to comment further about this. The median postoperative OS as well as 3- and 5-year survival rates were 39 months (95% CI: 25.4–52.6), 55% and 10.9%, respectively. These results were similar to that seen in the entire cohort.
Discussion
ASC is an uncommon histological type of lung carcinoma, comprising only 2.1%–3.4% of NSCLC.Citation3,Citation5 The cumulative 5-year postoperative survival rate of patients with ASC is approximately 20%, worse than that of patients with ADC or SCC (40% for both groups).Citation2,Citation5 In addition, there are few studies targeting the efficacy of EGFR-TKIs for ASC patients with an EGFR mutation, with most focusing on the clinical features and gene mutation frequency. Thus, an investigation of the relationship between histological subtypes of ASC and tumor response to EGFR-TKIs was needed. In this study, we summarized the clinical outcomes of ASC patients with EGFR mutations who underwent EGFR-TKI therapy. To our knowledge, the number of EGFR-mutant patients is more than that of previous studies. We found that the prognosis of EGFR-mutant ASC patients treated with EGFR-TKIs was satisfactory and the clinical significance of EGFR testing for ASCs was promising. Detection of EGFR mutation helped to select a subgroup of ASC patients who exhibited the best response to TKIs.
Previous studies have shown that the EGFR mutation rate in ASC patients was similar to that of ADC patients (13%–44%)Citation7,Citation8,Citation13–Citation15 but higher than that of SCC (<4%).Citation16,Citation17 The study of Jia and ChenCitation7 demonstrated that EGFR mutations were identified in 38.2% of Chinese ASC patients (n=55), which corresponded with a tumor size of ≥3 cm. More importantly, the same EGFR mutation was observed in both adenocarcinomatous and squamous cell carcinomatous components. Similar results were found in previous studies. Wang et alCitation14 reported an EGFR mutation rate of 31.6% in Chinese patients with ASC (n=76), which was found more frequently in nonsmokers and in tumors larger than 3 cm. The clinicopathological and mutational characteristics of ASC were similar to that of ADC, especially for poorly differentiated ADC. Kang et al,Citation8 in a study of the Korean population, found similar frequencies of EGFR mutations in ASC (44%) and ADC. In addition, female nonsmokers had high frequency of the EGFR mutation, which was also confirmed by Sasaki et al, Toyooka et al, and Shi et al.Citation15,Citation18,Citation19
Multiple clinical studies have demonstrated that gefitinib is effective as second- or third-line therapy for advanced or metastatic NSCLC and first-line therapy for EGFR-mutant patients. On the contrary, the efficacy of ASC with TKIs was not well known until now, owing to the rarity of this carcinoma subtype. In our study, the prognosis of EGFR-mutant ASC patients treated with EGFR-TKIs was satisfactory.
The prognosis of patients with ASC who underwent surgery and received no target therapy from previous studies was worse compared with our results. Filosso et alCitation6 reported 3- and 5-year survival rates of 25% and 15%, respectively. The median survival for patients with distant metastasis or local recurrence was 8 months compared with our results (51.9%, 15.3%, and 39 months). In the Wang et al study,Citation14 the 2-year survival for patients with ASC was 51.3% compared with ours of 63%. Additionally, a few studies have investigated the effectiveness of EGFR-TKIs for ASC patients. A retrospective study conducted by Song et alCitation10 demonstrated that the median PFS of EGFR-mutant ASC patients was significantly higher than that of the wild-type group with TKI treatment (8.7 vs 2.1 months). And the median PFS and median OS for all patients receiving TKIs was 4.3 and 17.6 months, respectively. Our results were superior to what has been reported, perhaps because all the patients in our study were EGFR mutation-positive. In addition, a case report by Iwanaga et alCitation9 showed that a stage IIIB patient with EGFR-mutant ASC who experienced a relapse after surgery and four cycles of chemotherapy achieved 3 years response with TKI treatment. However, a pooled analysis by Shukuya et alCitation11 demonstrated that non-ADC NSCLC patients harboring EGFR mutations with gefitinib treatment had a median PFS of 3 months (n=33) while that of ADC was 9.4 months. Those patients included only three ASC patients. The sample of ASC patients was too small and most of these non-ADC patients were SCC patients (n=27), thus resulting in the inferior efficacy.
Considering that ASC has some similarities with ADC as mentioned previously, it is necessary to discuss the efficacy of EGFR-TKIs for ADC. According to previous studies, for patients with NSCLC who received gefitinib or erlotinib as second- or third-line treatment, the median PFS and OS ranged from 1.4–3.6 and 5.3–14.8 months, with a DCR and ORR of approximately 54.1% and 27.5%, respectively.Citation20–Citation22 In addition, for patients of advanced NSCLC harboring EGFR mutations who were treated by TKIs as first-line therapy, the median PFS, OS, and ORR, respectively, ranged from 8–13.7 months, 19.3–36 months, and 56%–84.6%.Citation23–Citation28 Most patients in these studies were ADC patients. However, treatment efficacy was much lower in patients with SCC.Citation29 In this study, the median PFS, median postoperative OS, DCR, and ORR were, respectively, 12 months, 39 months, 74.1%, and 33.3%, which were similar to that of ADC patients with EGFR mutations, especially for group 2 patients who had metastasis (median PFS =10 months), but better than those of unselected ADC patients. This result might owe to the patients in our study who were in a relatively early stage (IIIA) and received surgery, compared with patients with advanced disease (IIIB and IV) and no surgery in those clinical trials mentioned above.Citation20–Citation28 Therefore, ASC patients could benefit from TKI therapy, thus suggesting that ASC could be subclassified into distinct subtypes with different pathogenetic mechanisms according to EGFR mutation status.
Above all, ASC patients with an EGFR mutation do respond to EGFR-TKI therapy; however, which structural components within ASC, either the adenocarcinomatous or squamous cell carcinomatous components alone or both, contributed more to the EGFR-TKI treatment response was unclear. As mentioned previously, the clinical and mutational characteristics of ASC appear to have more similarities with those of ADC (EGFR mutation rate and response to TKIs), which indicated that the two components may share a common mechanism of pathogenesis. Previous studies showed that EGFR mutations occurred in both adenocarcinomatous and squamous cell carcinomatous components. These findings implied that ASC is a monoclonal tumor, suggesting that squamous cell carcinomatous and adenocarcinomatous components may derive from common tumor stem cells and subsequently differentiate into separate histological types. In other words, ASC is a transitional stage between ADC and SCC.Citation13,Citation14,Citation19,Citation30,Citation31 It has been supported by a case report, in which a young light ex-smoker with ASC relapsed twice with histological changes (from ASC to pure SCC to pure ADC).Citation32
There are some advantages to our study compared with previous studies focusing on the efficacy of EGFR-TKI in ASC.Citation10,Citation11 Most importantly, all patients in our study underwent surgery, which means that all specimens used to perform EGFR mutation examination analysis were obtained from resected tumor tissue. This modality has more advantages with regard to diagnostic accuracy for ASC, considering the limitation of cytological or histological transbronchial needle aspiration or transthoracic needle aspiration data. Chen et alCitation33 found that the accuracy of transbronchial needle aspiration for the histological classification of lung cancer was only 63.6%, and 25% for ASC. Next, all cases in this study were EGFR-mutant patients with ASC who received TKIs after surgery.
The major limitation of the present study is its retrospective nature and its small sample size. Additionally, there is no control group in this study for comparison. However, even with a few cases in limited clinical trials focusing on the EGFR-TKI therapy for ASC, our retrospective study can be considered to be meaningful. Large sample multicenter randomized clinical trials should be conducted in the future to verify the efficacy of EGFR-TKIs in ASC patients.
Conclusion
EGFR mutation-positive ASC patients achieved a good response to EGFR-TKI therapy. Routine EGFR mutation analysis is important and valuable for selecting the appropriate patients who would best respond to TKI treatment. Further studies on TKIs versus chemotherapy alone for EGFR-mutant ASCs are required.
Acknowledgments
The authors would like to acknowledge support from the China Charity Federation for their provision of study materials and patients for this study. This study was also supported by Grant No 81572693 from the National Natural Science Foundation of China and Grant No YG2015ZD14 from Medicine and Engineering Cross Foundation of Shanghai Jiao Tong University.
Disclosure
The authors report no conflicts of interest in this work.
References
- CookeDTNguyenDVYangYChenSLYuCCalhounRFSurvival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomyAnn Thorac Surg201090394394820732522
- GawrychowskiJBrulinskiKMalinowskiEPaplaBPrognosis and survival after radical resection of primary adenosquamous lung carcinomaEur J Cardiothorac Surg200527468669215784375
- NakagawaKYasumituTFukuharaKShionoHKikuiMPoor prognosis after lung resection for patients with adenosquamous carcinoma of the lungAnn Thorac Surg20037561740174412822609
- TravisWDWHO Classification of Tumours of the Lung, Pleura, Thymus and HeartInternational Agency for Research on Cancer2015
- ShimizuJOdaMHayashiYNonomuraAWatanabeYA clinicopathologic study of resected cases of adenosquamous carcinoma of the lungChest199610949899948635382
- FilossoPLRuffiniEAsioliSAdenosquamous lung carcinomas: a histologic subtype with poor prognosisLung Cancer2011741252921371773
- JiaXLChenGEGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lungLung Cancer201174339640021592614
- KangSMKangHJShinJHIdentical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lungCancer2007109358158717186532
- IwanagaKSueoka-AraganeNNakamuraTMoriDKimuraSThe long-term survival of a patient with adenosquamous lung carcinoma harboring EGFR-activating mutations who was treated with gefitinibInt Med2012511927712774
- SongZLinBShaoLZhangYTherapeutic efficacy of gefitinib and erlotinib in patients with advanced lung adenosquamous carcinomaJ Chin Med Assoc201376948148523769878
- ShukuyaTTakahashiTKairaREfficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reportsCancer Sci201110251032103721272159
- GoldstrawPCrowleyJChanskyKThe IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumoursJ Thorac Oncol20072870671417762336
- TochigiNDacicSNikiforovaMCieplyKMYousemSAAdenosquamous carcinoma of the lung: a microdissection study of KRAS and EGFR mutational and amplification status in a western patient populationAm J Clin Pathol2011135578378921502435
- WangRPanYLiCAnalysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomasJ Thorac Oncol20149676076824481316
- SasakiHEndoKYukiueHKobayashiYYanoMFujiiYMutation of epidermal growth factor receptor gene in adenosquamous carcinoma of the lungLung Cancer200755112913017156891
- TaoDHanXZhangNGenetic alteration profiling of patients with resected squamous cell lung carcinomasOncotarget Epub2016429
- DeardenSStevensJWuYLBlowersDMutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap)Ann Oncol20132492371237623723294
- ToyookaSYatabeYTokumoMMutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lungInt J Cancer200611861588159016187277
- ShiXWuHLuJDuanHLiuXLiangZScreening for major driver oncogene alterations in adenosquamous lung carcinoma using PCR coupled with next-generation and Sanger sequencing methodsSci Rep201662229726923333
- CiuleanuTStelmakhLCicenasSEfficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 studyLancet Oncol201213330030822277837
- MaruyamaRNishiwakiYTamuraTPhase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancerJ Clin Oncol200826264244425218779611
- KimESHirshVMokTGefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trialLancet200837296521809181819027483
- ZhouCWuYLChenGFinal overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802)Ann Oncol20152691877188326141208
- RosellRCarcerenyEGervaisRErlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trialLancet Oncol201213323924622285168
- HanJYParkKKimSWFirst-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lungJ Clin Oncol201230101122112822370314
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- SoriaJCFelipECoboMAfatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trialLancet Oncol201516889790726156651
- VassellaELangschSDettmerMSMolecular profiling of lung adenosquamous carcinoma: hybrid or genuine type?Oncotarget2015627239052391626068980
- ShiozawaTIshiiGGotoKClinicopathological characteristics of EGFR mutated adenosquamous carcinoma of the lungPathol Int2013632778423464964
- BurkartJShiloKZhaoWOzkanEAjamAOttersonGAMetastatic squamous cell carcinoma component from an adenosquamous carcinoma of the lung with identical epidermal growth factor receptor mutationsCase Rep Pulmonol20152015
- ChenJGaoYDCaoYYangJLuoGWSurgical specimen histology revealed inadequacy of conventional transbronchial needle aspiration sample in the diagnosis of adenosquamous lung carcinomaJ Thorac Dis20157468068625973234
