Abstract
Primary pancreatic lymphoma (PPL) is an extremely rare disease, with only a few cases reported in the literature. Clinical manifestations of PPL are often nonspecific and may mimic other pancreatic diseases. Because of the limited experience of PPL, clinicopathological features, differential diagnosis, optimal therapy, and outcomes are not well defined. We described two cases diagnosed as PPL and confirmed by histological examination and immunohistochemical analysis. Case 1 was a young man with obstructive jaundice and upper abdominal malaise mimicking a pancreatic adenocarcinoma. A computed tomography (CT) scan revealed a diffuse heterogeneous mass in the head of the pancreas along with dilated bile ducts, no dilated pancreatic duct, no liver or splenic involvement, or evident retroperitoneal adenopathies. The patient underwent a pancreatico-duodenectomy, and the postoperative histopathology confirmed diffuse large B-cell non-Hodgkin lymphoma. Postoperatively, he received six courses of the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisolone). Case 2 was an older man with left flank pain. A CT confirmed a mass with irregular margins at the tail of the pancreas and the hilum of the spleen. The mass was heterogeneous, with no clear boundary between lesions, spleen, stomach, and pancreas, with nearby blood vessels wrapped around it, and multiple enlarged lymph nodes in the abdominal cavity. A CT-guided biopsy was performed. The immunohistological findings of the specimen revealed a diffuse large B-cell lymphoma. The size of the tumor was significantly reduced after four cycles of the CHOP chemotherapy regimen. These two cases were different in clinical manifestation, location, and treatment. We reviewed the literature and discussed the clinicopathological features, differential diagnosis, optimal therapy, and outcomes of this neoplasm.
Introduction
Primary pancreatic lymphoma (PPL) is an extremely rare entity, accounts for <0.5% of malignant pancreatic tumors,Citation1–Citation3 and is difficult to preoperatively diagnose even with various modalities. Clinically, the presentation of patients with PPL arising from the pancreatic head can often overlap that of patients with pancreatic adenocarcinoma and is difficult to radiographically distinguish. However, due to a much better prognosis for PPL, proper diagnosis is very important. To confirm the diagnosis of PPL, histological analysis is required. Management of PPL involves chemotherapy and radiotherapy,Citation4 yet uncertainty regarding the diagnosis of a mass in the pancreatic head usually results in surgical resection as well. For patients with symptoms caused by obstruction of the biliary tract, surgical intervention is effective.Citation4 Furthermore, the combination of surgical treatment and adjuvant chemotherapy has a better survival benefit than chemotherapy alone.Citation5,Citation6 Despite these efforts, surgical intervention is still controversial. We present two cases of PPL that were different in clinical manifestation, location, and treatment, and reviewed this neoplasm. Written informed consent was obtained from both patients to publish the case reports and any accompanying images.
Case 1
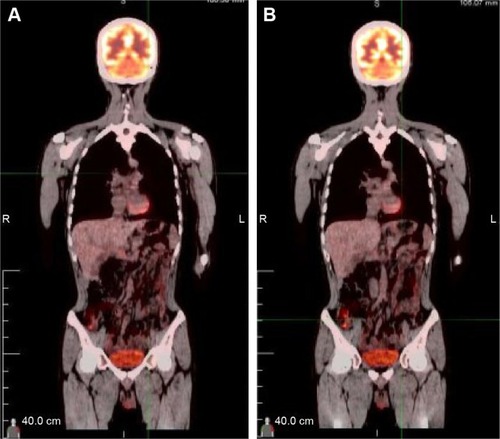
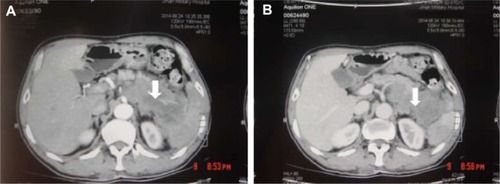
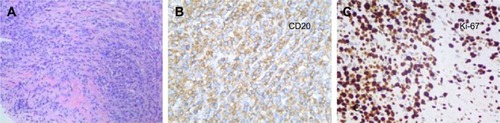
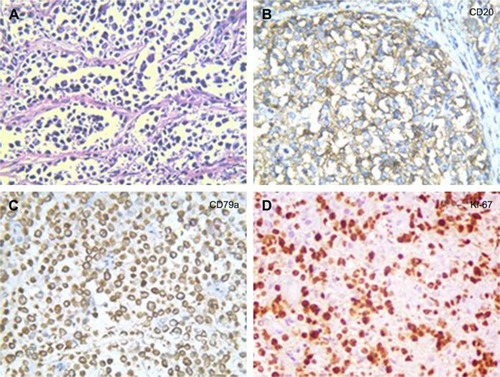
A 32-year-old man was admitted to our hospital after feeling ill for 10 days with complaints of jaundice and upper abdominal malaise. A physical examination revealed jaundice. Organomegaly or lymphadenopathy was not detected. Laboratory test results revealed indirect hyperbilirubinemia (total bilirubin: 154.1 mg/dL, conjugated bilirubin: 90.9 mg/dL). The liver function enzymes showed that alanine transaminase, aspartate transaminase, and lactate dehydrogenase (LDH) levels were increased to 465, 268, and 248 U/L, respectively. The β2-microglobulin level of the patient was 1.89 mg/L. Both his carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) were within the normal limits. Abdominal computed tomography (CT) showed an irregular enlargement of the pancreas and a 3.0×3.5 cm heterogeneous enhanced mass arising from the pancreatic head (). The main pancreatic duct was of normal caliber, and there was no connection between the mass and the pancreatic duct. The bile duct was in distention because of the compression by the mass in the head of the pancreas. No focal enhanced mass was observed in the liver, and there were no enlarged lymph nodes in the abdominal cavity. The initial diagnosis was pancreatic head cancer or a neuroendocrine tumor of the pancreas. The patient underwent a pancreatico-duodenectomy. Macroscopically, the mass had arisen from the pancreatic head and was limited to the pancreas. The cut surface of the tumor was yellowish (). A histological report confirmed diffuse large B-cell lymphoma (). Immunohistochemical staining showed the cells to be positive for CD20 and CD79a () and negative for CD3, CD10, and BCL-2. The histology also confirmed a proliferative index of over 50%–60% (). Finally, based on pathological and immunohistochemical findings, the tumor was diagnosed as PPL. Postoperatively, the patient received six cycles of treatment with doxorubicin, cyclophosphamide, vincristine, and prednisolone (CHOP regimen). He is still alive and asymptomatic after 16 months of follow-up. The last positron emission tomography-CT did not detect any signs of disease recurrence ().
Figure 1 Abdominal CT findings. A CT scan showing diffuse hypodense enlargement of the pancreatic head (arrow).
Abbreviation: CT, computed tomography.
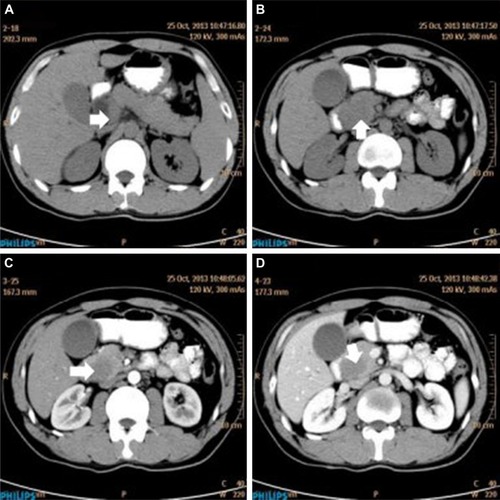
Figure 2 A gross pathological examination revealed a 3.0 × 4.0 cm multiseptated mass in the pancreatic head (arrow). The cut surface of the tumor was yellowish (arrow).
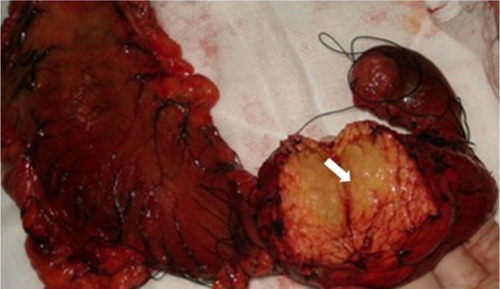
Figure 3 Histopathology showed small tumor cells without cell adhesion or tissue structure proliferation (A), and HE immunochemical staining was positive for B-cell markers CD20 and CD79a (B, C), compatible with the diagnosis of diffuse large B-cell non-Hodgkin lymphoma. HE immunochemical staining confirmed a proliferative index of over 50–60% (D). (A–C ×400; D ×200).
Case 2
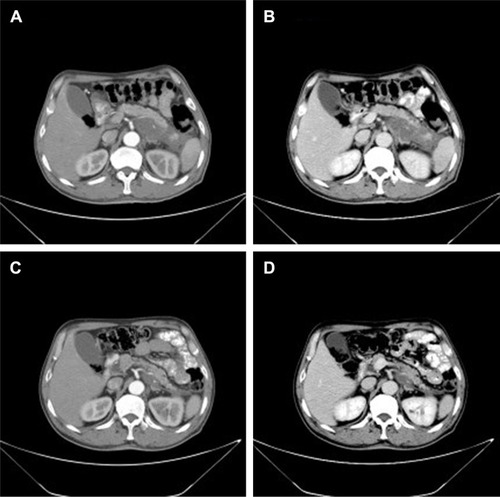
A 62-year-old man presented with a 1-month history of left flank pain. He presented to another hospital and had a CT which revealed a lesion in the tail of the pancreas. He was then transferred to our institution for further workup and evaluation. His past medical history was unremarkable. A physical examination revealed an upper abdominal mass but did not detect peripheral lymphadenopathy. His liver function enzymes were normal. However, LDH was 236 U/L. The β2-microglobulin level of the patient was 2.37 mg/L. Serum tumor markers CA 19-9, CEA, and chromogranin A were normal. An abdominal CT scan confirmed that a mass with irregular margins was in the hilum of the spleen. The mass was heterogeneous, with no clear boundary between lesions, spleen, stomach, and pancreas, nearby blood vessels were wrapped around it, and there were multiple enlarged lymph nodes in the abdominal cavity (). A CT-guided biopsy was taken from the mass. The pathological evaluation of the CT-guided biopsy revealed that the tumor was CD20-positive () and CD3- and pancytokeratin-negative, with a high Ki-67 proliferation index (80%) (), and findings are consistent with diffuse large B-cell lymphoma (). Due to the ill-defined mass, it was difficult to judge whether the tumor originated from the pancreas or spleen. However, a CHOP chemotherapy regimen is the preferred regimen for diffuse large B-cell lymphoma regardless of the location of the primary site. He consented to chemotherapy with the CHOP regimen. Lesions were significantly reduced after two cycles of treatment. A CT confirmed that the lymph nodes of the splenic hilum are significantly narrowed. The spleen was normal, and a low-density lesion at the tail of the pancreas was still present. Under these circumstances, we confirmed our diagnosis of PPL arising from the pancreatic tail. The size of the tumor was significantly reduced after four cycles of the CHOP chemotherapy regimen (), and further follow-up is being carried out.
Discussion
PPL is extremely rare, comprising 1% of extra-nodal lymphomas and 0.5% of malignant pancreatic tumors.Citation1–Citation3,Citation7 From our review of the English literature (),Citation4,Citation5,Citation8–Citation31 the most common lymphoma variant in the pancreas is diffuse large B-cell lymphoma, accounting in one study for as much as 80% of all the PPL cases.Citation29 Abdominal pain, jaundice, acute pancreatitis, small bowel obstruction, and diarrhea are the most commonly presenting symptoms,Citation7,Citation30 but these are nonspecific and overlap those of pancreatic adenocarcinoma. Interestingly, the common presentations of non-Hodgkin lymphoma, such as fever, chills, and night sweats, are rare (2%) in PPL.Citation7 The widely accepted criteria for diagnosis of PPL were established by Dawson et al.Citation32 They include a dominant mass in the pancreas, the absence of superficial or mediastinal lymphadenopathy on chest imaging, a normal leukocyte count in the peripheral blood, and the absence of hepatic or splenic involvement. Most PPLs occur in the pancreatic head, although they may also be found in other parts of the pancreas.Citation1,Citation2,Citation7 In our patients, case 1 presented with jaundice and upper abdominal malaise and the mass was located in the head of the pancreas, whereas case 2 presented with left flank pain and the mass was located at the tail of the pancreas.
Table 1 Reported cases of primary pancreatic leiomyosarcoma in the English literature of our review
Nonspecific biochemical markers can help a physician to make an accurate diagnosis of PPL. CA 19-9, a serum tumor marker, is generally increased in patients with pancreatic adenocarcinoma,Citation33 but it is rarely raised in patients with PPL. Lin et al reported that 80% of the patients with pancreatic adenocarcinoma have a high CA 19-9 level, whereas in patients with PPL, the serum CA 19-9 level is normal or only slightly elevated unless a biliary obstruction is presented.Citation4 LDH and β2-microglobulin levels are thought to be elevated in lymphoproliferative disorders, but they are not necessarily elevated in PPL.Citation34 However, a higher β2-microglobulin and LDH level are indicators of a poor prognosis for PPL patients.Citation35
Various imaging modalities have been used in the evaluation of pancreatic mass lesions. PPL can be divided into two morphological groups on CT: the pancreas has a similar appearance to pancreatitis, with an infiltrative mass and peripancreatic fat stranding or the pancreas seems to have a well-circumscribed tumor.Citation22 Some radiological findings that point to PPL (instead of adenocarcinoma) include a bulky, localized pancreatic head tumor; no significant pancreatic duct dilatation; no enlarged lymph nodes below the level of the renal veins; and no retroperitoneal, hepatic, or splenic involvement.Citation36 Furthermore, the presence of calcification or necrosis can rule out PPL.Citation7 With regard to the results of endoscopic ultrasonography (EUS), a retrospective analysis by Ramesh et alCitation2 determined that none of the patients showed features of pancreatic ductal dilation. Surrounding lymphadenopathy is common with any lymphoma. However, this feature would not exclude adenocarcinoma. Concerning endoscopic retrograde cholangiopancreatography (ERCP) findings, moderate-to-severe ductal dilation that is often seen in pancreatic adenocarcinoma has not been reported in patients with PPL.Citation36 Therefore, patients with moderate-to-severe ductal dilation may help to exclude the diagnosis of PPL. Magnetic resonance imaging is quite nonspecific.Citation36
Certain clinical and imaging characteristics may help to suggest a diagnosis of PPL, but they are both nonspecific. Thus, pathology is necessary. Initially, the biopsy can be performed by fine-needle aspiration (FNA). However, EUS-guided FNA was shown to improve diagnostic accuracy, and the sensitivity of diagnosing PPL was a significant increase compared with that of FNA alone.Citation15,Citation37,Citation38 CT-guided biopsy, which is minimally invasive, is another option for obtaining specimens.Citation13 Alternatively, a laparoscopy or laparotomy can be performed to biopsy the pancreatic lesion or lymph nodes. In our cases, diagnoses were based on CT-guided biopsy and surgical biopsy.
The strategy for PPL consists of surgery, chemotherapy, radiotherapy, or a combination of them. Most chemotherapy consists of CHOP and sometimes rituxan plus CHOP (R-CHOP). The majority of patients with PPL receiving only chemotherapy can achieve long-term disease remission.Citation39 However, Behrns et alCitation5 reported that it is difficult to obtain long-term survival by chemotherapy alone. Battula et alCitation6 also reported that the 5-year survival rate of PPL treated with current chemotherapy was inferior to the combination of surgery and adjuvant chemotherapy. Surgical intervention, however, is still controversial. It has been proven that pancreatic resection alone does not improve the survival rate of PPL.Citation22 For patients with jaundice, choledochojejunostomy is considered an effective treatment option.Citation4,Citation36,Citation40 But, in recent years, various nonoperative strategies can relieve this complication with a high rate of success. The role of radiation therapy has also not yet been defined except as an adjuvant to chemotherapy. A study by Behrns et al showed some evidence that an initial surgical resection, when coupled with chemotherapy and radiotherapy, was associated with increased long-term survival of PPL.Citation5 In case 1, the patient was not diagnosed with PPL preoperatively, but since there was obstruction of the biliary tract, a pylorus-preserving pancreatico-duodenectomy was performed. His postoperative course was uneventful, and six cycles of CHOP were performed with no signs of recurrence after 16 months. In case 2, the size of the tumor was significantly reduced after four cycles of CHOP chemotherapy. Therefore, it is important to choose the appropriate treatment depending on the disease progression and the patient’s condition.
Conclusion
We presented two rare cases of PPL. Certain clinical and imaging characteristics as well as biochemical markers are not specific for PPL. Since patients with PPL have a much better prognosis than those with adenocarcinoma and the treatment widely differs from those of other pancreatic neoplasms, a definite diagnosis should be sought based on the histological examination. Combined therapy may be the optimal treatment approach for PPL, but it needs further evaluation.
Acknowledgments
The authors thank Kai Cui for his valuable discussion. This work was supported by Natural Science Foundation of Shandong Province (Grant No ZR2015HZ004).
Disclosure
The authors report no conflicts of interest in this work.
References
- MishraMVKeithSWShenXBarAVChampCEBiswasTPrimary pancreatic lymphoma: a population-based analysis using the SEER programAm J Clin Oncol2013361384322134518
- RameshJHebert-MageeSKimHTrevinoJVaradarajuluSFrequency of occurrence and characteristics of primary pancreatic lymphoma during endoscopic ultrasound guided fine needle aspiration: a retrospective studyDig Liver Dis201446547047324560534
- BaylorSMBergJWCross-classification and survival characteristics of 5,000 cases of cancer of the pancreasJ Surg Oncol1973543353584355621
- LinHLiSDHuXGLiZSPrimary pancreatic lymphoma: report of six casesWorld J Gastroenterol200612315064506716937508
- BehrnsKESarrMGStricklerJGPancreatic lymphoma: is it a surgical diseasePancreas1994956626677809023
- BattulaNSrinivasanPPrachaliasARelaMHeatonNPrimary pancreatic lymphoma: diagnostic and therapeutic dilemmaPancreas200633219219416868486
- SaifMWPrimary pancreatic lymphomasJOP20067326227316685107
- ShtamlerBBickelAManorEBen ShaharMKutenASuprunHPrimary lymphoma of the head of the pancreasJ Surg Oncol198838148513287006
- HirabayashiKKawakamiHAizawaTA case of the primary pancreatic lymphomaRinsho Ketsueki19913244144182067088
- JolyIDavidAPayanMJSahelJSarlesHA case of primary non-Hodgkin lymphoma of the pancreasPancreas1992711181201557340
- Van BeersBLalondeLSoyerPDynamic CT in pancreatic lymphomaJ Comput Assist Tomogr199317194978419447
- EzzatAJamshedAKhafagaYPrimary pancreatic non-Hodgkin’s lymphomasJ Clin Gastroenterol19962321091128877636
- SalvatoreJRCooperBShahIKummetTPrimary pancreatic lymphoma: a case report, literature review, and proposal for nomenclatureMed Oncol200017323724710962538
- VolmarKERoutbortMJJonesCKXieHBPrimary pancreatic lymphoma evaluated by fine-needle aspiration: findings in 14 casesAm J Clin Pathol2004121689890315198364
- NayerHWeirEGShethSAliSZPrimary pancreatic lymphomas: a cytopathologic analysis of a rare malignancyCancer2004102531532115386314
- JiYKuangTTTanYSChenYZengHYJinDYPancreatic primary lymphoma: a case report and review of the literatureHepatobiliary Pancreat Dis Int20054462262616286277
- ArcariAAnselmiEBernuzziPPrimary pancreatic lymphoma. Report of five casesHaematologica2005902ECR09
- LeeMKJeonSWLeeYDA case of primary pancreatic non-Hodgkin’s lymphomaKorean J Intern Med200621212312616913443
- WangYJJengCMWangYCChangPPWangTHPrimary pancreatic Burkitt’s lymphoma mimicking carcinoma with obstructive jaundice and very high CA19-9Eur J Gastroenterol Hepatol200618553754016607151
- BasuAPatilNMohindraPIsolated non-Hodgkin’s lymphoma of the pancreas: case report and review of literatureJ Cancer Res Ther20073423623918270400
- SaifMWKhubchandaniSWalczakMSecondary pancreatic involvement by a diffuse large B-cell lymphoma presenting as acute pancreatitisWorld J Gastroenterol200713364909491117828824
- LiakakosTMisiakosEPTsapralisDNikolaouIKaratzasGMacherasAA role for surgery in primary pancreatic B-cell lymphoma: a case reportJ Med Case Rep2008216718489739
- HashimotoMUmekitaNNodaKNon-Hodgkin lymphoma as a cause of obstructive jaundice with simultaneous extrahepatic portal vein obstruction: a case reportWorld J Gastroenterol200814254093409518609698
- SağlamMYilmazMIMasMRA case of pancreatic Burkitt lymphoma: radiological findingsDiagn Interv Radiol2009151394219263373
- BautistaFMorenoLAndrésMMFernández-NavarroJMFernández-SanmartínMVerdeguerAAbdominal pain as the first manifestation of primary pancreatic lymphomaJ Pediatr Hematol Oncol200931322222319262254
- HajiAGSharmaSMajeedKAVijaykumarDKPavithranKDineshMPrimary pancreatic lymphoma: report of three cases with review of literatureIndian J Med Paediatr Oncol2009301202320668602
- SugishitaHWatanabeYYamamotoYPrimary pancreatic lymphoma: the role of surgical treatmentCase Rep Gastroenterol20104110411021103236
- AbeYTamuraKSakataIUnique intense uptake demonstrated by (18)F-FDG positron emission tomography/computed tomography in primary pancreatic lymphoma: a case reportOncol Lett20101460560722966351
- AlexanderRENakeebASandrasegaranKPrimary pancreatic follicle center-derived lymphoma masquerading as carcinomaGastroenterol Hepatol (N Y)201171283483822347825
- AnderloniAGencoCBallarèMCarmagnolaSBattistaSRepiciAA case of primary pancreatic non-Hodgkin B-cell lymphoma mimicking autoimmune pancreatitisJ Gastrointestin Liver Dis201524224524826114186
- FukubaNMoriyamaIIshiharaSPrimary pancreatic malignant lymphoma diagnosed from endoscopic ultrasound-guided fine-needle aspiration findingsIntern Med2016551313526726082
- DawsonIMCornesJSMorsonBCPrimary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosisBr J Surg196149808913884035
- BergerACGarciaMHoffmanJPPostresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704J Clin Oncol200826365918592219029412
- DuXZhaoYZhangTPrimary pancreatic lymphoma: a clinical quandary of diagnosis and treatmentPancreas2011401303620683215
- ChenWLuoRCFanWWMaSDClinical value of combined detection of LDH, TPS, CEA and beta2-MG in patients with non-Hodgkin’s lymphomaNan Fang Yi Ke Da Xue Xue Bao200626222722823016503538
- MerkleEMBenderGNBrambsHJImaging findings in pancreatic lymphoma: differential aspectsAJR Am J Roentgenol2000174367167510701607
- KhashabMMokademMDeWittJEndoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma – a case seriesEndoscopy201042322823120101569
- McCauleyAMGottliebKTPrimary pancreatic lymphoma coexisting with chronic lymphocytic leukemia: EUS findingsGastrointest Endosc200868118818918291383
- TuchekJMDe JongSAPicklemanJDiagnosis, surgical intervention, and prognosis of primary pancreatic lymphomaAm Surg19935985135188338282
- ZitvogelLTesniereAKroemerGCancer despite immunosurveillance: immunoselection and immunosubversionNat Rev Immunol200661071572716977338