Abstract
Objective
To investigate the correlations of two hepatocyte growth factor (HGF) gene polymorphisms (rs5745652 and rs2074725) and their protein expression levels with the efficacy of transhepatic arterial chemotherapeutic embolism (TACE) and prognosis in patients with primary liver cancer (PLC).
Methods
From March 2011 to June 2012, 109 PLC patients (the case group) who chose TACE as primary treatment and 80 healthy people (the control group) who had undergone physical examination in The First Affiliated Hospital, Zhejiang University were selected during the same period. Gene polymorphisms of HGF rs5745652 and HGF rs2074725 were detected. Serum HGF level, treating efficacy, survival quality, and 3-year survival rate for PLC patients who received TACE were observed.
Results
There were significant differences in genotype and allele frequencies of HGF rs5745652 and HGF rs2074725, between the case and control groups (all P<0.05). Compared with CT+TT genotype of HGF rs5745652, patients carrying CC genotype had lower serum HGF levels, higher efficacy, better survival quality, and prolonged 3-year survival rate (all P<0.05). In rs2074725, patients carrying CA+AA genotype had lower serum HGF levels, higher efficacy, better survival quality, and prolonged 3-year survival rate compared with patients carrying rs2074725 CC genotype (all P<0.05). Gene polymorphisms of HGF rs5745652 and HGF rs2074725, tumor size, and Barcelona Clinic Liver Cancer stage were independent prognostic factors for PLC (P<0.05).
Conclusion
Our results indicated that HGF gene polymorphisms affect TACE efficacy and survival quality of PLC patients. Patients with HGF CC genotype of rs5745652 and CA+AA genotype of rs2074725 had decreased HGF level, better curative effect, high survival quality, and a good prognosis after TACE treatment.
Introduction
Primary liver cancer (PLC) is the second most common malignant tumor and leads to 350,000 deaths a year in People’s Republic of China.Citation1 As the fifth most common cancer in men and the ninth in women, liver cancer is estimated to be the reason for nearly 745,000 deaths in 2012.Citation2 The onset of liver cancer is occult and most of the symptoms will not appear until the middle and late stages, thus making it rather difficult to diagnose liver cancer at the early stage.Citation3 The main cause of PLC is hepatitis B virus and hepatitis C virus infection, and aflatoxin, alcohol intake, smoking, obesity, and diabetes.Citation4 Because of the disease severity or the rarity of suitable organ donors at the time of diagnosis, only a minority of subjects can be treated by the potentially effective therapies such as liver transplantation and surgical resection.Citation5 Moreover, due to tumor size and tumor numbers, it is also difficult to apply surgical removal; therefore, transhepatic arterial chemotherapeutic embolism (TACE), a minimally invasive treatment, is the main therapeutic method for patients suffering from liver cancer.Citation6 After TACE therapy, tumor markers are needed to predict the prognosis of patients with liver cancer.Citation7
Hepatocyte growth factor (HGF), also known as scatter factor, is a multifunctional growth factor and mesenchyme-derived cytokine with potent neurotrophic, angiogenic, and antiapoptotic effects.Citation8 Through the activation of HGF-Met pathway, it can affect tumorigenesis and tumor invasion, and promote the growth, regeneration, and morphogenesis of various types of tissues and cells.Citation9 The activation and over-expression of autocrine HGF in cancer cells are considered to be a contributory factor for tumor formation and growth.Citation10 Evidence showed that the high expressions of HGF mRNA and protein in breast cancer tissue were associated with poor survival rate.Citation11 In addition, another study pointed out that the activity of HGF affects dissemination and ascite formation and HGF secreted by ovarian cancer cells plays an important role in cancer peritoneal implantation.Citation12 However, researches about, whether, and how HGF gene polymorphisms (rs5745652 and rs2074725) and its protein expression level affect the efficacy of TACE and survival of PLC are still very scarce. Therefore, this paper intends to assess the correlations of efficacy of TACE therapy with HGF gene polymorphisms (rs5745652 and rs2074725) in patients with PLC.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University, and written informed consent was obtained from each patient or guardians prior to study.
Research subjects
From March 2011 to June 2012, a total of 109 PLC patients were recruited from The First Affiliated Hospital, Zhejiang University who chose TACE as primary treatment (case group, n=109). There were 95 male and 14 female aging 25–76 years (52.27±5.21 years). According to Child-Pugh class,Citation13 the patients in case group were classified into: class A, 16 cases and class B, 93 cases. Based on Barcelona Clinic Liver Cancer (BCLC) staging classification,Citation14 there were 53 cases in stage B and 56 cases in stage C. In terms of the diameter of the tumor, 36 cases were <5 cm and 73 cases were <5 cm. Besides, 47 cases undergone <4 times of TACE treatment and 62 cases <4 times. Inclusion criteria were as follows: 1) patients were diagnosed with PLC based on American Association for the Study of Liver Diseases (AASLD);Citation15 2) expected survival time <3 months; 3) primary treatment was TACE; 4) patients could obtain at least one TACE treatment; 5) volunteer to participate in this research. Exclusion criteria: 1) patients have other malignant tumors or complication with serious chronic diseases; 2) pregnant or breast-feeding women; 3) patients have TACE treatment contraindications or allergic to contrast agent; 4) patients have severe liver dysfunction, active bleeding tendency or severe coagulation disorders; 5) patients refuse to participate in the research. All patients in the study met the inclusion criteria. Meanwhile, 80 healthy people undergone physical examination in our hospital during the same period were selected as control group, including 69 males and 11 females, aging 22–74 years (51.63±6.94 years). The inclusion criteria for the control group are as follows: 1) no history of cancer; 2) no siblingship with patients in case group; 3) volunteer to participate in the study. There was no significant difference in gender or age between the two groups (both P<0.05).
Sample collection
After fasting for 12 h, all subjects were extracted with 8 mL of peripheral venous blood the next morning. Half of the blood samples were anticoagulated with ethylenediaminetetraacetic acid. After conventional even mixing, the whole blood samples were temporarily stored in refrigerator at 4°C. Whole-genome DNA kit (Shanghai SaiBaiSheng Bioengineering Co., Ltd., Shanghai, People’s Republic of China) was used for extraction of blood samples in accordance with the instruction. The other half samples were placed at room temperature for 2 h before 10 min of 3,000 rpm/min centrifugation. Then the supernatant was stored at −80°C, which was used for the detection of serum HGF protein levels.
The serum HGF protein level was detected before and after TACE treatment. The serum HGF protein was measured by double antibody sandwich enzyme-linked immunosorbent assay. Kits were purchased from Rapdbio Company (San Diego, CA, USA) and the operation procedures in strict accordance with the instructions.
Genotyping
Polymerase chain reaction (PCR) restriction fragment length polymorphism was used to detect the polymorphism of HGF rs5745652. Primers were designed as follows: upstream 5′-CACGTAGGCTGGAACTGAGT-3′; downstream 5′-ACAGCATTCCAGTA-GTCCCC-3′. PCR primers were designed and synthesized by Takara Biotechnology Co., Ltd.; Dalian, People’s Republic of China. PCR amplification was applied to whole-genome DNA, extracted from blood samples. PCR amplification reaction system includes: double-distilled water (ddH2O) 17.5 μL, upstream primer 0.5 μL, downstream primer 0.5 μL, Taq DNA polymerase 0.125 μL, deoxy-nucleotide-tri phosphate 2 (dNTP 2) μL, 10× ESTaq slow release liquid 2.5 μL, and DNA 1 μL. PCR reaction conditions: pre-denaturation for 5 min at 95°C, then a total of 36 cycles of denaturation for 30 s at 95°C, annealing for 45 s at 56°C, extending for 1 min at 72°C, and at last extending for 5 min at 72°C. Conditions for PCR product restriction enzyme reaction were ddH2O 1.0 μL, 10× buffer 1.0 μL, 10 μg/μL bovine serum albumin 0.5 μL, template 7 μL, and HpaII endonuclease 0.5 μL. After being placed into thermostat at 37°C for a night, products of restriction enzyme digestion were analyzed with 3% agarose gel electrophoresis.
Polymorphism of HGF rs2074725 was detected by single allele-specific primer (SASP-PCR). The primer sequences are as follows: upstream primer P1-C (wild): 5′-CAAATTATAGTCCAGAGCTTACC-3′; P1-A (mutant): 5′-CAAATTATAGTCCAGAGCTTACA-3′; downstream primer P2: 5′-TCTTGTGCCAAAACGAAAC-3′. PCR primers were designed and synthesized by Takara Biotechnology (Dalian) Co., Ltd. PCR reaction: two PCR reactions are needed to detect the gene type of every research object, one reaction using P1-C and the other P1-A with all other conditions the same. PCR total volume is 20 μL with dNTP 1 μL, 10× buffer 2 μL, Taq enzyme 0.2 μL DNA, primers each 1 μL. PCR amplification was performed after short-time centrifugation. PCR reaction conditions: pre-denaturation for 5 min at 94°C, then denaturation for 40 s at 94°C, annealing for 40 s at 56°C, extending for 1 min at 72°C, for a total of 35 cycles, and at last extending for 10 times at 72°C before being stored at 4°C. Seven percent agarose gel electrophoresis was used as amplifier, ethidium bromide was used for staining, and electrophoresis results were detected under ultraviolet light. Experimental reagents were all purchased from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, People’s Republic of China).
TACE regimens
The right femoral artery of patients was punctured and conventional skin disinfection was performed. The percutaneous transarterial access to the hepatic artery or its branches was obtained through Seldinger technique. Hepatic artery angiography was performed to identify the proper hepatic artery supplying the tumor. Then microcatheter was sent into the blood supplying artery to inject 40–60 mg cisplatin, 6–10 mg mitomycin C, and 1,000 mg fluorouracil per square meter of body surface area, and the mixture of 30 mg pirarubicin and 8–20 mL of 40% iodized oil was injected per square meter of body surface area. Gelatin sponge embolization of the target artery was the last step of the TACE treatment. The treatment was performed every 4–6 weeks. The reagents used in the experiment were purchased from Sangon Biotech Co., Ltd. (Shanghai, People’s Republic of China).
Efficacy criteria of TACE
The curative effect of TACE therapy for PLC was evaluated according to the response evaluation criteria in solid tumors.Citation16 Complete response (CR) means that liver tumor disappears without new lesions occurred, and tumor markers are normal for at least 4 weeks. Partial response (PR) means that the decrease in the sum of the longest diameter (LD) of liver tumor is over 30% for at least 4 weeks. Stable disease (SD) means that the decrease in the sum of the LD of the liver tumor was not up to PR or the increase was not up to Progress disease (PD). PD means that the increase in the sum of the LD of the liver tumor is at least 20%, or new lesions appear. Effective includes CR and PR and ineffective includes SD and PD.
Follow-up
All patients were followed-up for 3 years until December 31, 2015. Life quality of patients was observed and Karnofsky scoreCitation17 was used to evaluate the survival quality of patients before and after treatment and 3 months after treatment. The survival time and 3-year survival rate of the patients were observed. Calculation criterion of survival time is taken from treatment ending to the last follow-up or patients’ death.
Statistical analysis
The statistical analyses were conducted with SPSS Version 21.0 (SPSS Inc., Chicago, IL, USA). Measurement data presented by x–± s were compared using an unpaired t-test. Categorical data presented by ratio or percentage were compared by chi-square test. Chi-square goodness-of-fit test used to confirm whether genotype distribution in the control group is consistent with the Hardy–Weinberg equilibrium or not. Kaplan–Meier estimator was used to analyze survival quality, and survival time was compared using log-rank test. P-value was two-tailed test and <0.05 was considered statistically different.
Results
Electrophoresis results of PCR products
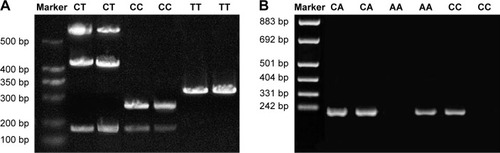
The PCR products were digested by restriction enzyme HpaII and polymorphism was detected in HGF gene rs5745652 (). Gene typing was performed according to the electrophoresis results of enzyme digestion products. The enzyme digestion products of HGF rs5745652 gene included gene fragments of 541, 417, and 123 bp, which were mutant heterozygous CT; gene fragments of 252 and 124 bp, which were mutant homozygous CC; gene fragment of 376 bp, which was wild-type homozygous TT. SASP-PCR results showed the existence of polymorphism at rs2074725 site of HGF gene. The 1, 3, and 5 lanes were the electrophoresis results of PCR with the addition of P1-C primer, and the 2, 4, and 6 lanes were the electrophoresis results of PCR with the addition of P1-A primer, including wild type CC (5 and 6 lanes), mutant heterozygous CA (1 and 2 lanes), and mutant homozygous AA (3 and 4 lanes) ().
Distribution of genotype and allele frequencies of two HGF gene polymorphisms
Both in the control group and the case group, rs5745652 and rs2074725 of HGF gene achieved Hardy–Weinberg genetic equilibrium in terms of polymorphism genotype and allele frequency distribution. shows the TT genotype, CT genotype, CC genotype, and allele frequency of HGF gene rs5745652 in case group were significantly different from those in the control group (all P<0.05), and allele C was a protective gene (P<0.001, odds ratio [OR] =0.338, 95% confidence interval [CI]: 0.207–0.553). The CC genotype, CA genotype, AA genotype, and allele frequency of HGF gene rs2074725 in the case group were significantly different from those in the control group (all P<0.05), and allele A was a protective gene (P<0.001, OR =0.301, 95% CI: 0.181–0.502).
Table 1 Distribution of genotype and allele frequencies of two HGF gene polymorphisms in the case and control groups
Serum HGF protein level changes of two HGF gene polymorphisms before and after treatment
The changes of serum HGF protein level in the patients of different HGF genotype before and after treatment are shown in . The pretreatment serum HGF protein levels in patients carrying different genotypes of HGF gene rs5745652 and rs2074725 were not different from posttreatment levels (both P<0.05), and HGF levels of all patients were significantly reduced after treatment compared with before treatment (all P<0.05). CT genotype at HGF rs5745652 was too few in number, so we combined genotype CT and TT as CT+TT genotype. After treatment, the serum HGF level of CC genotype was significantly lower than that of CT+TT genotype (P<0.05). Since AA genotype at HGF rs2074725 was also too few in number, we combined AA and CA genotypes as CA+AA genotype. After treatment, the serum HGF level of CA+AA genotype was significantly lower than that of CC genotype (P<0.05) ().
Table 2 Serum HGF protein level changes of each HGF genotypes before and after treatment between the case and control groups
Comparisons of TACE curative effect of each HGF genotype between the case and control groups
Three months after treatment, 36 cases of patients with HGF rs5745652 CC genotype were effective with a total efficacy rate of 78.26% and 26 cases of CT+TT genotype were effective with a total efficacy rate of 41.27%, indicating that in terms of treatment efficacy, patients carrying CT+TT genotype were much lower than those carrying CC genotype (P<0.05). When it comes to the HGF rs2074725, 20 cases of CA+AA genotype were effective with a total efficacy rate of 80.00% and 42 cases of CC genotype were effective with a total efficacy rate of 50.00%, which demonstrated that the total treatment efficacy in patients carrying CC genotype was much lower than those carrying CA+AA genotype (P<0.05) ().
Table 3 Comparisons of TACE curative effect of each HGF genotypes between the case and control groups
Comparisons of survival quality of each HGF genotype between the case and control groups
As shown in , there was no significant difference in the Karnofsky scores before and after the treatment on different HGF genotype (P<0.05). Three months after TACE treatment, patients carrying CC genotype at HGF rs5745652 or CA+AA genotype at HGF rs2074725 got higher Karnofsky score than before treatment (P<0.05). Patients of CC genotype had higher Karnofsky score than those of CT+TT genotype at HGF rs5745652, and patients of CA+AA genotype had higher Karnofsky score than those of CC genotype at HGF rs2074725 (all P<0.05).
Table 4 Comparisons of survival quality of each HGF genotypes between the case and control groups
Analysis of HGF rs5745652 polymorphism and the survival rate of patients with PLC
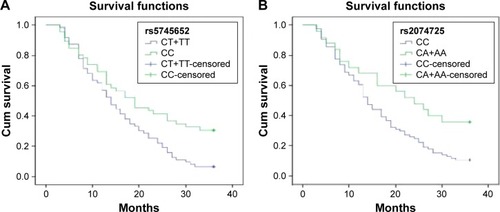
All patients were followed-up by telephone or regular outpatient follow-up and the last follow-up was conducted on July 30, 2015. Survival curves of patients carrying CT+TT genotype or CC genotype at rs5745652 site of HGF are shown in . Patients carrying CC genotype at HGF rs5745652 had a 3-year survival rate of 30.43% (14/46), while patients carrying CT+TT genotype at HGF rs5745652 had a 3-year survival rate of 6.35% (4/63), indicating that patients with CC genotype had higher 3-year survival rate than CT+TT genotype in this aspect (P<0.05). Patients carrying CA+AA genotype at HGF rs2074725 had a 3-year survival rate of 44.00% (11/25), which was significantly higher than those carrying CC genotype at HGF rs2074725 of 8.00% (7/84) (P<0.05) ().
Cox regression analyses
Factors included in the Cox proportional risk model were HGF rs5745652 and HGF rs2074725, age, gender, tumor size, BCLC stage, and TACE treatment frequency. The results showed that rs5745652 and rs2074725 polymorphisms, tumor size, and BCLC stage were independent factors for prognosis of patients with PLC (all P<0.05) ().
Table 5 Cox regression analysis of prognostic factors of PLA TACE therapy
Discussion
It was reported that genetic change and expression change accordingly can affect the malignant progression and prognosis of tumors.Citation18,Citation19 Therefore, study on the relation of HGF gene polymorphisms with TACE efficacy and survival has great significance. This study proved that HGF polymorphisms affect the efficacy of TACE and survival quality of PLC patients. Patients carrying HGF CC genotype of rs5745652 or CA+AA genotype of rs2074725 have lower HGF level, better curative effect, higher survival quality, and better prognosis after treatment.
In this study, we found that the allele C of HGF rs5745652 and the A allele of the HGF rs2074725 are protective genes. HGF is a kind of multifunctional and heterogeneous polypeptide produced by mesenchymal cells and it can mediate the growth and dispersion of different types of cells.Citation20 HGF can promote the generation of new blood vessels in tumors through activation mechanism and create conditions for the growth and transfer of tumor.Citation21,Citation22 Evidence has shown that patients with liver cancer had significantly higher serum HGF levels than those without liver cancer.Citation23 HGF and its receptor c-Met will form a paracrine signaling cycle to mediate the development and progression of cancers.Citation24 A study has confirmed that upregulated expression of growth factors, including HGF, and the activation of their signaling pathway play an important role in the formation of liver cancer.Citation25 Another study noted that the “A” allele is a protective gene, which is consistent with the result of this paper.Citation26 Besides, a previous study has demonstrated that the function of the spleen is deteriorating and serum HGF protein levels can be elevated in patients with liver cirrhosis due to overexpression of HGF protein by the spleen.Citation27 Furthermore, patients with chronic hepatitis C have also proved that higher HGF concentrations were correlated with increased fibrosis and angiogenesis and have indicated a higher risk of PLC development.Citation28–Citation31
In this study, we found that the serum HGF level in patients carrying rs5745652 CC genotype was significantly lower than those carrying the CT+TT genotype, and the total efficacy rate of patients carrying CC genotype was significantly higher than those carrying CT+TT genotype. Serum HGF level of patients carrying rs2074725 CA+AA genotype was significantly lower than those carrying the CC genotype, and the total efficacy rate of CA+AA genotype patients was significantly higher than those carrying the CC genotype. It has been demonstrated that HGF levels were higher in cancer cell lines than in normal hepatocyte cell lines, and HGF upregulation can directly promote the mesenchymal and tumorigenic properties in liver cancer through the activation of Akt and COX-2 pathways.Citation32 Therefore, serum HGF increase may implicate in the occurrence and progression of liver cancer. A study has shown that the transcription activity of HGF-1652 T allele was less than that of C allele so TT genotype carriers were more likely to get end-stage liver disease (ESLD) than CT or CC genotype carriers, and accordingly the total efficacy rate of T genotype carriers will be lower.Citation33 A research by Motone et al showed that serum HGF level in rs2074725 CC genotype carriers was much higher than that in CA or AA genotype carriers and the liver HGF secretion of CC genotype carriers increased indicating that the total efficacy rate of TACE therapy will be lower in CC genotype carriers than in CA+AA genotype carriers.Citation34
The study found that the Karnofsky scores of patients carrying HGF rs5745652 CC genotype or carrying HGF rs2074725 CA+AA genotype were significantly enhanced than before TACE treatment, and they were higher than those of counterpart CT+TT genotype or CC genotype carriers, respectively. The 3-year survival rates of the former two were also higher than that of the latter ones. Chemoembolization is an important therapy for patients with liver cancer, and the continuous elevation of serum HGF level after TACE may be associated with the postoperative tumor metastasis.Citation32 Serum HGF level, as an important tumor marker, is closely related to metastasis and recurrence of some tumors, and high HGF level is not beneficial to the prognosis.Citation35–Citation37 There fore, with a relatively low serum HGF level, rs5745652 CC genotype and rs2074725 CA+AA genotype are conducive to the treatment of PLC and the improvement of patients’ survival rate.
This study pointed out that the HGF gene polymorphisms can influence the efficacy of TACE and survival quality of PLC patients. Specifically, the HGF level of patients carrying HGF CC genotype of rs5745652 or HGF CA+AA genotype of rs2074725 was decreased after TACE, which was related to superior curative effect, survival quality, and prognosis. However, the mechanism of how HGF gene rs5745652 site and rs2074725 affect the occurrence, development, and prognosis of PLC has not been clarified, and follow-up study is still needed.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenJGZhangSWLiver cancer epidemic in China: past, present and futureSemin Cancer Biol2011211596921144900
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- PircherAMedingerMDrevsJLiver cancer: targeted future optionsWorld J Hepatol201132384421423913
- FanJHWangJBJiangYAttributable causes of liver cancer mortality and incidence in chinaAsian Pac J Cancer Prev201314127251725624460283
- Hernandez-AlcocebaRSangroBPrietoJGene therapy of liver cancerAnn Hepatol20076151417297424
- LoffroyRFavelierSCherblancVEstivaletLC-arm dual-phase cone-beam CT: a revolutionary real-time imaging modality to assess drug-eluting beads TACE success in liver cancer patientsQuant Imaging Med Surg20133419619924040615
- ZhangJJiangTYJiangBGRMP predicts survival and adjuvant TACE response in hepatocellular carcinomaOncotarget2015653432344225605019
- KesslerJASmithAGChaBSDouble-blind, placebo-controlled study of HGF gene therapy in diabetic neuropathyAnn Clin Transl Neurol20152546547826000320
- SakaiKAokiSMatsumotoKHepatocyte growth factor and Met in drug discoveryJ Biochem2015157527128425770121
- PetriniIBiology of MET: a double life between normal tissue repair and tumor progressionAnn Transl Med2015368225992381
- MaJDeFrancesMCZouCJohnsonCFerrellRZarnegarRSomatic mutation and functional polymorphism of a novel regulatory element in the HGF gene promoter causes its aberrant expression in human breast cancerJ Clin Invest2009119347849119188684
- NakamuraMOnoYJKanemuraMHepatocyte growth factor secreted by ovarian cancer cells stimulates peritoneal implantation via the mesothelial-mesenchymal transition of the peritoneumGynecol Oncol2015139234535426335595
- HellingGWahlinSSmedbergMPlasma glutamine concentrations in liver failurePLoS One2016113e015044026938452
- LlovetJMBruCBruixJPrognosis of hepatocellular carcinoma: the BCLC staging classificationSemin Liver Dis199919332933810518312
- BruixJShermanMPractice Guidelines Committee, American Association for the Study of Liver DiseasesManagement of hepatocellular carcinomaHepatology20054251208123616250051
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- ApostolopolouERaftopoulosVTerzisKPissakiKPagoniMDelibasiSInfection probability score, apache ii and karnofsky scoring systems as predictors of infection onset in haematology-oncology patientsJ Clin Nurs20101911–121560156820384664
- LiuYHuHZhangCCo-expression of mitosis-regulating genes contributes to malignant progression and prognosis in oligodendrogliomasOncotarget2015635382573826926468983
- WuGWilsonGZhouGHebbardLGeorgeJQiaoLOct4 is a reliable marker of liver tumor propagating cells in hepatocellular carcinomaDiscov Med20152011021922926562475
- CaoBSuYOskarssonMNeutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal modelsProc Natl Acad Sci U S A200198137443744811416216
- KawaguchiMKataokaHMechanisms of hepatocyte growth factor activation in cancer tissuesCancers (Basel)2014641890190425268161
- KaraFYildirimAGumusdereMKarataySYildirimKBakanEAssociation between hepatocyte growth factor (HGF) gene polymorphisms and serum HGF levels in patients with rheumatoid arthritisEurasian J Med201446317618125610321
- KarabulutSFTasFAkyuzFClinical significance of serum hepatocyte growth factor (HGF) levels in hepatocellular carcinomaTumour Biol20143532327233324142532
- HuangFIChenYLChangCNYuanRHJengYMHepatocyte growth factor activates Wnt pathway by transcriptional activation of LEF1 to facilitate tumor invasionCarcinogenesis20123361142114822436613
- LevreroMViral hepatitis and liver cancer: the case of hepatitis COncogene200625273834384716799625
- SahebjadaSSchacheMRichardsonAJSnibsonGDaniellMBairdPNAssociation of the hepatocyte growth factor gene with keratoconus in an Australian populationPLoS One201491e8406724416191
- PrystupaAKicinskiPSakJBoguszewska-CzubaraAToruń-JurkowskaAZałuskaWProinflammatory cytokines (il-1alpha, il-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosisGastroenterol Res Pract2015201553261526448742
- Marin-SerranoERodriguez-RamosCDiaz-GarciaFMartín-HerreraLFernández-Gutiérrez-Del-AlamoCGirón-GonzálezJAHepatocyte growth factor and chronic hepatitis cRev Esp Enferm Dig2010102636537120575596
- MedinaJCavedaLSanz-CamenoPHepatocyte growth factor activates endothelial proangiogenic mechanisms relevant in chronic hepatitis c-associated neoangiogenesisJ Hepatol200338566066712713878
- YamagamimHMoriyamaMMatsumuraHSerum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in c-viral chronic liver diseasesCancer200295482483412209727
- DaveauMScotteMFrancoisAHepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinomaMol Carcinog200336313014112619035
- OgunwobiOOLiuCHepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathwaysClin Exp Metastasis201128872173121744257
- HoshinoKSatohTKawaguchiYKuwanaMAssociation of hepatocyte growth factor promoter polymorphism with severity of interstitial lung disease in Japanese patients with systemic sclerosisArthritis Rheum20116382465247221520010
- MotoneMKatsuyaTIshikawaKAssociation between hepatocyte growth factor gene polymorphism and essential hypertensionHypertens Res200427424725115127882
- ToiyamaYMikiCInoueYOkugawaYTanakaKKusunokiMSerum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patientsInt J Cancer200912571657166219569242
- HosodaHIzumiHTukadaYPlasma hepatocyte growth factor elevation may be associated with early metastatic disease in primary lung cancer patientsAnn Thorac Cardiovasc Surg20121811721959198
- CanadasITausAGonzalezIHigh circulating hepatocyte growth factor levels associate with epithelial to mesenchymal transition and poor outcome in small cell lung cancer patientsOncotarget20145145246525625026301