Abstract
Background
Let-7 miRNAs are reported to play an inhibitory role in carcinogenesis, tumor progression, recurrence, and pluripotency of cancer. However, few studies have reported the relationship between let-7 and drug sensitivity, especially for let-7a (a subtype of let-7). This study aimed to investigate the function of let-7a in regulating the sensitivity of hepatocellular carcinoma (HCC) cell lines to cetuximab.
Methods
The cytotoxicity of cetuximab on HCC cell lines (Huh7, Hep3B, HepG2, SNU449, and SNU387) was evaluated using a cell viability assay (the Cell Counting Kit-8 assay) and a cell proliferation assay (the Click-iT EdU Imaging Kit) in the presence of a control, a let-7a mimic, and a let-7a inhibitor. Small interfering RNA to knockdown the expression of signal transducer and activator of transcription 3 (STAT3) were employed. Protein and mRNA expression levels were determined using quantitative polymerase chain reaction and Western blot analysis.
Results
It was found that let-7a enhances the sensitivity of HCC cells with an epithelial phenotype (Huh7, Hep3B, and HepG2) to cetuximab, but has no effect on cells with the mesenchymal phenotype (SNU449 and SNU387). It was determined that STAT3 was a target mRNA of let-7a using TargetScan. Expression of STAT3 and let-7a mRNA were negatively correlated in HCC cell lines. Moreover, let-7a altered the protein and mRNA expression of STAT3. Furthermore, STAT3 knockdown enhanced the function of cetuximab on HCC cell lines with epithelial phenotypes, but not on HCC cell lines with mesenchymal phenotypes. Finally, a rescue experiment confirmed that let-7a affected the sensitivity of HCC cell lines to cetuximab by interacting with STAT3.
Conclusions
There is a functional link between let-7a and STAT3 in enhancing the sensitivity of HCC cells with an epithelial phenotype to cetuximab. Our results provide novel insight into new methodologies for combating HCC drug resistance.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide, and has persistently increasing rates of both incidence and mortality.Citation1 Curative surgical procedures, such as tumor resection and liver transplantation, are not available for advanced HCC patients; instead, they can only turn to chemotherapeutic drugs to slow down the progress of the tumor.Citation2 Currently, sorafenib is the only chemotherapy drug that is widely used in clinical applications as a first-line treatment for advanced HCC.Citation3 However, there is increasing evidence of resistance to this drug.Citation4 As such, identification of curative second-line treatments for advanced HCC has become extremely urgent.
While cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, was shown to display satisfactory curative effect in patients with non-small cell lung cancer,Citation5 many clinical trials have indicated its modest activity in advanced HCC, even though some patients show high EGFR expression.Citation6,Citation7 However, a recent study revealed that rapamycin or an miR-146a mimic could enhance cetuximab cytotoxicity on HCC cell lines.Citation8,Citation9 Therefore, cetuximab may be a promising second-line treatment for HCC in combination with some other complementary medicines, such as microRNAs (miRNAs).
MiRNAs, endogenous noncoding RNA molecules (18–25 nucleotides in length), negatively regulate the expression of numerous target genes. For the past few years, miRNA profiling studies have indicated that many miRNAs are abnormally expressed in HCC tissues and affect the initiation and progression of HCC.Citation10,Citation11 Chiefly, the miRNA let-7 plays a vital role in tumor suppression in many cancers, including esophageal squamous cell carcinoma, lung cancer, nasopharyngeal carcinoma, and prostate cancer.Citation12–Citation15 Although miRNA let-7 is known to correlate with poor prognosis of hepatitis B virus-related HCC patients,Citation16 few studies have investigated its precise function in HCC.
Let-7 has multiple subtypes (a, b, c, d, e, f, and g); however, the let-7a subtype has been correlated with tumor proliferation and differentiation.Citation17–Citation19 Therefore, in this study, we investigated whether the let-7a subtype could increase the sensitivity of HCC cell lines to cetuximab, and aimed to unravel its mechanism of action.
Materials and methods
Cell culture
HCC cell lines Huh7, Hep3B, HepG2, SNU449, and SNU387 were purchased from the Chinese Academy of Science Cell Bank (Shanghai, People’s Republic of China). The ethics committee and institutional review board of the Chinese Academy of Science Cell Bank gave ethical approval for this study. The HepG2 cell line was maintained in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA), Huh7 and Hep3B were maintained in Minimum Essential Medium (Thermo Fisher Scientific), and SNU449 and SNU387 were maintained in RPMI 1640 (Thermo Fisher Scientific). All culture media were supplemented with 10% fetal bovine serum (FBS), and cells were incubated at 37°C in a humidified atmosphere of 5% CO2. Cetuximab was obtained from Merck (Darmstadt, German) and dissolved in double-distilled water for the purpose of this research.
RNA oligoribonucleotides and transfection
The let-7a mimic, inhibitor, control, siRNA-signal transducer and activator of transcription 3 (STAT3), and negative control siRNA were synthesized by Qiagen (Venlo, the Netherlands). Cells were transfected using lipofectamine 2000 (Thermo Fisher Scientific) according to manufacturer’s instructions.
Cell viability assay
HCC cell lines (Huh7, Hep3B, HepG2, SNU449, and SNU387) were seeded at a density of 3,000 cells/well in 96-well plates. After the cells completely adhered to the well, the culture medium was replaced with media containing 10% FBS and a certain concentration of cetuximab (0, 500, 1,000, 1,500, or 2,000 µg/mL) and cultivated at 37°C, 5% CO2 for 48 hours. Cell viability was subsequently measured using a Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Tokyo, Japan) after 1–2 hours, according to manufacturer’s instructions. An MRX II microplate reader (Dynex, Chantilly, VA, USA) was used to measure the optical density at 450 nm.
Western blot
HCC cell lines (Huh7, Hep3B, HepG2, SNU449, and SNU387) were seeded at a density of 2.0×105 cells/well in 6-well plates. After the cells completely adhered to the well, the let-7a mimic, inhibitor, control, si-STAT3, and negative control siRNA were added to the media, respectively. After 48 hours, cells were washed with phosphate-buffered saline three times, and lysed in radio immunoprecipitation assay lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% sodium deoxycholate; 0.1% sodium dodecyl sulphate; and 1 mM phenylmethylsulfonyl fluoride) for 2 hours on ice. Sample proteins were quantified using a Pierce bicinchoninic acid protein assay (Thermo Fisher Scientific). Equal amounts of proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with the antibody of interest. The primary antibody (stat3) concentration was 1:1,000, and the secondary antibody concentration was 1:2,000. All antibodies were purchased from Abcam company (Cambridge, MA, USA).
Cell proliferation assay
The proliferation of HCC cell lines (Huh7, Hep3B, HepG2, SNU449, and SNU387) was determined using the Click-iT EdU Imaging Kit (Thermo Fisher Scientific), according to the manufacturer’s protocol. Briefly, cells were treated with miRNA let-7a mimics, inhibitors, or control for 24 hours, then incubated with an half maximal inhibitory concentration (IC50) (Huh7: 2,564 µg/mL, Hep3B: 2,299 µg/mL, HepG2: 7,167 µg/mL, SNU449: 2,450 µg/mL, and SNU387: 3,182 µg/mL) of cetuximab for 24 hours, and finally with 10 µM EdU for 2 hours before fixation, permeabilization, and EdU staining. Cell nuclei were stained with Hoechst 33342 (Thermo Fisher Scientific) at a concentration of 5 µg/mL for 30 minutes.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from HCC cell lines using TRIzol reagent following the manufacturer’s protocol (Thermo Fisher Scientific). First-strand complementary DNA was generated using a Reverse Transcription System Kit (Promega Corporation, Madison, WI, USA). Real-time and polymerase chain reaction (PCR) primers for miRNA let-7a and STAT3 were purchased from TAKARA Biotechnology (Dalian, People’s Republic of China). Expression of each gene was measured by quantitative PCR with the Applied Biosystems 7,500/7,500 Fast Real-Time PCR system (Thermo Fisher Scientific) and SYBR green dye (TAKARA Biotechnology). Glyceraldehyde 3-phosphate dehydrogenase and U6 were used as internal controls, and the 2−ΔΔCt method was used for relative quantification. All reactions were performed in triplicate.
Statistical analysis
SPSS17.0 software was used for statistical analysis. The experimental data were expressed as mean ± standard deviation, and assessed by a two-tailed Student’s t-test. A P<0.05 level of statistical significance was used.
Results
Let-7a enhances the sensitivity of HCC cells with epithelial phenotypes to cetuximab
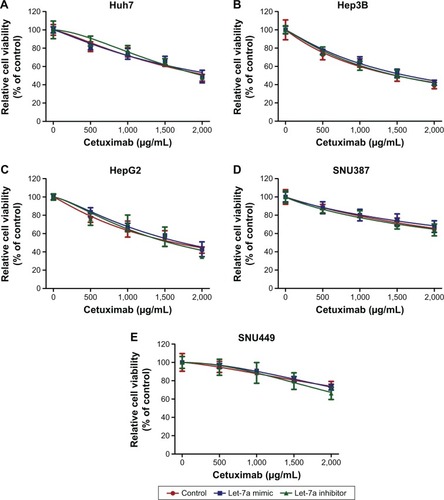
To investigate the function of let-7a on the sensitivity of HCC cell lines to cetuximab, we used a cell viability assay (the CCK-8 assay) and a cell proliferation assay (the EdU assay). We detected the viability of HCC cell lines incubated in the presence of different cetuximab concentrations. Each cell line was divided into three groups: a let-7a mimic, a let-7a inhibitor, and a control group. Compared with the control, the let-7a mimic enhanced the function of cetuximab in HCC cells with epithelial phenotypes (ie, Huh7, Hep3B, and HepG2), while the let-7a inhibitor displayed the opposite results (). However, no difference in the function of cetuximab in HCC cells with mesenchymal phenotypes (ie, SNU387 and SNU449) was observed (). Under the same condition, the EdU assay also revealed that the let-7a mimic could enhance the function of cetuximab on the proliferation of HCC cells with epithelial phenotypes but not in those with mesenchymal phenotypes ( and ).
Figure 1 Let-7a can enhance the sensitivity of HCC cells with epithelial phenotypes to cetuximab.
Abbreviations: CCK-8, Cell Counting Kit-8; Cet, cetuximab; IC50, half maximal inhibitory concentration; HCC, hepatocellular carcinoma.
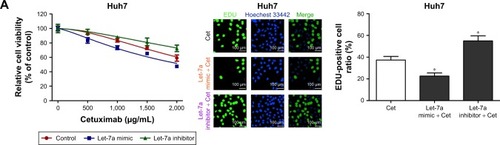
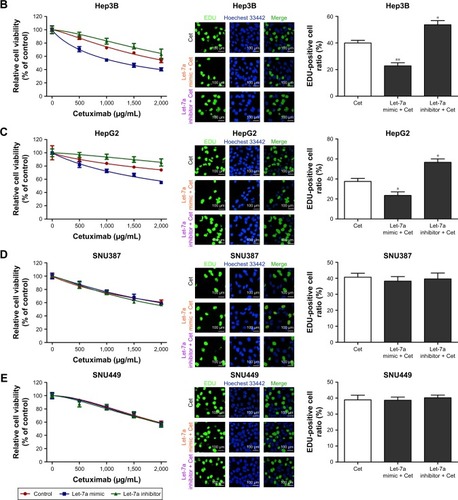
Table 1 IC50 values for cetuximab in HCC cell lines with or without let-7a mimic or let-7a inhibitor treatment
STAT3 is the target gene of let-7a and is negatively regulated by let-7a
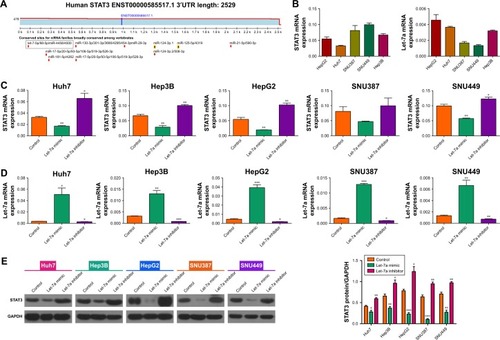
Recent studies have reported that STAT3 expression is correlated with the effect of anti-EGFR therapeutic drugs in solid tumors.Citation20 Therefore, we hypothesized that STAT3 is regulated by the let-7a. We used TargetScan (www.targetscan.org) to predict the associated miRNAs for STAT3. As expected, STAT3 is a target gene of let-7a (). Then, we detected the expression of let-7a and STAT3 mRNA in the HCC cell lines (Huh7, Hep3B, HepG2, SNU387, and SNU449) by PCR. We found the mRNA expression of let-7a was negatively correlated with mRNA expression of STAT3 (). Next, we treated these HCC cell lines separately with a let-7a mimic, inhibitor, and control, and detected the expression of STAT3 and let-7a by PCR. Compared with the control, expression of STAT3 was suppressed in the presence of the let-7a mimic (). However, STAT3 was overexpressed in the presence of the let-7a inhibitor (). These changes in STAT3 expression were further confirmed by our Western blot analysis of STAT3 protein expression (). Therefore, our results suggest that STAT3 is the target gene of let-7a.
Figure 2 Let-7a negatively regulates the expression of STAT3.
Abbreviations: HCC, hepatocellular carcinoma; miRNA, microRNA; mRNA, messenger RNA; STAT3, signal transducer and activator of transcription 3.
Suppression of STAT3 could enhance the sensitivity of epithelial phenotype HCC cells to cetuximab
As STAT3 was negatively regulated by let-7a, and a let-7a mimic can enhance sensitivity of HCC cells with epithelial phenotypes to cetuximab, we hypothesized that the suppression of STAT3 could lead to the same result. To examine this, first we employed siRNA to knockdown the expression of STAT3 and verified the efficiency by Western blot (). Then, we used the CCK-8 assay to detect the cell viability of HCC cell lines in different cetuximab concentrations. Each cell line was divided into two groups: a STAT3 siRNA group and a negative siRNA group. Compared to the control, suppression of STAT3 enhanced the function of cetuximab on HCC cell lines with epithelial phenotypes (ie, Huh7, Hep3B, and HepG2), but not on HCC cell lines with mesenchymal phenotypes (ie, SNU387 and SNU449; and ). Therefore, negative regulation of STAT3 is correlated with the increased sensitivity of HCC cells with epithelial phenotypes to cetuximab.
Figure 3 Suppression of STAT3 could enhance the sensitivity of epithelial phenotype HCC cells to cetuximab.
Abbreviations: CCK-8, Cell Counting Kit-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3.
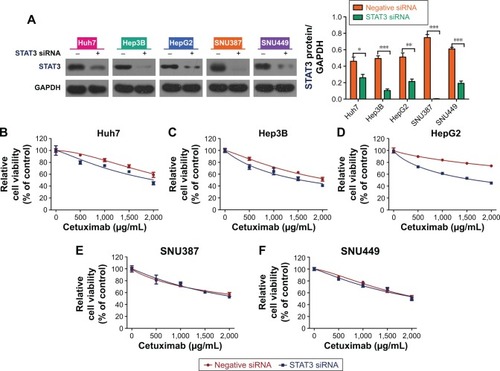
Table 2 IC50 values for cetuximab in HCC cell lines with negative siRNA plus cetuximab treatment or cetuximab plus STAT3 siRNA treatment
STAT3 suppression impairs the role of let-7a on increasing the sensitivity of HCC cells to cetuximab
To verify that let-7a enhances sensitivity of HCC cells with epithelial phenotype to cetuximab through negatively regulating STAT3 expression, we conducted a rescue experiment. We adopted siRNA to knockdown the expression of STAT3 in HCC cell lines. Then we used the CCK-8 assay to detect the cell viability of HCC cell lines in different cetuximab concentrations. Again, each cell line was divided into three groups (a let-7a mimic, a let-7a inhibitor, and a control group). All HCC cell lines showed the same result: that is, the regulatory ability of let-7a on the sensitivity of HCC cell lines to cetuximab was reversed when STAT3 was suppressed (). This indicates that let-7a enhances the sensitivity of HCC cell lines with epithelial phenotypes to cetuximab by regulating STAT3 expression.
Figure 4 The role of let-7a on increasing the sensitivity of HCC cells to cetuximab is impaired after STAT3 suppression.
Abbreviations: CCK-8, cell counting kit-8; HCC, hepatocellular carcinoma; STAT3, signal transducer and activator of transcription 3.
Discussion
Traditional surgical treatment or liver transplantation are the only potentially curative therapies for HCC; however, in the case of advanced HCC, chemotherapy is the only available option.Citation1–Citation3 Moreover, with the increase in resistance to first-line chemotherapy treatments (such as for sorafenib),Citation4 the identification of curative second-line treatments for advanced HCC is urgently required. Furthermore, it has been determined that EGFR activation is a potential determinant of primary resistance of HCC cells to sorafenib, while inhibition of EGFR by its specific inhibitor cetuximab promotes the efficacy of sorafenib.Citation20 So recent studies indicate that cetuximab may be a promising second-line drug for advanced HCC, although it only shows moderate activity.Citation7–Citation9 MiRNAs have shown some promise for enhancing cetuximab cytotoxicity on HCC cell lines.Citation8,Citation9 Therefore, we examined whether the well-known tumor suppressor, let-7a,Citation12–Citation15 could enhance the cytotoxicity of cetuximab in HCC cells. We found that exogenous let-7a overexpression could enhance the sensitivity of HCC cells with epithelial phenotypes to cetuximab, while no difference in sensitivity was observed on HCC cell lines with mesenchymal phenotypes.
Several recent studies have reported that anti-EGFR therapeutic efficacy correlates directly with inhibition of STAT3 activity. In particular, patients who develop cetuximab resistance always have overexpression of STAT3.Citation21 Furthermore, a novel STAT3 inhibitor NSC 74859 enhances the antiproliferative activity of cetuximab in HCC.Citation22 Moreover, the activity of STAT3 was correlated with tumor proliferation, migration, and invasion.Citation23 Cetuximab, an anti-EGFR monoclonal antibody that targets the extracellular domain of the EGFR, is used to treat several cancers.Citation24 Furthermore, the previous research demonstrated that activation of STAT3 was shown to play a role in tumor resistance to anticancer drugs, and downregulation of pSTAT3 increased the sensitivity of human cancer cells to cetuximab. Therefore, we examined whether the function of let-7a on HCC sensitivity to cetuximab was related to STAT3. We used TargetScan to search the correlated miRNAs of the STAT3 3′-UTR segment, and found that STAT3 is a target gene of let-7a. Furthermore, we found that STAT3 expression was negatively correlated with let-7a. This suggests that STAT3 is negatively regulated by let-7a. Indeed, in the presence of a let-7a mimic, STAT3 expression was decreased. To further verify our hypothesis, we used STAT3 siRNA to knockdown STAT3 expression in HCC cell lines. When the HCC cell lines were incubated in the presence of a control, a let-7a mimic, and a let-7a inhibitor, we found no differences in the sensitivity (cytoxicity) of the cells to cetuximab.
In conclusion, our study found that let-7a can enhance the sensitivity of HCCs with an epithelial phenotype to cetuximab by regulating STAT3 expression, but has no effect on HCC cells with the mesenchymal phenotype. This finding suggests a crucial role of let-7a in future miRNA-based combined chemotherapy. However, the validity and security of combined chemotherapy based on let-7a should be carefully evaluated in well-designed clinical trials.
Acknowledgments
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, CA, USA) for editing our manuscript. This work was supported by grants from the National Natural Science Foundation of People’s Republic of China (number 81273260) and the Henan provincial key scientific and technological project (number 132102310088).
Disclosure
The authors report no conflicts of interest in this work.
References
- FinnRSCurrent and future treatment strategies for patients with advanced hepatocellular carcinoma: role of mTOR inhibitionLiver Cancer201213–424725624159589
- ZhouYLiangCXueFSalinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the β-catenin/TCF complex association via FOXO3a activationOncotarget2015612103501036525871400
- FornerAGilabertMBruixJRaoulJLTreatment of intermediate-stage hepatocellular carcinomaNat Rev Clin Oncol201411952553525091611
- WuQYangZNileYShiYFanDMulti-drug resistance in cancer chemotherapeutics: mechanisms and lab approachesCancer Lett2014347215916624657660
- PirkerRPereiraJRvon PawelJEGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX studyLancet Oncol2012131334222056021
- ZhuAXStuartKBlaszkowskyLSPhase 2 study of cetuximab in patients with advanced hepatocellular carcinomaCancer2007110358158917583545
- SanoffHKBernardSGoldbergRMPhase II study of capecitabine, oxaliplatin, and cetuximab for advanced hepatocellular carcinomaGastrointest Cancer Res201143788322043322
- ChenWHuQDXiaXFRapamycin enhances cetuximab cytotoxicity by inhibiting mTOR-mediated drug resistance in mesenchymal hepatoma cellsCancer Biol Ther201415899299924800850
- HuangSHeRRongMDangYChenGSynergistic effect of MiR-146a mimic and cetuximab on hepatocellular carcinoma cellsBiomed Res Int2014201438412124895573
- AugelloCVairaVCarusoLMicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster as a novel prognostic biomarker in hepatocellular carcinomaLiver Int201232577278222429613
- SatoFHatanoEKitamuraKMicroRNA profile predicts recurrence after resection in patients with hepatocellular carcinoma within the Milan CriteriaPLoS One201161e1643521298008
- LiuQLvGDQinXRole of microRNA let-7 and effect to HMGA2 in esophageal squamous cell carcinomaMol Biol Rep20123921239124621598109
- WagnerSNgezahayoAMurua EscobarHNolteIRole of miRNA let-7 and its major targets in prostate cancerBiomed Res Int2014201437632625276782
- WongTSManOYTsangCMMicroRNA let-7 suppresses nasopharyngeal carcinoma cells proliferation through downregulating c-Myc expressionJ Cancer Res Clin Oncol2011137341542220440510
- GuanHZhangPLiuCCharacterization and functional analysis of the human microRNA let-7a2 promoter in lung cancer A549 cell linesMol Biol Rep20113885327533421365266
- LiZGuoYZhouLAssociation of a functional RAD52 genetic variant locating in a miRNA binding site with risk of HBV-related hepatocellular carcinomaMol Carcinog201554985385824729511
- RoushSSlackFJThe let-7 family of microRNAsTrends Cell Biol2008181050551618774294
- BruecknerBStresemannCKunerRThe human let-7a-3 locus contains an epigenetically regulated microRNA gene with oncogenic functionCancer Res20076741419142317308078
- LuLKatsarosDde la LongraisIASochircaOYuHHypermethylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-II expression and favorable prognosisCancer Res20076721101171012217974952
- EzzoukhryZLouandreCTrecherelEEGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenibInt J Cancer2012131122961296922514082
- UngNPutoczkiTLStylliSSAnti-EGFR therapeutic efficacy correlates directly with inhibition of STAT3 activityCancer Biol Ther201415562363224556630
- ChenWShenXXiaXNSC 74859-mediated inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinomaLiver Int2012321707722098470
- WheelerSESuzukiSThomasSMEpidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activationOncogene201029375135514520622897
- IidaMBrandTMStarrMMOvercoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapyMol Cancer20141324225344208
