Abstract
Despite multimodal therapeutic treatments of osteosarcoma (OS), some patients develop resistance to currently available regimens and eventually end up with recurrent or metastatic outcomes. Many attempts have been made to discover effective drugs for improving outcome; however, due to the heterogeneity of the disease, new therapeutic options have not yet been identified. This study aims to explore potential targeted therapy related to protein profiles of OS. In this review of proteomics studies, we extracted data on differentially expressed proteins (DEPs) from archived literature in PubMed and our in-house repository. The data were divided into three experimental groups, DEPs in 1) OS/OB: OS vs osteoblastic (OB) cells, 2) metastasis: metastatic vs non-metastatic sublines plus fresh tissues from primary OS with and without pulmonary metastasis, and 3) chemoresistance: spheroid (higher chemoresistance) vs monolayer cells plus fresh tissues from biopsies from good and poor responders. All up-regulated protein entities in the list of DEPs were sorted and cross-referenced with identifiers of targets of US Food and Drug Administration (FDA)-approved agents and chemical inhibitors. We found that many targets of FDA-approved antineoplastic agents, mainly a group of epigenetic regulators, kinases, and proteasomes, were highly expressed in OS cells. Additionally, some overexpressed proteins were targets of FDA-approved non-cancer drugs, including immunosuppressive and antiarrhythmic drugs. The resulting list of chemical agents showed that some transferase enzyme inhibitors might have anticancer activity. We also explored common targets of OS/OB and metastasis groups, including amidophosphoribosyltransferase (PPAT), l-lactate dehydrogenase B chain (LDHB), and pyruvate kinase M2 (PKM2) as well as the common target of all categories, cathepsin D (CTSD). This study demonstrates the benefits of a text mining approach to exploring therapeutic targets related to protein expression patterns. These results suggest possible repurposing of some FDA-approved medicines for the treatment of OS and using chemical inhibitors in drug screening tests.
Introduction
Osteosarcoma (OS) is the most common primary tumor of bone and has a high incidence in children and adolescents.Citation1 An overall incidence is accounted for one to three per million annually worldwide. Multimodal treatment of OS currently includes surgery and multi-agent chemotherapy (ie, doxorubicin, cisplatin, and methotrexate), which results in an overall survival of ~60%–70% in patients with localized disease.Citation2 Nevertheless, for the past three decades, the 5-year survival rate of patients with metastasis remained stable at 30%.Citation3 The survival expectancy of OS patients with poor response to preoperative chemotherapy is even lower compared to the good responders.Citation4 An effective therapy is a need to improve survival of those patients with unfavorable prognosis.
Many attempts have been made to develop targeted medicines with low levels of adverse effects, which confer benefits to individual patients. Some clinical trials of targeted medicines in OS patients showed an improvement of outcome in subgroups of patients, whereas others demonstrated only low or no response (https://ClinicalTrials.gov). This lack of effectiveness is mainly due to OS genomic instability, the result of as yet unidentified driver genes.Citation5 Dramatic heterogeneity of OS exists both among patients and in intra-tumor masses. Whole-genome sequencing (WGS) of OS specimens has revealed a high rate of somatic structural variation and gene copy number alterations.Citation6 Various oncogenic pathways have been reported to be involved in the pathogenesis of this disease. All these facts suggest that to improve a response to targeted therapy in OS, it will be necessary to be able to match the right patient with the right treatment.
In an era of an advanced “omics” technology and big data analysis, proteomics is a major technique of choice for studying proteins expressed by certain types of cancer.Citation7 Aberrant protein expression is an important characteristic of malignant transformation that involves changes in various cellular processes.Citation8 Not only does proteomics determine the alteration of protein abundance, it also serves as a valuable tool for investigating diversity of proteomes, which arise largely from alternative splicing and post-translational modification.Citation9 This technique allows scientists to gain additional insights into the molecular mechanisms that relate to oncogenic pathways.
This study aims to explore potential targeted therapy related to protein profiles of OS that possibly determine oncogenic phenotypes. To target putative oncogenic drivers, the list of up-regulated proteins was cross-referenced with antineoplastic agents approved by the US Food and Drug Administration (FDA). This method identified a potential targeted therapy based on an oncogene addiction model. This study also provided likely new targets for the treatment of OS using a drug repurposing approach. Interestingly, many groups of non-FDA-approved protein inhibitors were also explored as candidates for targeted therapy of OS. Based on these findings, we suggest further experimental testing of FDA-approved medicines for the treatment of OS and reveal possible uses of chemical agents as anticancer modality.
Methods
Literature on mining of proteomics of OS
In this study, we searched proteomics of OS data through the PubMed database using the search term “osteosarcoma AND Proteomics” for articles available before January 31, 2016. Studies that examined non-human models were excluded. All proteomics information on OS cell lines and tissue specimens were retrieved and categorized according to their comparative models. Those models are 1) OS/OB: OS vs osteoblastic (OB) cells, 2) metastasis: metastatic vs non-metastatic sublines plus fresh tissues from primary OS with and without pulmonary metastasis, and 3) chemoresistance: spheroid (higher chemoresistance) vs monolayer cells plus fresh tissues from biopsies from good and poor responders. Differentially expressed proteins (DEPs) with significant statistical power were extracted from the original articles as well as from supplementary data sources. Identifiers of genes and/or proteins that were not provided in the articles were added to the list by searching the UniProt database.
Annotation of biological functions and pathways of DEPs
To explore enriched biological functions and pathways of DEPs, we used an available bioinformatics approach for proteomics data analysis. Biological processes relating to DEPs were designated as Gene Ontology (GO) entities following the GO database released May 20, 2016.Citation10 Cluster analysis of DEPs regarding their related biological process was also performed using the PANTHER classification system Version 10.0 released May 15, 2015.Citation11 Pathway information from the KEGG and BIOCARTA databases was accessed via DAVID Bioinformatics Resources 6.7.Citation12
Generating lists of therapeutic targets in OS
Up-regulated proteins were sorted from the list of DEPs in each of the experimental groups (OS/OB, metastasis, and chemoresistance). The proteins were cross-referenced with lists of FDA-approved antineoplastic agents, FDA-approved non-antineoplastic agents, and non-FDA-approved chemical agents, reported elsewhere.Citation13
Results
Literature mining
According to the PubMed database, proteomics studies of OS have been performed both in cell cultures and in clinical specimens including the study of 1) OS and OB cells, 2) OS and benign tumor tissues, 3) phenotypic characteristics of OS cells including metastasis and chemoresistance, 4) responsiveness to various medicines or specific induction, 5) OS cell lines, and 6) serum or plasma from OS patients (). The primary literature mining unveiled a number of DEPs. We found that DEPs in specific models might relate to molecular mechanisms of relapse events in OS patients, so we focus primarily on proteomics analysis of DEPs in three experimental groups: OS/OB, metastases, and chemoresistance.
Table 1 Proteomics studies of osteosarcoma in PubMed database
Eight proteomics studies of OS/OB were published from 2006 through early 2016 (). Those proteomics studies of OS cells were performed mainly using a gel-based approach, and most studied biological mechanisms of the disease as well as seeking potential diagnostic biomarkers and novel therapeutic targets. From our previous work, we have performed proteomics study in primary OS cells (n=7) and OB cells of cancellous bone (n=7) using two-dimensional gel electrophoresis (2DE) and liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.Citation14 We successfully identified DEPs in OS compared to OB cells. Therefore, to generate a list of DEPs in OS, proteomics data from literature and in-house results were combined. The end result was the successful identification of ~2,300 DEPs.Citation15–Citation22 Proteomics studies of metastatic and chemoresistant phenotypes of OS have previously been reported by different research groups ( and ). Although the number of publications on these events has been limited, we found that the data mined from text database resources were informative. Overall, 49 and 29 DEPs were identified in metastatic and chemoresistant studies, respectively. Most proteomics studies of metastases have been performed in cell culture experiments (). DEPs have been identified in non-metastatic and metastatic sublines that have included several models, including one report that examined DEPs in primary OS tissue with and without metastasis.
Table 2 Proteomics studies of OS/OB experimental groups
Table 3 Proteomics studies of metastasis in OS
Table 4 Proteomics studies of chemoresistance in OS
The list generated in this study also includes DEPs that exhibit chemoresistance. Proteomics studies of chemoresistance of OS included investigation of eleven OS cell lines treated with doxorubicin. Notably, DEPs resistant to doxorubicin were observed in most of the cells examined. Other papers have reported DEPs in frozen tissues of both good and poor responders before treatment with methotrexate, doxorubicin, and cisplatin. We included DEPs in both those groups as these identified proteins provide an indication of cellular responsiveness after chemotherapy. DEPs identified in these reports are potentially useful as biomarkers that may be able to predict chemotherapy responsiveness in OS.
Enriched biological processes and pathways in OS
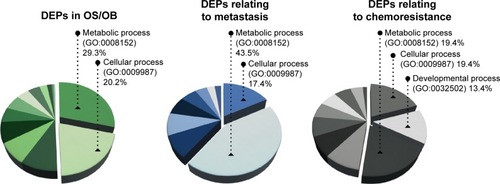
To the list of DEPs identified from literature mining, we added information about each protein including gene entities (eg, from the UniProtKB database). DEPs in each of the experimental groups (OS/OB, metastasis, and chemoresistance) were additionally categorized on the basis of their biological processes using GOCitation10 and were designated as GO terms (). It was found that more than half the DEPs in all the experimental groups were involved in metabolic and cellular processes. Lower pathway enrichment involved developmental processes, localization, biological regulation, etc. The diversity of these pathways varied slightly among the experimental groups. The complete list of biological processes is provided in the Supplementary materials.
Figure 1 Enriched biological processes (GO annotation) of DEPs in OS/OB, metastasis, and chemoresistance.
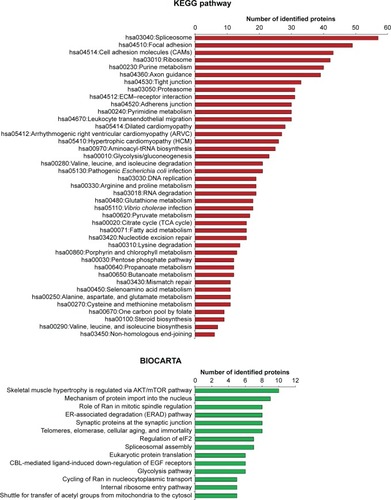
Various functions of all DEPs that were related to signaling pathways were accessed through two pathway databases, KEGG and BIOCARTA, using the DAVID bioinformatics resource. It was found that DEPs in OS/OB were involved in pivotal metabolisms of molecular building blocks, including carbohydrates, amino acids, and nucleotides. Some played roles in genetic information processes including translation, transcription, replication, and repair, as well as folding, sorting, and degradation, whereas others were associated with cardiovascular diseases. By considering individual pathways (child categories), spliceosomes accounted for the most enriched pathways among all OS/OB DEPs according to the KEGG database (). Additionally, the BIOCARTA database revealed an association between DEPs in OS with AKT/mTOR signaling pathways.
Figure 2 Pathway analysis of DEPs in OS/OB (from KEGG and BIOCARTA databases).
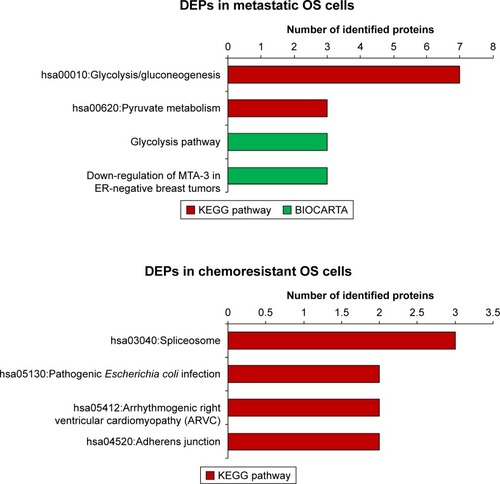
Due to the limited number of DEPs identified in metastases and chemoresistance, pathway analyses using KEGG pathways and BIOCARTA were not very revealing for most DEPs. However, we did find that most DEPs in metastatic outcomes were involved in both glycolysis/gluconeogenesis and pyruvate metabolism (). Additionally, analysis of the BIOCARTA database revealed an association between DEPs and pathways of down-regulated MTA-3 in estrogen receptor (ER)-negative breast tumors. Small amounts of DEPs in chemoresistance were associated with several pathways including spliceosomes ().
Figure 3 Pathway analysis of DEPs in metastasis and chemoresistance (from KEGG and BIOCARTA databases).Citation75,Citation76
Novel targets for the treatment of OS
In this study, our ultimate aim was to use proteomics data to seek potential target remedies for precision treatment. All the up-regulated proteins extracted from the list of DEPs were converted to gene identifiers. The proteins were then cross-referenced with three groups of available medicines and chemical agents including FDA-approved antineoplastic drugs, FDA-approved non-antineoplastic drugs, and non-FDA-approved chemical agents. The result was 14, 5, and 37 proteins up-regulated in OS/OB that could be matched to FDA-approved antineoplastic drugs, FDA-approved non-antineoplastic drugs, and non-FDA-approved chemical agents, respectively. The flow of generating the list of targeted treatments is shown in .
Figure 4 Generating the list of druggable targets for the treatment of OS: (A) overview of all steps used in generating the list and (B) diagrams of targets of FDA-approved non-antineoplastic drugs and non-FDA-approved chemical agents from studies of proteomics in three experimental groups.
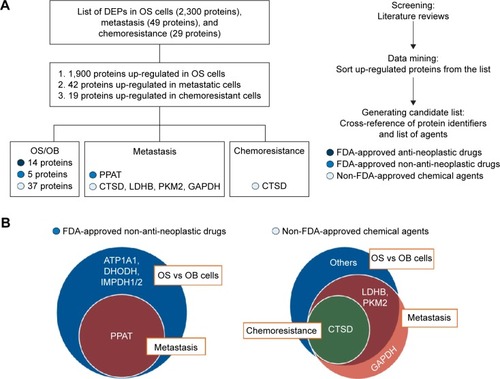
We also found that several overexpressed proteins in the metastatic and chemoresistant groups were targets of either FDA-approved non-antineoplastic drugs or non-FDA-approved chemical agents. The resulting lists revealed that the target of the FDA-approved non-antineoplastic drug amidophosphoribosyltransferase (PPAT) was a common target of OS/OB and metastatic sublines (). In addition, targets of the non-FDA-approved chemical agents L-lactate dehydrogenase B chain (LDHB) and pyruvate kinase M2 (PKM2) were found as common targets between OS/OB and metastases, while cathepsin D (CTSD) was the intersected target of all scenarios. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was exclusively overexpressed in metastatic cells (). Details of individual targeted proteins and drugs are shown in and and the Supplementary materials.
Table 5 Up-regulated proteins and targets of FDA-approved antineoplastic drugs
Table 6 Up-regulated proteins and genes that are targets of FDA-approved non-antineoplastic drugs
Discussion
In this study, we used bioinformatics tools to analyze proteomics data retrieved from “PubMed”. It was found that “metabolic pathway” was the most enriched biological process of DEPs in the three experimental groups: 1) OS/OB: OS vs OB cells, 2) metastasis: OS with metastasis vs non-metastasis, and 3) chemoresistance: OS with higher vs lower chemoresistance (). Among those, the primary metabolic processes of proteins, nucleobase-containing compounds, lipids, carbohydrates, and amino acids were the most frequently altered. In addition, KEGG pathway analysis demonstrated that aberrant expression of proteins in the OS/OB group was mainly related to the “genetic information process”, which consists of translation, transcription, replication, and repair, as well as folding, sorting, and degradation ().
This study identified metabolic processes potentially associated with progression of the disease. Growing evidence of metabolic reprogramming in cancer cells has uncovered the important role of oncogenic signaling pathways in the regulation of metabolic activities. Cancer cells require more energy and need specific building blocks for macromolecule biosynthesis during growth and proliferation. All metabolic changes effectively support and maintain the uncontrollable growth, migration, and metastatic processes of cancer cells. However, it is worth noting that not all metabolic shifts are a consequence of oncogenic driven processes: they might possibly also reflect cellular adaptation to high proliferation rates.
In order to identify alternative targets for OS treatment, we sorted lists of up-regulated proteins from DEPs in each experimental group and then cross-referenced those with three groups of drugs and chemical agents. Overexpression of proteins in the OS/OB group defined as targets of FDA-approved antineoplastic drugs were classified into one of four categories based on their functions: 1) Epigenetic regulators: DNMT1, HDAC1, and HDAC2; 2) Kinases: ERBB2, FGFR1, KIT, MET, MTOR, and PDGFRα; 3) Proteasomes: PSMC5 and PSMC6, and 4) Others: GSR and PARP1 (). These proteins have been previously studied as promising targets for the treatment of various cancer types including OS. Recently, Yu et alCitation23 screened 54 FDA-approved agents for their antigrowth activity in five pediatric OS cell lines carrying p53 inactivation. Among the tested agents, inhibitors targeting HDACs (romidepsin and panobinostat) showed significant single-agent activity and synergistic effects when combined with proteasome inhibitors (carfilzomib and bortezomib) that work effectively within a range of clinically achievable concentrations.
Other promising oncogenic targets discovered in this study were tyrosine kinases. Attempts at using multi-kinase inhibitors for the treatment of OS were carried on in clinical trials. The outcomes were very promising as seen in Phase II clinical trials that assessed the effectiveness of sorafenib in patients with high-grade and unresectable OS.Citation24,Citation25 Those trials, which resulted in a 48% progression-free survival rate of patients at 4 months, demonstrated that sorafenib could potentially be used as a second- or third-line treatment. A small molecule kinase inhibitor, imatinib mesylate, induced significant cytotoxic activity in OS primary cells that were overexpressing PDGFRα.Citation26,Citation27 Phase II clinical trials conducted in OS patients with recurrence, chemoresistance, or lung metastasis found moderately clinical responses in subgroup of patients.Citation28,Citation29 Ideally, experimental investigation of expression of tyrosine kinases is the prerequisite for selecting patients who will gain a true benefit from this targeted treatment. One of these trials examined the expression and mutation profiles of targeted kinases including “cKIT, PDGRα, AKT, pAKT, PTEN, and pFKHR” in formalin-fixed, paraffin-embedded (FFPE) tissues from eligible patients.Citation29 However, an association between the laboratory evaluation and a responsiveness to imatinib was not well established due to a limitation of available retained tissue samples. Therefore, further systemic experimental evaluation is necessary for suggesting therapeutic options to specific groups of patients. Other clinical trials of kinase inhibitors in OS patients including pazopanib (Phase II; NCT01532687) and lenvatinib (Phase I/II; NCT02432274) are ongoing.
Carmustine works as an antagonist of GSR and as a DNA and RNA alkylator.Citation30 The FDA has approved the use of carmustine in the treatment of brain tumors, multiple myeloma, Hodgkin’s disease, and non-Hodgkin’s lymphomas. Some evidence has indicated an antitumor activity of carmustine in androgen-independent prostate cancer cells and various human solid tumor cell lines.Citation30,Citation31 Another promising target is MET oncoprotein. Overexpression and amplification of MET have been observed frequently in OS, with this aberrancy associated with poorer outcomes.Citation32 Moreover, it was found that up-regulation of MET potentially induced transformation of osteoblasts into OS carrying typical OS characteristics and neovascularization ability.Citation33
The process of drug development starting from basic research through FDA approval normally requires >10 years and an average investment of US$1.8 billion.Citation34 Unfortunately, most new agents fail to reach the market stage due to a lack of efficacy in Phase II clinical trials. This issue in current cancer drug development has been addressed by scientific communities, with the result that the strategy of using approved non-cancer drugs for the treatment of various cancers (known as “drug repurposing” or “drug repositioning”) has gained much attentionCitation35 as the necessary toxicity testing as well as pharmacokinetic and pharmacodynamics profile development has already been assessed and approved in the preclinical and Phase I clinical trials. Thus, the potential drugs are in a position to move into Phase II and III clinical trials within a shorter period and at a lower cost. This study has identified a group of immunosuppressant and antiarrhythmia agents that are promising candidates for the repurposing approach ().
Among the immunosuppressants, leflunomide is the likely candidate for repurposing as an anticancer agent. Leflunomide is an inhibitor of DHODH that, in turn, modulates pyrimidine synthesis that is a major target in the treatment of rheumatoid arthritis.Citation36 Interestingly, this study found that the expression profile of DHODH was aberrant in some malignancies including OS. There is growing evidence of the anticancer activity of leflunomide in preclinical trials with neuroblastoma, medullary thyroid cancer, and other cancers.Citation36,Citation37 Besides being a potent inhibitor of DHOH, it has been reported that leflunomide also inhibits PDGFR and other tyrosine kinases.Citation38 Using leflunomide in cancer therapy would likely be of great benefit since the direct relationship between tyrosine kinases and oncogenesis has been well documented.
In this study, we also explored the possibility that digoxin could be a potential candidate for repurposing. This drug is in a group of cardiac glycosides, an inhibitor of Na+/K+-ATPase pump that has been commonly used in heart failure and which has worked as an antiarrhythmic. Treating cancer cell lines with digoxin resulted in cytotoxicity in several types of cancer cells including prostate, breast, renal, and lung cancers, melanoma, and leukemia.Citation39 Notably, the IC50 of this drug in tests with the aforementioned cancer cells is lower (0.1–1 µM) than the dose used for the treatment of heart disease, thereby warranting further clinical testing.
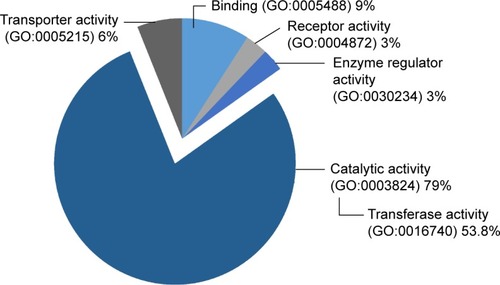
Apart from FDA-approved agents, this study also investigated various targets of chemical inhibitors (Supplementary materials). Most of the targeted proteins were catalytic enzymes (GO:0003824), in particular, the group of transferases (). Interestingly, we found that some transferases are associated with cancer-related signaling pathways including the Wnt, MAPK, VEGF, and ErbB signaling pathways. This finding provides a list of targeted proteins that are potential candidates for further screening tests. We also identified common overexpressed proteins in the OS/OB and metastatic groups including LDHB and PKM2 as well as a shared target among all categories: CTSD ().
Figure 5 Groups of up-regulated proteins, targets of non-FDA-approved chemical agents.
LDHB is an enzyme catalyzing the conversion of pyruvate to lactate via the glycolysis pathway.Citation40 The association between LDHB and the etiology of OS was studied through integrated analysis of gene expression data in OS.Citation41 The results showed higher expression of LDHB in OS tissues with single-nucleotide polymorphisms (SNPs) and copy number variants (CNVs). In addition, another study reported that high levels of serum LDH in OS was significantly related to lower overall survival.Citation42 These all suggest a possible role of LDHB in tumorigenesis and the progression of the disease that might be linked to worsened outcomes.
PKM2 is one of the key potential targets for cancer therapy. It catalyzes the end step in the glycolysis pathway by converting phosphoenolpyruvate (PEP) to pyruvate.Citation43 A great quantity of evidence has emerged suggesting a pivotal role of PKM2 in the metabolic phenotype of various cancers.Citation44 Additionally, some studies have revealed the function of PKM2 as a protein kinase that is involved in cell migration and angiogenesis of colon and gastric carcinoma.Citation45,Citation46 Even though there have been only limited studies of the association of PKM2 and OS, this study positions PKM2 as a potential target in the treatment of OS.
In this study, CTSD was the only protein identified as a potential target in all experimental groups. CTSD is a lysosomal aspartic endopeptidase that plays multi-faceted roles in the normal physiological state as well as in the pathogenesis of diverse diseases.Citation47 Furthermore, many studies have demonstrated roles of CTSD in a wide range of cancers. It seems like this lysosomal enzyme is involved in multiple stages of tumorigenesis as well as in the progression of the disease including cell proliferation, invasion, angiogenesis, and metastasis.Citation48 Increased expression of CTSD in OS, lung metastases, and chemoresistance are evidence that CTSD has important functions in pathological processes of OS.
Conclusion
With a growing understanding of biological mechanisms, the oncogenic driver of various cancers is being unveiled, leading to the development of a wide variety of targeted medicines. In addition, advancement in proteomics with the emergence of big data has identified key events in tumorigenesis. Therapeutic agents identified by protein expression profiles in OS were explored through a text mining and systemic review of proteomics data set. As the result, we successfully identified and explored 1) potential anticancer drugs for targeting OS-related pathways, 2) non-cancer drug repurposing, and 3) new targets for the treatment of OS. The use of targeted therapy alone or in a combination regimen as well as testing the effectiveness of drug repurposing in clinical trials can be beneficial for OS patients, especially for those who experience relapse after the use of conventional therapeutic options.
Acknowledgments
We would like to thank Prof Apiwat Mutirangura for his valuable advice. This study was supported by the National Science and Technology Development Agency (NSTDA), code P-15-50265, Faculty of Medicine, Chiang Mai University, National Research University (NRU) fund, and the Excellence Center in Osteology Research and Training Center (ORTC). The authors would also like to express their sincere thanks to Dr G Lamar Robert and Assoc Prof Chongchit Sripun Robert for editing the English manuscript. The abstract (not full version) of this paper was presented at the conference, RCOST&AOA 2016, as an oral presentation with interim findings. The abstract was published in “RCOST&AOA 2016 Abstract book”.
Disclosure
The authors report no conflicts of interest in this work.
References
- SettakornJLekawanvijitSArpornchayanonOSpectrum of bone tumors in Chiang Mai University Hospital, Thailand according to WHO classification 2002: a study of 1,001 casesJ Med Assoc Thai200689678078716850677
- FriebeleJCPeckJPanXAbdel-RasoulMMayersonJLOsteosarcoma: a meta-analysis and review of the literatureAm J Orthop (Belle Mead NJ)2015441254755326665241
- AllisonDCCarneySCAhlmannERA meta-analysis of osteosarcoma outcomes in the modern medical eraSarcoma2012201270487222550423
- MooreDDLuuHHOsteosarcomaCancer Treat Res2014162659225070231
- MoriarityBSOttoGMRahrmannEPA sleeping beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasisNat Genet201547661562425961939
- ChenXBahramiAPappoARecurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcomaCell Rep20147110411224703847
- HanashSTaguchiAThe grand challenge to decipher the cancer proteomeNat Rev Cancer201010965266020733593
- ShuklaHDMahmoodJVujaskovicZIntegrated proteo-genomic approach for early diagnosis and prognosis of cancerCancer Lett20153691283626276717
- LaranceMLamondAIMultidimensional proteomics for cell biologyNat Rev Mol Cell Biol201516526928025857810
- Gene OntologyCGene Ontology Consortium: going forwardNucleic Acids Res201543Database issueD1049D105625428369
- MiHPoudelPMuruganujanACasagrandeJTThomasPDPANTHER version 10: expanded protein families and functions, and analysis toolsNucl Acids Res2015
- Huang daWShermanBTLempickiRASystematic and integrative analysis of large gene lists using DAVID bioinformatics resourcesNat Protoc200941445719131956
- TermglinchanVWanichnopparatWSuwanwongseKCandidate cancer-targeting agents identified by expression-profiling arraysOnco Targets Ther2013644745823637543
- PruksakornDTeeyakasemPKlangjorhorJOverexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcomaInt J Oncol201649310
- SpreaficoAFredianiBCapperucciCA proteomic study on human osteoblastic cells proliferation and differentiationProteomics20066123520353216705754
- GuoQCShenJNJinSComparative proteomic analysis of human osteosarcoma and SV40-immortalized normal osteoblastic cell linesActa Pharmacol Sin200728685085817506944
- FolioCMoraMIZalacainMProteomic analysis of chemonaive pediatric osteosarcomas and corresponding normal bone reveals multiple altered molecular targetsJ Proteome Res2009883882388819492781
- LiuXZengBMaJWanCComparative proteomic analysis of osteosarcoma cell and human primary cultured osteoblastic cellCancer Invest200927334535219212829
- ZhangZZhangLHuaYComparative proteomic analysis of plasma membrane proteins between human osteosarcoma and normal osteoblastic cell linesBMC Cancer20101020620470422
- PosthumaDeboerJPiersmaSRPhamTVSurface proteomic analysis of osteosarcoma identifies EPHA2 as receptor for targeted drug deliveryBr J Cancer201310982142215424064975
- GemollTEppingFHeinrichLIncreased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignanciesOncotarget2015618165171652626203049
- HuaYJiaXSunMPlasma membrane proteomic analysis of human osteosarcoma and osteoblastic cells: revealing NDRG1 as a marker for osteosarcomaTumour Biol20113251013102121706236
- YuDKahenECubittCLIdentification of synergistic, clinically achievable, combination therapies for osteosarcomaSci Rep201551699126601688
- GrignaniGPalmeriniEDileoPA phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group studyAnn Oncol201223250851621527590
- GrignaniGPalmeriniEFerraresiVSorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trialLancet Oncol20151619810725498219
- KuboTPiperdiSRosenblumJPlatelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcomaCancer2008112102119212918338812
- GobinBMoriceauGOryBImatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine modelsPLoS One201493e9079524599309
- BondMBernsteinMLPappoAA phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children’s Oncology Group studyPediatr Blood Cancer200850225425817262795
- ChughRWathenJKMakiRGPhase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a Bayesian hierarchical statistical modelJ Clin Oncol200927193148315319451433
- ThamilselvanVMenonMThamilselvanSCarmustine enhances the anticancer activity of selenite in androgen-independent prostate cancer cellsCancer Manag Res2012438339523204869
- KuoCCLiuTWChenLTCombination of arsenic trioxide and BCNU synergistically triggers redox-mediated autophagic cell death in human solid tumorsFree Radic Biol Med201151122195220922001324
- ScotlandiKBaldiniNOlivieroMExpression of Met/hepatocyte growth factor receptor gene and malignant behavior of musculoskeletal tumorsAm J Pathol19961494120912198863670
- PataneSAvnetSColtellaNMET overexpression turns human primary osteoblasts into osteosarcomasCancer Res20066694750475716651428
- PaulSMMytelkaDSDunwiddieCTHow to improve R&D productivity: the pharmaceutical industry’s grand challengeNat Rev Drug Discov20109320321420168317
- GuptaSCSungBPrasadSWebbLJAggarwalBBCancer drug discovery by repurposing: teaching new tricks to old dogsTrends Pharmacol Sci201334950851723928289
- ZhuSYanXXiangZDingHFCuiHLeflunomide reduces proliferation and induces apoptosis in neuroblastoma cells in vitro and in vivoPLoS One201388e7155523977077
- AlhefdhiABurkeJFRedlichAKunnimalaiyaanMChenHLeflunomide suppresses growth in human medullary thyroid cancer cellsJ Surg Res2013185121221623816245
- KoYJSmallEJKabbinavarFA multi-institutional phase ii study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancerClin Cancer Res20017480080511309325
- ElbazHAStueckleTATseWRojanasakulYDinuCZDigitoxin and its analogs as novel cancer therapeuticsExp Hematol Oncol201211423210930
- GalloMSapioLSpinaANaviglioDCalogeroANaviglioSLactic dehydrogenase and cancer: an overviewFront Biosci (Landmark Ed)2015201234124925961554
- XiongYWuSDuQWangAWangZIntegrated analysis of gene expression and genomic aberration data in osteosarcoma (OS)Cancer Gene Ther2015221152452926427513
- DurnaliAAlkisNCangurSPrognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patientsMed Oncol201330362423749307
- MazurekSPyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cellsInt J Biochem Cell Biol201143796998020156581
- ChanetonBGottliebERocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancerTrends Biochem Sci201237830931622626471
- ZhouCFLiXBSunHPyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cellsIUBMB Life201264977578222807066
- WangLYLiuYPChenLGPyruvate kinase M2 plays a dual role on regulation of the EGF/EGFR signaling via E-cadherin-dependent manner in gastric cancer cellsPLoS One201386e6754223840737
- HasilikANeufeldEFBiosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weightJ Biol Chem198025510493749456989821
- BenesPVetvickaVFusekMCathepsin D–many functions of one aspartic proteaseCrit Rev Oncol Hematol2008681122818396408
- SueharaYKondoTFujiiKProteomic signatures corresponding to histological classification and grading of soft-tissue sarcomasProteomics20066154402440916807943
- KawaiAKondoTSueharaYKikutaKHirohashiSGlobal protein-expression analysis of bone and soft tissue sarcomasClin Orthop Relat Res200846692099210618535868
- LiYLiangQWenYQComparative proteomics analysis of human osteosarcomas and benign tumor of boneCancer Genet Cytogenet201019829710620362224
- RaoUNHoodBLJones-LaughnerJMSunMConradsTPDistinct profiles of oxidative stress-related and matrix proteins in adult bone and soft tissue osteosarcoma and desmoid tumors: a proteomics studyHum Pathol201344572573323063503
- BonaAPapaiZMaaszGMass spectrometric identification of ancient proteins as potential molecular biomarkers for a 2000-year-old osteogenic sarcomaPLoS One201491e8721524475253
- FloresRJLiYYuAA systems biology approach reveals common metastatic pathways in osteosarcomaBMC Syst Biol201265022640921
- ChenXYangTTZhouYProteomic profiling of osteosarcoma cells identifies ALDOA and SULT1A3 as negative survival markers of human osteosarcomaMol Carcinog201453213814422949271
- TangJShenLYangQZhangCOverexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transitionCell Prolif201447542743425174891
- AraiKSakamotoRKubotaDKondoTProteomic approach toward molecular backgrounds of drug resistance of osteosarcoma cells in spheroid culture systemProteomics201313152351236023712969
- KubotaDMukaiharaKYoshidaATsudaHKawaiAKondoTProteomics study of open biopsy samples identifies peroxiredoxin 2 as a predictive biomarker of response to induction chemotherapy in osteosarcomaJ Proteomics20139139340423911960
- IzbickaECamposDMartyJCarrizalesGMangoldGTolcherAMolecular determinants of differential sensitivity to docetaxel and paclitaxel in human pediatric cancer modelsAnticancer Res2006263A1983198816827133
- KangJHParkKKLeeISProteome analysis of responses to ascochlorin in a human osteosarcoma cell line by 2-D gel electrophoresis and MALDI-TOF MSJ Proteome Res20065102620263117022633
- ChangYCParkWHMinKSKimTKimCHKangJHProteome profiling of U2OS cell line in response to a prenylphenol antibiotic isolated from a phytopathogenic fungusBiol Pharm Bull20083191696170318758062
- XieXBYinJQWenLLCritical role of heat shock protein 27 in bufalin-induced apoptosis in human osteosarcomas: a proteomic-based researchPLoS One2012710e4737523091618
- KansaraMTengMWSmythMJThomasDMTranslational biology of osteosarcomaNat Rev Cancer2014141172273525319867
- SauAFilomeniGPezzolaSTargeting GSTP1-1 induces JNK activation and leads to apoptosis in cisplatin-sensitive and -resistant human osteosarcoma cell linesMol Biosyst201284994100622068640
- KlevebringDFagerbergLLundbergEEmanuelssonOUhlenMLundebergJAnalysis of transcript and protein overlap in a human osteosarcoma cell lineBMC Genomics20101168421126332
- KirkwoodKJAhmadYLaranceMLamondAICharacterization of native protein complexes and protein isoform variation using size-fractionation-based quantitative proteomicsMol Cell Proteomics201312123851387324043423
- GemeiMCorboCD’AlessioFDi NotoRVentoRDel VecchioLSurface proteomic analysis of differentiated versus stem-like osteosarcoma human cellsProteomics201313223293329724106197
- LaranceMAhmadYKirkwoodKJLyTLamondAIGlobal subcellular characterization of protein degradation using quantitative proteomicsMol Cell Proteomics201312363865023242552
- NiforouKMAnagnostopoulosAKVougasKKittasCGorgoulisVGTsangarisGTThe proteome profile of the human osteosarcoma U2OS cell lineCancer Genomics Proteomics200851637818359981
- LiGZhangWZengHAn integrative multi-platform analysis for discovering biomarkers of osteosarcomaBMC Cancer2009915019445706
- LiYDangTAShenJIdentification of a plasma proteomic signature to distinguish pediatric osteosarcoma from benign osteochondromaProteomics20066113426343516673437
- LiYDangTAShenJPlasma proteome predicts chemotherapy response in osteosarcoma patientsOncol Rep201125230331421165584
- SavitskayaYARico-MartinezGLinares-GonzalezLMSerum tumor markers in pediatric osteosarcoma: a summary reviewClin Sarcoma Res20122922587902
- JinSShenJNGuoQC2-D DIGE and MALDI-TOF-MS analysis of the serum proteome in human osteosarcomaProteomics Clin Appl20071327228521136678
- KanehisaMGotoSKEGG: Kyoto encyclopedia of genes and genomesNucleic Acids Res200028273010592173
- NishimuraDBiotech Software & Internet ReportNYMary Ann Liebert, Inc200123117120
- TermglinchanVWanichnopparatWSuwanwongseKCandidate cancer-targeting agents identified by expression-profiling arraysOnco Targets Ther2013644745823637543
- WishartDSKnoxCGuoACDrugBank: a comprehensive resource for in silico drug discovery and explorationNucleic Acids Res200634Database issueD668D67216381955
