Abstract
Objective
The value of antiangiogenic inhibitors for patients with recurrent ovarian cancer has not been completely affirmed. Therefore, we aimed to assess the effectiveness and toxicities of various antiangiogenic drugs for the treatment of recurrent ovarian cancer.
Methods
In this meta-analysis, we searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials databases for complete randomized controlled trials. The searches were extended to May 15, 2016. The risk of bias of the included studies was evaluated via a Cochrane systematic evaluation, and the statistical analyses were performed using RevMan 5.2 software.
Results
In total, we included 8 randomized controlled trials involving 3,211 patients and divided them into 3 groups, vascular endothelial growth factor receptor inhibitors (VEGFRIs), vascular endothelial growth factor (VEGF) inhibitors (bevacizumab), and angiopoietin inhibitors (trebananib). The progression-free survival improved significantly in all the groups being given antiangiogenic drugs (hazard ratio [HR]: 0.55, 95% confidence interval [CI]: 0.45–0.67, I2=0%, P<0.00001 for the VEGFRI group; HR: 0.53, 95% CI: 0.45–0.63, I2=51%, P<0.00001 for the VEGF inhibitor group; HR: 0.67, 95% CI: 0.58–0.77, I2=0%, P<0.00001 for the trebananib group). Overall survival was obviously prolonged in the VEGFRI (HR: 0.76, 95% CI: 0.59–0.97, I2=0%, P=0.03), the VEGF inhibitor (HR: 0.87, 95% CI: 0.77–0.99, I2=0%, P=0.03), and trebananib groups (HR: 0.81, 95% CI: 0.67–0.99, I2=0%, P=0.04). The incidence of grade 3/4 side effects was different among the 3 groups, for example, proteinuria, hypertension, gastrointestinal perforation, and arterial thromboembolism were presented in the VEGF inhibitor group. Increased incidences of fatigue, diarrhea, and hypertension were seen in the VEGFRI group, and the trebananib group had a higher incidence of hypokalemia.
Conclusion
This meta-analysis showed that antiangiogenic drugs improved the progression-free survival. The VEGFRI, bevacizumab, and trebananib groups showed increased overall survival. Adding antiangiogenic drugs to chemotherapy treatment resulted in a higher incidence of grade 3/4 side effects, but these were manageable.
Introduction
Currently, ovarian cancer is the leading cause of cancer-related death in middle-aged and elderly females.Citation1 Despite the significantly improved prognosis of advanced ovarian cancer, it will recur in >50% of women within 18–24 months.Citation2 The treatment of relapsing ovarian cancer mainly consists of a single or a combination of intravenous chemotherapy. The addition of antiangiogenic drugs in the treatment of relapsed ovarian cancer has not yet been fully defined.Citation3
According to our search results, 8 randomized controlled trials (RCTs) have been conducted on this topic.Citation4–Citation11 To the best of our knowledge, there are 2 pathways for neovascularization, including the vascular endothelial growth factor (VEGF) and angiopoietin pathways. VEGF signaling through VEGF receptors (VEGFRs) activated downstream signal transduction molecules phospholipase C-γ(PLC-γ), PI3K, Akt, Ras, Src, and MAPK and regulated cell proliferation, migration, survival, and vascular permeability.Citation10,Citation12–Citation15 Therefore, we divided these RCTs into 3 groups, including a VEGF receptor inhibitor (VEGFRI) group, VEGF inhibitor group, and angiopoietin group.
Several meta-analyses have been conducted on a single antiangiogenic drug or advanced ovarian cancer. However, this meta-analysis aimed to estimate the efficacy and toxicity of various antiangiogenic drugs for the treatment of patients with recurrent ovarian cancer.
Methods
The PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases were comprehensively searched from January 2000 to May 2016, without language restrictions. The search was limited to RCTs with or without antiangiogenic therapy for recurrent ovarian cancer. The search terms included “ovarian cancer”, “ovarian carcinoma”, “ovarian neoplasm”, “ovarian tumor”, “angiogenesis”, “angiogenic”, and “randomized controlled trial”. Abstracts from the annual meetings of the American Society of Clinical Oncology, the European Society of Medical Oncology, and the Society of Gynecologic Oncology from within the past five years were also searched.
Study selection and inclusion criteria
The inclusion criteria were as follows: 1) the research subjects were patients with recurrent ovarian cancer, including platinum-sensitive and platinum-resistant patients; 2) chemotherapy interventions with or without antiangiogenic drugs; and 3) RCTs. The articles were obtained for an independent assessment of eligibility by 2 of the authors (SY Yi and LJ Zeng). A difference of opinion was resolved via consultation with a third author (Y Kuang), if necessary.
Data extraction and quality assessment
Two of the authors (SY Yi and LJ Zeng) independently extracted the data on the basis of the following: first author, year of publication, age, pathology, sample size, intervention, and outcome data. As shown in , we assessed the quality of the eligible studies according to the Cochrane Collaboration’s risk of bias tool in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. We resolved any disagreements by discussing them with a third review author (Y Kuang).
Statistical analysis
The pooled hazard ratios (HRs) and 95% confidence interval (95% CI) for the progression-free survival (PFS) or overall survival (OS) and the relative risks (RRs) for adverse events from all the articles were calculated using RevMan 5.2. The heterogeneity among the studies was estimated using the I2 index. We use the fixed-effects model if I2≤50%, otherwise the random-effects model was applied. The statistical analyses were performed using the RevMan 5.2 software.
Results
Search and study characteristics
The search process of this study is presented in , and the characteristics of the RCTs used are presented in . After searching the literature, only 8 RCTs containing 3,211 patients with recurrent ovarian cancer were included in this analysis. We divided them into the following 3 groups according to the different active targets of the antiangiogenic drugs: 3 RCTs with VEGFRIs,Citation4–Citation6 3 RCTs with VEGF inhibitors,Citation7–Citation9 and 2 RCTs with angiopoietin inhibitors.Citation10,Citation11 One RCT applied antiangiogenic drugs during the maintenance phase,Citation4 but the other drugs were fully employed from the beginning of therapy to disease progression in the other 7 RCTs. The evaluation of the quality of the RCTs included in this study is shown in . We were not concerned about publication bias due to the fact that only 8 articles were included.Citation16
Figure 2 Flowchart showing study selection procedure.
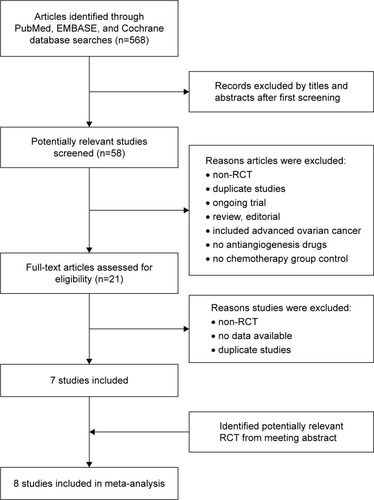
Table 1 The basic characteristics of the included randomized controlled trials
PFS
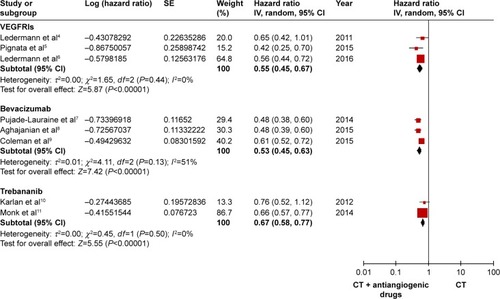
A random-effects model was applied with high heterogeneity, and all the RCTs included reported the HRs and 95% CIs of the PFS. As illustrated in , compared with chemotherapy alone, the PFS improved significantly in all the groups, as follows: HR: 0.55, 95% CI: 0.45–0.67, I2=0%, P<0.00001 for the VEGFRI group; HR: 0.53, 95% CI: 0.45–0.63, I2=51%, P<0.00001 for the VEGF inhibitor group; and HR: 0.67, 95% CI: 0.58–0.77, I2=0%, P<0.00001 for the trebananib group.
OS
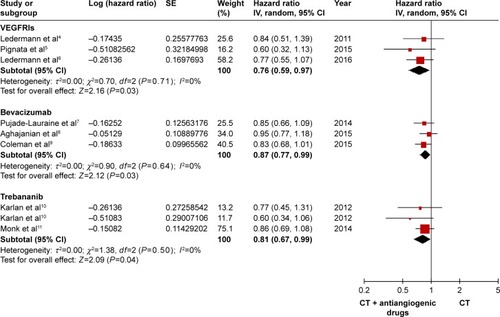
The OS was determined by using a fixed-effects model attributed to low heterogeneity among the studies. Only 1 study did not report the OS.Citation5 The OS was obviously prolonged in the VEGFRI (HR: 0.76, 95% CI: 0.59–0.97, I2=0%, P=0.03), the VEGF inhibitor group (HR: 0.87, 95% CI: 0.77–0.99, I2=0%, P=0.03), and the trebananib group (HR: 0.81, 95% CI: 0.67–0.99, I2=0%, P=0.04). The outcomes are shown in .
Toxicity (adverse effect grade ≥3, except gastrointestinal perforation [GIP] grade ≥1)
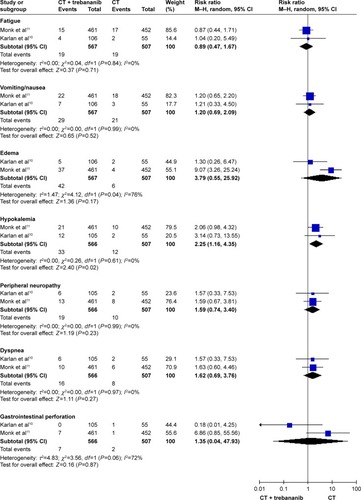
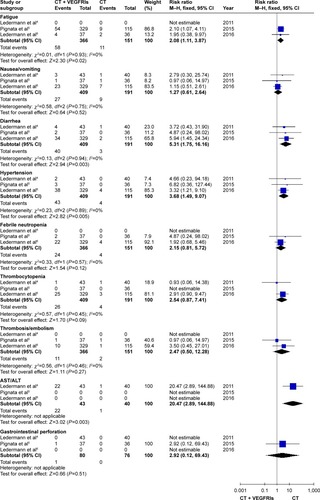
As presented in , the rates of hypertension (RR: 3.68, 95% CI: 1.49–9.07, I2=0%, P=0.005), fatigue (RR: 2.08, 95% CI: 1.11–3.87, I2=0%, P=0.02), and diarrhea (RR: 5.31, 95% CI: 1.75–16.16, I2=0%, P=0.003) were numerically higher in the VEGFRI treatment group. In addition, the nintedanib as a VEGFRI was associated with higher incidences of hepatotoxicity (RR: 20.47, 95% CI: 2.89–144.88, P=0.003).
Figure 5 The forest plot for evaluating the toxicity (the VEGFRI group).
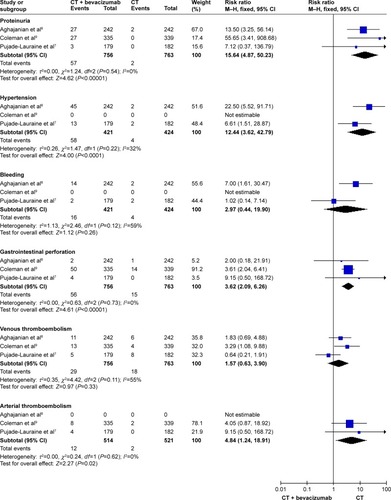
With regard to the VEGF inhibitor group, the proteinuria (RR: 15.64, 95% CI: 4.87–50.23, I2=0%, P<0.00001), hypertension (RR: 12.44, 95% CI: 3.62–42.79, I2=32%, P<0.0001), arterial thromboemboli (RR: 4.84, 95% CI: 1.24–18.91, I2=0%, P=0.02), and GIP (RR: 3.62, 95% CI: 2.09–6.26, I2=0%, P<0.00001) were significantly different ().
Figure 6 The forest plot for evaluating the toxicity (the bevacizumab group).
As shown in , the trebananib treatment may cause a higher risk of hypokalemia (RR: 2.25, 95% CI: 1.16–4.35, I2=0%, P=0.02).
Subgroup analysis
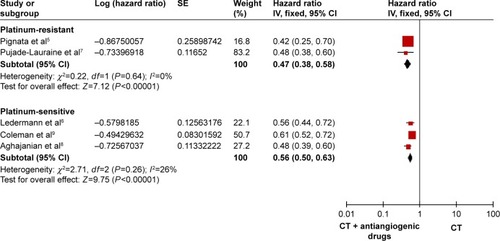
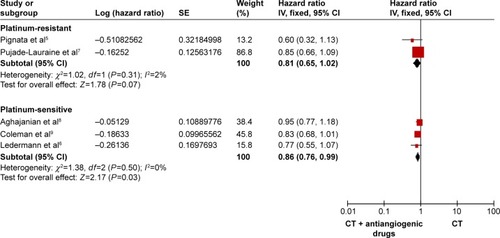
We divided the RCTs into 2 subgroups, 2 RCTs with platinum-resistantCitation5,Citation7 and 3 RCTs with platinum-sensitive.Citation6,Citation8,Citation9 As shown in , the PFS improved significantly both the platinum-resistant and the platinum-sensitive recurrent ovarian cancer (HR: 0.47, 95% CI: 0.38–0.58, I2=0%, P<0.00001 for the platinum-resistant group; HR: 0.56, 95% CI: 0.50–0.63, I2=26%, P<0.00001 for the platinum-sensitive group). As presented in , the OS was clearly better in the platinum-sensitive group (HR: 0.86, 95% CI: 0.76–0.99, P=0.03), with no obvious statistical significance for OS in the platinum-resistant group (HR: 0.81, 95% CI: 0.65–1.02, I2=2%, P=0.07).
Discussion
Ovarian cancer is the leading cause of cancer deaths in women,Citation1 and many patients with advanced ovarian cancer will undergo recurrences with chemotherapy resistance. Ovarian carcinoma is a complicated disease, controlled by multiple mutating genes, and there are no clear molecular therapeutic targets. Therefore, a new promising therapy targeting the tumor microenvironment has been proposed. Neovascularization is crucial for tumor growth and metastasis, and various antiangiogenic drugs have subsequently emerged.Citation17–Citation19
To the best of our knowledge, VEGF plays an important role in neovascularization. VEGF signals through VEGFRs and upregulates downstream signaling pathways. Another pathway uses angiopoietin, which is a regulator of tumor angiogenesis. Based on the mechanism of vascularization, we divided the RCTs into 3 groups to perform our analysis.Citation10,Citation12–Citation14 In this meta-analysis, the PFS showed significant improvement with antiangiogenic therapy in those patients with relapsed ovarian cancer. The OS was clearly better in the VEGFR inhibitor, the bevacizumab and trebananib groups. In addition, both the platinum-resistant and the platinum-sensitive subgroups had a benefit in the PFS. The OS was obviously improved in the platinum-sensitive group; however, it was not statistically significant in the platinum-resistant group.
In our study, the incidences of grade 3/4 toxicity were higher when compared with chemotherapy alone but were manageable; however, the type and incidence of side effects (≥ grade 3) varied with the different anti-angiogenic drugs. Furthermore, the incidence of GIP in the bevacizumab group was higher than the VEGFRIs and trebananib groups. Oncologists gradually realized the significant risk of GIP. They reported that the rate of GIP in recurrent ovarian cancer varies from 0% to 11.4%. However, in large Phase III clinical trials, the rate of perforation is 0%–2.6%. GIPs carry a high rate of mortality, especially in the setting of recurrent, platinum-resistant ovarian cancer.Citation20 Overall, the application of antiangiogenic drugs remains controversial. Bevacizumab is the most successful antiangiogenic drug, but it is not currently the upfront chemotherapy, because it has shown no statistical significance in improving the OS in recurrent ovarian cancer according to original studies.Citation3,Citation7–Citation9
Antiangiogenic therapy could be applied for recurring ovarian cancer, if the patient has previously received antiangiogenic therapy; however, the impact on the efficacy is not known. Both Huijbers’s and Bergers’s studies have shown the role of tumor stromal cells in the resistance to antiangiogenic drugs, and the mechanisms for use are diverse, including endothelial (progenitor) cells, smooth muscle cells, pericytes, fibroblasts, immune cells, and platelets. Multiple mechanisms develop evasive or intrinsic resistance and finally become resistant to antiangiogenic therapy. Although the incidence of resistance to antiangiogenic therapy after previous antiangiogenic therapy is not clear, it is closely related to the prognosis of the patient with recurrent ovarian cancer. Currently, there are no relevant trials in the literature.Citation3,Citation21,Citation22
Which recurrent patients benefit most from antiangiogenic therapy remains unclear. Abu-Jawdeh et al discovered that VEGF and its receptors were not expressed in benign ovarian tumors, with intermediate levels of expression shown in borderline ovarian tumors and strong expression shown in malignant cancer, via detection in the serum, ovarian tumor tissue, and ascites. Recently, Pasquet et al reported that ascites-derived stromal cells play a role in promoting tumor growth by inducing angiogenesis. Antiangiogenic therapy alone delays tumor tissue growth, narrows metastatic lesions, and reduces malignant peritoneal effusion. The research by Ferriss et al showed that patients with ascites treated with bevacizumab had significantly better PFS and OS, but the issue has not reached consistency.Citation23–Citation26
In addition to bevacizumab, there were several antiangiogenic drugs described in our meta-analysis. Therefore, the decision about selecting an appropriate antiangiogenic drug to obtain the most benefit for recurrent patients will be complicated. Both the characteristics of the patients and the toxicity of the drugs must be taken into account. Currently, only bevacizumab and pazopanib have been recommended for application in ovarian cancer by the National Comprehensive Cancer Network.Citation3
In addition, the optimal doses and therapeutic time period have not yet been determined. In our meta-analysis, the doses and durations differed among the 8 RCTs we included in this study. The survival outcomes were improved with the use of antiangiogenic therapy as a maintenance therapy for patients with recurrent ovarian cancer. A previous meta-analysis, conducted by Qian et al, also confirmed that both the PFS and OS were improved in the antiangiogenic maintenance therapy group, when compared with the chemotherapeutic group alone.Citation27,Citation28 According to preclinical researches, tumor vessels were known to be leaky and dysfunctional, which disturb delivery of chemotherapy to tumor. Vascular normalization by crossover application of antiangiogenic therapy was proposed to increase the density of chemotherapeutic agents in tumor. Currently, clinical studies were not enough to prove the vascular normalization theory. Therefore, the effect of difference was still unknown between crossover and subsequent antiangiogenic therapy.Citation29,Citation30 In a word, a standard antiangiogenic regime has not yet been established.
Directly predicting the clinical efficacy of antiangiogenic drugs using biomarkers is currently under study, but the results do not look good. The measurement of serum-free VEGF was proposed first based on its easy accessibility and reasonable price. However, there is little predictive value as a serum biomarker due to its dynamic change between the total and free VEGF levels. Recently, 2 studies have recognized several biomarkers (mesothelin, FLT4, AGP, CA-125, and miR-378) that seem predictive of the benefits from the antiangiogenic treatment of ovarian cancer, but further larger population studies are required.Citation31–Citation33
Despite the fact that we only included RCTs to minimize bias in our meta-analysis, several potential limitations remain. Due to the disparate design of the research studies, characteristics of the recurrent patients, various ovarian tumor types, unclear International Federation of Gynecology and Obstetrics staging, and different doses and durations of the drugs, there was heterogeneity among all 8 studies.
Conclusion
The antiangiogenic therapy showed a clear improvement in the PFS in the treatment of relapsed ovarian cancer patients. In addition, the bevacizumab and trebananib groups showed prolonged OS. Antiangiogenesis as a targeted therapy seems to be promising, despite the many uncertainties put forth in our study.
Disclosure
The authors report no conflicts of interest in this work.
References
- LuveroDMilaniALedermannJATreatment options in recurrent ovarian cancer: latest evidence and clinical potentialTher Adv Med Oncol20146522923925342990
- American Cancer SocietyCancer facts and figures 2008 Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfacts-figures2008/indexAccessed December 31, 2008
- National Comprehensive Cancer NetworkNCCN Clinical practice Guidelines in Oncology: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 22015 Available from: http://www.nccn.orgAccessed 2, 2015
- LedermannJAHackshawAKayeSRandomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancerJ Clin Oncol201129283798380421859991
- PignataSLorussoDScambiaGPazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomized, open-label, phase 2 trialLancet Oncol201516556156825882986
- LedermannJAEmbletonACRajaFCediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomized, double-blind, placebo-controlled phase 3 trialLancet2016387100231066107427025186
- Pujade-LauraineEHilpertFWeberBBevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trialJ Clin Oncol201432131302130824637997
- AghajanianCGoffBNycumLRWangYVHusainABlankSVFinal overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancerGynecol Oncol20151391101626271155
- ColemanRLBradyMFHerzogTJGynecologic oncologyPresented at: Society of Gynecologic Oncology 2015 Annual Meeting on Women’s CancerMarch 28–31, 2015Chicago, IL, USA Abstract 3
- KarlanBYAmitMOGaryERRandomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancerJ Clin Oncol201230436237122184370
- MonkBJAndrésPIgnaceVAnti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomized, multicenter, double-blind, placebo-controlled phase 3 trialLancet Oncol201415879980824950985
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab, and bevacizumabAngiogenesis201215217118522302382
- HolashJMaisonpierrePCComptonDVessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGFScience199928454221994199810373119
- LokadasanRJamesFVNarayananGPrabhakaranPKTargeted agents in epithelial ovarian cancer: review on emerging therapies and future developmentsEcancer medical science20161062627110282
- NilssonMHeymachJVVascular endothelial growth factor (VEGF) pathwayJ Thorac Oncol2006176877017409958
- SongFEustwoodAJGilbodySDuleyLSuttonAJPublication and related biasesHealth Technol Assess20004101115
- ColemanRLMonkBJSoodAKHerzogTJLatest research and treatment of advanced-stage epithelial ovarian cancerNat Rev Clin Oncol201310421122423381004
- Cancer Genome Atlas Research NetworkIntegrated genomic analyses of ovarian carcinomaNature2011474735360961521720365
- HansenJMColemanRLSoodAKTargeting the tumor microenvironment in ovarian cancerEur J Cancer20165613114326849037
- McClungECWenhamRMProfile of bevacizumab in the treatment of platinum-resistant ovarian cancer: current perspectivesInt J Womens Health20168597527051317
- HuijbersEJvan BeijnumJRThijssenVLSabrkhanySNowak-SliwinskaPGriffioenAWRole of the tumor stroma in resistance to anti-angiogenic therapyDrug Resist Updat201625263727155374
- BergersGHanahanDModes of resistance to anti-angiogenic therapyNat Rev Cancer20088859260318650835
- Abu-JawdehGMFaixJDNiloffJStrong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasmsLab Invest1996746110511158667614
- YamamotoSKonishiIMandaiMExpression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levelsBr J Cancer1997769122112279365173
- PasquetMGolzioMMeryEHospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesisInt J Cancer201012692090210119739074
- FerrissJSJavaJJBookmanMAAscites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube, and peritoneal cancers: an NRG oncology/GOG studyGynecol Oncol20151391172226216729
- KhaliqueSHookJMLedermannJAMaintenance therapy in ovarian cancerCurr Opin Oncol201426552152825033374
- QianXQinJPanSLiXPanYMaSMaintenance therapy in ovarian cancer with targeted agents improves PFS and OS: a systematic review and meta-analysisPLoS One2015109e013902626402447
- JainRKNormalization of tumor vasculature: an emerging concept in antiangiogenic therapyScience2005307586215637262
- JainRKNormalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapyNat Med2001798798911533692
- JubbAMHarrisALBiomarkers to predict the clinical efficacy of bevacizumab in cancerLancet Oncol201011121172118321126687
- ChanJKKietTKBlansitKMiR-378 as a biomarker for response to anti-angiogenic treatment in ovarian cancerGynecol Oncol2014133356857424680769
- CollinsonFHutchinsonMCravenRAPredicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selectionClin Cancer Res201319185227523923935036