Abstract
Micro-abstract
In a Phase I dose-finding study of metronomic daily oral vinorelbine in advanced non-small-cell lung cancer, a recommended dose was established for this therapeutic approach. In addition, this trial revealed promising efficacy data and an acceptable tolerability profile. The observed vinorelbine blood concentrations suggest continuous anti-angiogenic coverage.
Introduction
We present a Phase I dose-finding study investigating metronomic daily oral vinorelbine (Navelbine® Oral, NVBo) in advanced non-small-cell lung cancer (NSCLC).
Patients and methods
Patients with stage III/IV NSCLC received daily NVBo at fixed dose levels of 20–50 mg/d for 21 days of each 4-week cycle. Primary end point was the maximum tolerated dose. Secondary end points included tumor response, time to progression (TTP), overall survival (OS) and tolerability.
Results
Twenty-seven patients with advanced NSCLC were enrolled. Most of them were extensively pretreated. Daily NVBo was well tolerated up to 30 mg/d. At 40 mg/d, two of five patients experienced dose-limiting toxicities (DLTs). Three of six patients had DLTs at the 50 mg/d level. The recommended dose was established at 30 mg/d in cycle 1, with escalation to 40 mg/d in cycle 2, if tolerated. Pharmacokinetic analyses showed continuous blood exposure over 21 days and only marginal accumulation. The tolerability profile was acceptable (all dose levels – all grades: decreased appetite 33%, diarrhea 33%, leukopenia 33%, nausea 30%, vomiting 26%; ≥grade 3: leukopenia 30%, lymphopenia 19%, neutropenia 19%, febrile neutropenia 15%). Disease control rate, OS and TTP signaled a treatment effect.
Conclusion
Daily metronomic NVBo therapy in extensively pretreated patients with advanced NSCLC is feasible and safe at the recommended dose of 30 mg/d. Escalation to 40 mg/d in the second cycle is possible. The blood concentrations of vinorelbine after daily metronomic dosing reached lower peaks than intravenous or oral conventional dosing. Blood concentrations were consistent with anti-angiogenic or immune modulating pharmacologic properties of vinorelbine. Further studies are warranted to evaluate the safety and efficacy of this novel approach in specific patient populations.
Introduction
Lung cancer remains a major burden to patients and their communities. With 1.8 million new cases and 1.59 million deaths in 2012, lung cancer is one of the most common, and most fatal, cancers worldwide.Citation1,Citation2
Non-small-cell lung cancer (NSCLC) accounts for >80% of lung cancer cases. Although patients with early-stage disease may be cured by surgical resection, most patients with NSCLC present with advanced, inoperable disease. These patients, in particular those whose tumors do not respond to treatment, have a poor prognosis.Citation3,Citation4
Vinorelbine, a semisynthetic vinca-alkaloid, has demonstrated a good safety profile and consistent treatment efficacy across randomized trials in advanced NSCLC.Citation5–Citation9 The combination of cisplatin and vinorelbine is considered a standard of care in this setting.Citation10–Citation12
Most cytotoxic chemotherapy treatments are administered intravenously (iv). In NSCLC, single doses of iv chemotherapeutics are often administered on the first day of each 3- or 4-week cycle or more frequently, for example, on a weekly basis.
However, as the availability of oral cancer treatments increases,Citation13 potential advantages of this form of dosing are becoming clear. In addition to eliminating the discomfort, stress and potential complications associated with iv lines,Citation14 oral treatments can be administered at home, increasing convenience for patients and reducing the costs associated with visits to chemotherapy clinics.Citation15,Citation16 Oral treatments may be administered frequently without the burden associated with repeated infusions or continuous chemotherapy pumps.
Metronomic low-dosing schedules made possible by oral formulations may have biologic advantages compared to conventional chemotherapy boluses. The pharmacokinetics (PK) of metronomic administration allow for constant exposure to the cytotoxic agent, which may prevent tumor regrowth that may otherwise happen between conventional chemotherapy cycles. Furthermore, the toxic effects of chemotherapy might be lessened due to lower peak plasma concentrations. In addition, metronomic chemotherapy has been described to mediate antitumor effects by mechanisms other than cytotoxicity. The frequent administration of low-dose chemotherapy can induce anti-angiogenic effects, target tumor vasculature and strengthen the antitumor immune response by suppressing regulatory T cells and inducing the maturation of dendritic cells.Citation17,Citation18 Metronomic treatment strategies in various tumor entities were recently reviewed by Bocci and KerbelCitation19 in Nature Reviews. These authors emphasized the importance of including PK data in studies of metronomic chemotherapy in order to better understand dosing and treatment effects.
Oral vinorelbine (Navelbine® soft capsules; Pierre Fabre Médicament, Boulogne Billancourt, France; NVBo) has similar cytotoxic activity to iv vinorelbine.Citation20–Citation22 Initially, mimicking iv regimens, NVBo was given weeklyCitation23,Citation24; however, taking advantage of the oral formulation, more frequent and metronomic dosing has been recently introduced. Previous studies have shown that the administration of NVBo three times per week is feasible and well tolerated.Citation25,Citation26 Preliminary data indicate that fractionated doses of 70 mg/m2/wk split over days 1, 3 and 5 as well as fixed doses of up to 60 mg every other day are well tolerated and show activity in some patients.Citation27,Citation28
In this Phase I clinical trial, tolerability and preliminary efficacy of a metronomic treatment schedule, using fixed daily doses of 20–50 mg NVBo administered for 21 consecutive days of a 28-day cycle, were investigated in extensively pretreated patients with advanced NSCLC.
Patients and methods
Study design and objectives
This open-label Phase I clinical study recruited patients from April 2007 to December 2011. The primary objective was to determine the maximum tolerated dose (MTD) of daily NVBo. Secondary end points included tumor response, time to progression (TTP), overall survival (OS), tolerability profile and pharmacokinetic parameters. Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events v3.0.29.Citation29
Patients and study centers
Eligible patients were at least 18 years old and had histologically or cytologically confirmed stage IIIB/IV NSCLC (TNM 6) or recurrent disease after local therapy, with an indication for palliative chemotherapy. Both previously untreated patients and those who had received treatment with one or more lines of systemic therapy were eligible for inclusion. Patients were eligible if they had at least one measurable lesion (Response Evaluation Criteria in Solid Tumors [RECIST] v1.0Citation30), a Karnofsky index of at least 70%, a life expectancy of at least 12 weeks and adequate organ function.
Patients were excluded if they had metastases of the central nervous system, superior vena cava syndrome, symptomatic sensory neuropathy greater than grade 1 or any concomitant uncontrolled medical disorder. Moreover, patients could not have received previous treatment with vinorelbine and were required to be off systemic treatments and radiation for 30 days prior to enrollment. The study was conducted in four German centers.
Treatment
The patients were treated at four dose levels: 20, 30, 40 and 50 mg/d NVBo administered as levels I, II, III and IV, respectively. One treatment cycle was defined as a 28-day period, with daily administration on days 1–21 followed by a 7-day treatment-free interval. This schedule was selected to limit potential accumulation and associated toxicity.
In the dose escalation phase of the study, a 3+3 dose escalation design was applied. Three to six patients were treated at each dose level before escalation to the next dose level. The MTD was defined at the dose level, where at least two of six patients experienced dose-limiting toxicities (DLTs); the level below the MTD was defined as recommended dose for further evaluation. DLTs were assessed during cycle 1 at dose levels I–III. At dose level IV, the study was amended in order to include cycles 1 and 2 in the DLT assessment based on events fulfilling DLT criteria observed in cycle 2. DLTs were defined as follows: fever (with or without clinical infection), three elevations of oral temperature to >38°C during a 24-hour period or a single elevation in oral temperature to >38.5°C concomitant with grade 4 neutropenia and requiring iv antibiotics and/or hospitalization; grade 4 neutropenia and/or grade 2 thrombocytopenia lasting for ≥7 days; grade 4 or symptomatic grade 3 thrombocytopenia and any non-hematological event higher than grade 2 (excluding nausea/vomiting and AEs considered unrelated to treatment).
After MTD identification, it was planned to enroll a maximum of 12 patients at the recommended dose level. Due to slow accrual, only seven patients were included at this level.
PK assessment
Vinorelbine blood levels were assessed on days 1 and 21 of cycle 1, with additional trough levels on days 8 and 15. Blood concentrations of vinorelbine and its only active metabolite 4-O-deacetylvinorelbine (DVRL) were quantified using a fully validated liquid chromatography–tandem mass spectrometry with a lower limit of quantification of 0.25 ng/mL. PK parameters, such as the area under the plasma drug–concentration time curve (AUC), were calculated by a model-independent approach using KINETICA® software (Thermo Fischer Scientific Inc., Waltham, MA, USA). Median AUC estimates were used to calculate the accumulation ratio based on exposure (RAUC). The accumulation ratios based on trough concentration levels at days 1, 8, 15 and 21 (RCtrough) were calculated as well.
Efficacy assessment
Standard imaging was performed after every second cycle. Assessments of tumor response were performed according to RECIST v1.0. After treatment discontinuation, patients were followed up every 3 months until death.
Statistics
Data were evaluated using SAS® version 9.2 (Cary, NC, USA). Standard descriptive methods were used for all corresponding data. Time-to-event parameters were analyzed using the Kaplan–Meier method.
Duration of disease control (DoDC) was measured from the start of treatment until progression or death in patients with response or stable disease. TTP was calculated from the start of treatment until progression. If progression or death did not occur during treatment or within 30 days after treatment termination, patients were censored with the date of the last tumor assessment. Survival was measured from the start of treatment until death. Patients who were alive or lost to follow-up were censored with the last date they were known to be alive.
Ethical principles
All patients provided written informed consent. Approval of the study was obtained from the responsible ethics committee (ethics committee of the Medical Department Ludwig-Maximilians-University Munich) and regulatory authority (Bundesinstitut für Arzneimittel und Medizinprodukte). The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and local ethical and legal requirements.
Results
Analysis populations
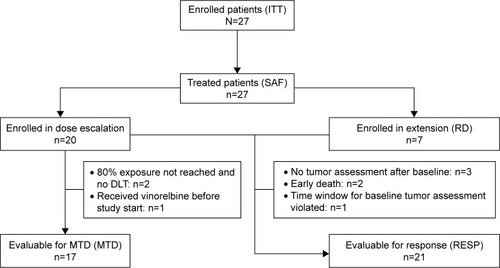
Twenty-seven patients were enrolled in the study; all patients received study treatment. The safety population (SAF) included all 27 patients and was identical with the intent-to-treat population (ITT). In addition, the following populations were analyzed: patients evaluable for response (RESP; 21 patients), patients evaluable for MTD (17 patients) and patients treated at the recommended dose level (RD; seven patients). The study population is summarized in a Consort diagram ().
Baseline parameters and disease characteristics
Of the 27 enrolled patients, 55.6% were male. Patient age ranged between 49 and 78 years (median: 65 years). Most patients (63.0%) had a Karnofsky index of 70%–80%. The median time from the first NSCLC diagnosis until enrollment was 20.7 months (range: 0.4–78.5 months). The distribution of histology was 44.4% for squamous cell carcinoma and 55.6% for adenocarcinoma. A total of 22.2% and 77.8% of patients were classified as stages IIIB and IV at study entry, respectively. At the time of baseline assessment, all patients had liver metastases: additional baseline tumor locations included lymph nodes (40.7%), pleura (33.3%) and bone (18.5%). Nearly all patients had received prior chemotherapy (92.6%) mainly in palliative intention (77.8%); the median number of prior palliative treatment lines was 3 (range: 0–5). About half of the patients (51.9%) had prior radiotherapy. Demographic data and disease characteristics are shown in .
Table 1 Demographic data and disease characteristics
Study treatment
The enrolled patients received a median of two treatment cycles (range: 1–33 cycles). The median treatment duration, absolute dose and relative dose intensity were 48 days (range: 3–912 days), 1,290 mg (range: 90–33,750 mg) and 97.9% (range: 40.0%–105.8%), respectively. The main reason for treatment discontinuation was progression or death (85.2%); discontinuations due to AEs occurred in 11.1% of the patients.
Maximum tolerated dose
A total of 17 patients were evaluable for MTD. provides a detailed overview on the dose escalation phase. At dose level III (40 mg/d), one of three patients experienced a DLT (neutropenia) in cycle 2, that is, after the protocol-specified DLT observation period. At dose level IV (50 mg/d), two of six patients showed DLTs during cycle 1 (fever and neutropenia) and one patient experienced DLTs during cycle 2 (fever and non-hematological event). Accordingly, the MTD was reached at dose level IV, and recruitment was extended at the draft recommended dose of 40 mg/d. However, one additional patient experienced a DLT during cycle 1 at this dose (fever).
Table 2 Dose escalation – DLTs observed during the dose escalation phase of the trial including toxicities observed at the MTD
Therefore, the following dose regimen was finally defined as recommended schedule for further assessment: 30 mg/d NVBo in cycle 1; escalation to 40 mg/d from cycle 2, if no DLT occurred during cycle 1. A total of seven patients were treated with this recommended schedule.
Pharmacokinetics
Cycle 1 PK could be assessed in a total of 21 patients. However, five of these patients were considered as non-evaluable on at least one of the PK days. Blood concentrations of vinorelbine generally increased with escalating dose levels. At 30 and 40 mg (), the daily oral dosing of vinorelbine provided a continuous blood exposure, as median trough concentrations were all >1 ng/mL.
Figure 2 Vinorelbine blood concentrations on day 1 and day 21 (cycle 1) after daily dosing of oral vinorelbine.
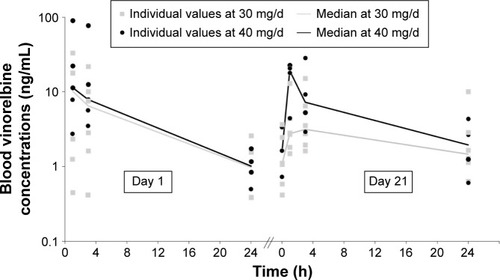
A marginal accumulation of vinorelbine was observed until day 8 based on residual concentrations (median RCtrough ranged from 1.96 to 2.03 between day 1 and either day 8, 15 or 21, all dose levels). This had no impact on global exposure over repeated dosing, as only minor differences in blood exposure were detected between days 1 and 21 (median RAUC day 21/day 1: 0.8, 1.6, 1.8 and 0.7 at 20, 30, 40 and 50 mg/d, respectively).
For DVRL, concentrations on day 1 were either not quantifiable or near the lower limit of quantitation for all patients. On day 21, DVRL concentrations were approximately or <1 ng/mL. As a result, no DVRL accumulation ratio could be calculated.
Tolerability
Of the 27 treated patients, 26 (96.3%) patients experienced at least one adverse event and 20 (74.1%) patients had at least one event with potential relationship to NVBo (). The most frequently reported related events were decreased appetite (33.3%), diarrhea (33.3%), leukopenia (33.3%), nausea (29.6%) and vomiting (25.9%). The most frequently reported related AEs of grade 3 or higher were leukopenia (29.6%), lymphopenia (18.5%), neutropenia (18.5%) and febrile neutropenia (14.8%). In the RD population (n=7), the most frequently reported related events were decreased appetite (42.9%) and vomiting (28.6%).
Table 3 AEs potentially related to vinorelbine occurring in at least 10% of the SAF and/or RD population
Serious AEs were observed in 17 (63.0%) patients of the SAF, and in 8 (29.6%) of those patients, the event was rated as potentially related to NVBo. The latter included the following cases: at dose level II (30 mg/d), one patient experienced grade 3 anorexia. At level III (40 mg/d), one patient experienced grade 4 febrile neutropenia and another patient grade 3 nausea. At level IV (50 mg/d), one patient experienced grade 4 leukopenia and grade 4 febrile neutropenia, one patient grade 4 neutropenic sepsis and another patient grade 4 leukopenia. At the recommended dose level (30–40 mg/d), one patient showed grade 2 vomiting and another patient experienced grade 4 febrile neutropenia, grade 4 pneumonia, grade 4 sepsis and grade 5 colitis. The latter patient was 74 years old, had several comorbidities (including hypertension, coronary heart disease and pulmonary embolism), was extensively pretreated (adenocarcinoma stage IV, two prior treatments with pemetrexed plus cisplatin followed by erlotinib) and experienced these events at the end of cycle 2 (40 mg/d). The management of this case was further complicated by the patients’ delayed reporting of the early symptoms and initial refusal to be admitted for treatment, so that treatment could not be delivered effectively.
Efficacy
In this heavily pretreated population, none of the patients experienced complete or partial response (). Four patients showed stable disease during the study, resulting in a disease control rate (DCR) of 19.0% in the RESP population (n=21). Kaplan–Meier analysis revealed a median DoDC of 8.7 months (95% confidence interval [95% CI]: 4.9–27.3 months) in the patients with stable disease.
Table 4 Efficacy parameters
In the intent-to-treat population (n=27), the median TTP and OS were 1.7 months (95% CI: 1.4–2.1 months) and 5.7 months (95% CI: 3.5–9.6 months), respectively.
Discussion
Metronomic chemotherapy is characterized by continuous long-term administration of chemotherapeutics at relatively low doses.Citation17 Potential advantages of this form of dosing include lower toxicity, avoidance of drug resistance and anti-angiogenic effects.Citation18,Citation31 In recent years, preclinical and clinical studies have investigated metronomic schedules using a variety of anti-neoplastic treatments including vinorelbine, cyclophosphamide, capecitabine, methotrexate, sorafenib, everolimus and temozolomide.Citation31 However, conflicting results demonstrated the need for optimized patient selection and stratification.
This Phase I trial confirmed that NVBo can be admini stered safely on a daily basis with only marginal PK accumulation. In a “three weeks on, one week off” schedule, the recommended dose was found to be 30 mg/d in cycle 1 with escalation to 40 mg/d from cycle 2, if tolerated. The results show acceptable tolerability and only marginal PK accumulation. Moreover, the observed vinorelbine blood concentrations at 30 and 40 mg/d are in the 1–10 ng/mL range >24 hours, which suggests that a continuous anti-angiogenic coverage is maintained using this dose regimens.Citation25 This vinorelbine concentration range is also consistent with the previously demonstrated effect of vinorelbine with platinum to sensitize tumor cells to cytotoxic T lymphocyte-mediated lysis.Citation32 More broadly, while sparing the cytotoxic T cells, vinorelbine daily metronomic administration may continuously stimulate the maturation of dendritic cells and kill the immunosuppressive Treg cells, as was described for related chemotherapeutic agents.Citation33,Citation34 On the contrary, those effects may be triggered only episodically with conventional dosing regimen, for which there are higher Cmax and larger peak/trough fluctuations of circulating concentrations.Citation35,Citation36
Because vinorelbine is predominantly metabolized by hepatic CYP3A4,Citation37 drug exposure may be higher in patients with impaired liver function. Patients with significantly impaired liver function were excluded from this study. This study included many patients who had undergone one or more lines of systemic chemotherapy. Although this population is clinically relevant, the MTD established in this setting may be lower than in a treatment-naive, first-line population. Careful investigation of the safety and efficacy in specific populations and treatment situations is required in future studies.
As this study was not primarily designed to assess efficacy of the tested treatment, efficacy results must be interpreted with caution, especially taking into account the extensive and mixed pretreatment of the included patient population. Considering these limitations, the observed results – with a DCR of 19.0%, a median TTP of 1.7 months and a median OS of 5.7 months – provide an initial signal with regard to clinical activity of the investigated regimen. The observed disease stabilization and lack of partial or complete responses in this study should be interpreted with consideration of the heavy pretreatment of most patients included. A retrospective analysis of third-line chemotherapy by Girard et alCitation38 described a response rate of 38% after first-line treatment but only 14% and 6% after second- and third-line treatments, respectively. In contrast, the rate of disease stabilization following third-line treatment was 30% and cancer-related symptoms and performance status improved during third-line treatment for many patients. A decreasing response rate with increasing number of lines of treatment was also reported in the large retrospective study by Massarelli et al,Citation39 who showed third-line response rates of only 2.3% and fourth-line response rates of 0% in contrast to first-line response rates over 20%. These results demonstrate that a low response rate is often seen following the administration of treatments in third or later line and should not in itself discourage further trials of a treatment. In this study, there was significant heterogeneity in the responses observed, as shown by the wide 95% CI for DCR (4.9–27.3 months), with some patients benefiting with >2 years of stable disease control. Molecular and clinical predictors of response to metronomic vinorelbine have yet to be established. Future clinical trials of metronomic vinorelbine should include the search for potential predictors of response in clearly defined patient populations.
Several other recent trials have investigated and further ongoing trials are currently evaluating the metronomic application of NVBo in solid tumors. In a recent Phase I–II study of metronomic NVBo plus capecitabine in patients with metastatic breast cancer, the MTD for NVBo was found to be 40 mg three times per week, combined with capecitabine 500 mg three times per day from days 1–14 of a 21-day cycle. Initial efficacy data revealed a clinical benefit in 58.1% of the patients.Citation26 In addition, Briasoulis et alCitation25 investigated a metronomic NVBo monotherapy in patients with various advanced solid tumors and suggested a NVBo dose of 50 mg given three times per week. This schedule was further investigated as first-line and salvage treatment in NSCLC by Camerini et al,Citation40 who demonstrated a clinical benefit in 58.1% of patients treated in first line, with a median TTP and OS of 5 and 9 months, respectively. As salvage treatment, this regimen showed a median TTP and OS of 2.2 and 9.4 months, respectively.Citation41 An adequate tolerability profile was observed in all of these studies. Subsequent Phase II studies investigating the three times per week as well as the continuous daily administration of metronomic NVBo in patients with metastatic breast cancer and advanced NSCLC are currently ongoing (EudraCT 2014-003860-19, EudraCT 2014-003859-61, NCT03007992). The exclusion of the chemotherapy-free week in these regimens may provide more continuous anti-neoplastic activity and offer additional biological benefit.
Conclusion
The results from this Phase I study in mostly extensively pretreated patients with advanced NSCLC show that daily administration of NVBo is feasible and safe at the recommended dose level (30–40 mg/d). PK assessment indicated that blood concentrations of vinorelbine after daily administration are continuously maintained at rather low levels, which would have a potential to trigger anti-angiogenic and immune-mediated antitumor mechanisms. Further studies are warranted to evaluate the efficacy and to further characterize the safety of this novel approach.
Acknowledgments
This study was designed, initiated, conducted and analyzed under the responsibility of the University Hospital of Munich, the sponsor of this trial. The project was supported by an unrestricted grant as well as by free-of-charge medication provided by Pierre Fabre Pharma GmbH, Freiburg, Germany. Moreover, Pierre Fabre Pharma GmbH also provided financial support for medical writing assistance and scientific input during the manuscript preparation. The authors also would like to thank the patients for their participation, the staff of the study sites and the clinical research organization ClinAssess GmbH (Leverkusen, Germany), all of whom significantly contributed to the success of the study. We further thank Dr Schreier and Dr Esser at co.faktor GmbH (Berlin, Germany), who provided medical writing assistance.
Disclosure
AT received conference registration and travel support from Pierre Fabre Pharma GmbH. PF is an employee of Pierre Fabre Pharmaceuticals (Toulouse, France), BE is an employee of Pierre Fabre Pharma GmbH (Freiburg, Germany) and RMH received funding from Pierre Fabre Pharma GmbH (Freiburg, Germany). The other authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIErvikM homepage on the InternetGLOBOCAN 2012 – Lung cancer incidence and mortality worldwide Available from: http://globocan.iarc.frAccessed September 15, 2015
- StewartBWWildCPWorld Cancer Report 2014Lyon, FranceInternational Agency for Research on Cancer (IARC) – World Health Organization2014
- Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical OncologyJ Clin Oncol1997158299630189256144
- MolinaJRYangPCassiviSDSchildSEAdjeiAANon-small cell lung cancer: epidemiology, risk factors, treatment, and survivorshipMayo Clin Proc200883558459418452692
- Le ChevalierTBrisgandDDouillardJYRandomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patientsJ Clin Oncol19941223603678113844
- DepierreAChastangCQuoixEVinorelbine versus vinorelbine plus cisplatin in advanced non-small cell lung cancer: a randomized trialAnn Oncol1994513742
- CrawfordJO’RourkeMSchillerJHRandomized trial of vinorelbine compared with fluorouracil plus leucovorin in patients with stage IV non-small-cell lung cancerJ Clin Oncol19961410277427848874339
- WozniakAJCrowleyJJBalcerzakSPRandomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group studyJ Clin Oncol1998167245924659667264
- Le ChevalierTBrisgandDSoriaJCLong term analysis of survival in the European randomized trial comparing vinorelbine/cisplatin to vindesine/cisplatin and vinorelbine alone in advanced non-small cell lung cancerOncologist20016suppl 1811
- ManegoldCChemotherapy for advanced non-small cell lung cancer: standardsLung Cancer200134suppl 2S165S17011720760
- ReckMPopatSReinmuthNMetastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201425suppl 3ii27ii39
- BesseBAdjeiABaasPPanel MembersESMO2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced diseaseAnn Oncol20142581475148424669016
- FindlayMvon MinckwitzGWardleyAEffective oral chemotherapy for breast cancer: pillars of strengthAnn Oncol200819221222218006898
- TwelvesCGollinsSGrieveRSamuelLA randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorou-racil/leucovorin regimens in patients with advanced colorectal cancerAnn Oncol200617223924516344278
- LiuGFranssenEFitchMIWarnerEPatient preferences for oral versus intravenous palliative chemotherapyJ Clin Oncol19971511101158996131
- CassidyJDouillardJYTwelvesCPharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: the X-ACT trialBr J Cancer20069481122112916622438
- KerbelRSKamenBAThe anti-angiogenic basis of metronomic chemotherapyNat Rev Cancer20044642343615170445
- TorimuraTIwamotoHNakamuraTMetronomic chemotherapy: possible clinical application in advanced hepatocellular carcinomaTransl Oncol20136551151924151531
- BocciGKerbelRSPharmacokinetics of metronomic chemotherapy: a neglected but crucial aspectNat Rev Clin Oncol2016131165967327184418
- TanEHRolskiJGrodzkiTGlobal Lung Oncology Branch trial 3 (GLOB3): final results of a randomised multinational phase III study alternating oral and i.v. vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small-cell lung cancerAnn Oncol20092071249125619276396
- De LenaMDRamlauRHansenOPhase II trial of oral vinorelbine in combination with cisplatin followed by consolidation therapy with oral vinorelbine in advanced NSCLCLung Cancer200548112913515777980
- GrallaRJGatzemeierUGebbiaVHuberRO’BrienMPuozzoCOral vinorelbine in the treatment of non-small cell lung cancer: rationale and implications for patient managementDrugs200767101403141017600389
- JassemJKosmidisPRamlauROral vinorelbine in combination with cisplatin: a novel active regimen in advanced non-small-cell lung cancerAnn Oncol200314111634163914581271
- FreyerGDelozierTLichinisterMPhase II study of oral vinorelbine in first-line advanced breast cancer chemotherapyJ Clin Oncol2003211354012506167
- BriasoulisEAravantinosGKouvatseasGDose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational studyBMC Cancer20131326323718900
- CazzanigaMETorriVVillaFEfficacy and safety of the all-oral schedule of metronomic vinorelbine and capecitabine in locally advanced or metastatic breast cancer patients: the phase I–II VICTOR-1 studyInt J Breast Cancer201420147 Article ID 769790
- AddeoRSgambatoACennamoGLow-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancerClin Breast Cancer201010430130620705563
- PallisAGChandrinosVPavlakouGA multicenter phase I trial of metronomic oral vinorelbine plus cisplatin in patients with NSCLCCancer Chemother Pharmacol20116761239124520697712
- DCTDNCINIHDHHSCancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0, 20032006 Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed December 19, 2016
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst2000923205216
- GnoniASilvestrisNLicchettaAMetronomic chemotherapy from rationale to clinical studies: a dream or reality?Crit Rev Oncol Hematol2015951466125656744
- GameiroSRCaballeroJAHodgeJWDefining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killingCancer Biother Radiopharm2012271233522316209
- TanakaHMatsushimaHNishibuAClausenBETakashimaADual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturationCancer Res200969176987699419706755
- TagliamonteMPetrizzoJNapolitanoMA novel multi-drug metronomic chemotherapy significantly delays tumor growth in miceJ Transl Med2016145826911136
- BourgeoisHVermorkenJDarkGEvaluation of oral versus intravenous dose of vinorelbine to achieve equivalent blood exposures in patients with solid tumoursCancer Chemother Pharmacol200760340741317541591
- KhayatDRixeOBrunetRPharmacokinetic linearity of i.v. vinorelbine from an intra-patient dose escalation study designCancer Chemother Pharmacol200454319320515160284
- KajitaJKuwabaraTKobayashiHKobayashiSCYP3A4 is mainly responsible for the metabolism of a new vinca alkaloid, vinorelbine, in human liver microsomesDrug Metab Dispos20002891121112710950859
- GirardNJacouletPGainetMThird-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practiceJ Thorac Oncol20094121544154919884862
- MassarelliEAndreFLiuDDA retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancerLung Cancer2003391556112499095
- CameriniAPuccettiCDonatiSMetronomic oral vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer: results of a phase II trial (MOVE trial)BMC Cancer20151535925943747
- KontopodisEHatzidakiDVarthalitisIA phase II study of metronomic oral vinorelbine administered in the second line and beyond in non-small cell lung cancer (NSCLC): a phase II study of the Hellenic Oncology Research GroupJ Chemother2013251495523433445