Abstract
Galectin-1 (Gal-1) is known to regulate cell signaling within the immune system and may be a target for new anticancer immune therapy. In patients with chronic lymphocytic leukemia (CLL) and classical Hodgkin lymphoma (cHL), high levels of Gal-1 within the tumor microenvironment were associated with worse disease state or poor outcome. Gal-1 can be secreted from cells by an unknown mechanism, and levels in blood samples were associated with high tumor burden and worse disease state in cHL and CLL patients. However, serum levels of Gal-1 have never been investigated in patients with multiple myeloma (MM). We measured serum Gal-1 levels in samples from patients with treatment demanding MM at the time of diagnosis (n=102) and after treatment (n=24) and examined associations of serum Gal-1 with clinicopathological information obtained from patient medical records, as well as data on bone marrow angiogenesis and the macrophage activation biomarkers soluble CD163 (sCD163) and soluble mannose receptor. Serum Gal-1 levels were not elevated in patients with MM at diagnosis compared with healthy donors (median values 8.48 vs 11.93 ng/mL, P=0.05), which is in contrast to results in cHL and CLL. Furthermore, Gal-1 levels did not show association with bone marrow angiogenesis, clinicopathological parameters, overall survival, or response to treatment. There was a statically significant association between Gal-1 and sCD163 levels (R=0.24, P=0.02), but not with soluble mannose receptor (P=0.92). In conclusion, our results indicate that Gal-1 is not an important serum biomarker in MM, which is in contrast to data from patients with cHL and CLL. However, the association with sCD163 is in line with previous data showing that Gal-1 may be involved in alternative (M2-like) activation of macrophages.
Introduction
Galectins comprise a 15-member family of lectins (carbohydrate-binding proteins), of which some are widely distributed in various tissues and others are more confined.Citation1,Citation2 Galectins exert diverse functions by binding to carbohydrate moieties on, for example, extracellular proteins, but they also function intracellularly where they are involved in cell signaling processes.Citation1 Furthermore, galectins have been linked with the regulation of hematopoiesis and modulation of the immune system in both acute and chronic inflammation.Citation1,Citation3
Galectin-1 (Gal-1) is involved in regulating homeostasis and differentiation of both T and B cells, can skew an immune reaction in a TH2-like (anti-inflammatory) direction, and may sway monocyte/macrophage polarization toward an alternatively activated (M2-like) phenotype.Citation4,Citation5 Importantly, studies have indicated that inhibition of Gal-1 signaling may affect immune activation, and thus could be a strategy for new anticancer therapy.Citation6,Citation7
In a study on classical Hodgkin lymphoma (cHL) patients, Gal-1 was expressed by Hodgkin and Reed–Sternberg cells as well as macrophages and endothelial cells. Interestingly, high expression of Gal-1 within the tumor microenvironment was associated with poor survival.Citation8
Gal-1 can be secreted from cells by an unknown mechanismCitation1 and can be measured in blood samples.Citation9,Citation10 In patients with chronic lymphocytic leukemia (CLL), both bone marrow and plasma levels of Gal-1 were increased in patients with progressive compared with stable disease, and CLL-supporting myeloid cells were found to be the major producers of Gal-1.Citation11 In cHL patients, serum Gal-1 levels were increased compared with healthy controls and associated with clinical features of high tumor burden.Citation9
Multiple myeloma (MM) is the second most common hematological malignancy, in which malignant plasma cells (PCs) proliferate within the bone marrow, leading to anemia, bone destruction, and renal failure.Citation12 The bone marrow microenvironment is known to be of major importance in the disease. Recently, macrophages have been reported as important supportive cells in MM, at least in part because of the promotion of MM cell growth and resistance to therapy,Citation13 and infiltration by CD163+ macrophages in the bone marrow is associated with poor outcome in MM.Citation14,Citation15 Within the few reports addressing the role of galectins in MM, one study indicated that Gal-1 increased proliferation and survival of CD45RAneg MM cells.Citation16 A recent study reported increased Gal-1 mRNA expression in CD138+ bone marrow cells of MM patients, and that Gal-1 may be important in MM-induced angiogenesis.Citation17 Increased bone marrow angiogenesis is a characteristic feature of MM, where high level of angiogenesis is associated with poor outcome,Citation18 and interestingly, Gal-1 may be involved in the regulation of angiogenesis in various human cancers.Citation19
However, levels of serum Gal-1 have never been reported for patients with MM. Therefore, the aim of this study was to determine Gal-1 levels in patients with newly diagnosed MM and investigate associations with clinicopathological parameters, bone marrow angiogenesis, markers of macrophage activation, and patient outcome.
Patients and methods
Patients
The cohort used in this study consists of 102 patients with newly diagnosed, treatment demanding MM, included at the Department of Hematology, Aarhus University Hospital, Aarhus, Denmark, from 1991 to 2004. With data from the same cohort of patients, we have previously published results on the macrophage-derived biomarkers soluble CD163 and soluble CD206/mannose receptor (sCD163 and sMR).Citation20,Citation21 Basic characteristics of the included patients have been thoroughly described previously.Citation20 Briefly, patients were treated with either conventional peroral chemotherapy (e.g., melphalan) and prednisolone (MP, 60%) or high-dose melphalan with autologous stem cells support (high dose therapy [HDT], 40%). We obtained a serum sample from the time of diagnosis and measured Gal-1 concentration. Of the included patients, 54% were male, median age at diagnosis was 60 years (range 39–84 years), and the heavy chain class subtypes were IgG: 58%, IgA: 25%, and light chain only: 17%. Furthermore, for 24 patients, we were able to measure serum Gal-1 in a sample taken 3 months after HDT. Data from a previously published study in which we have measured serum Gal-1 in 30 healthy donors were included as a control group: 53% male, median age 53 years (range 45–66 years).Citation10 All samples were stored at −80°C.
Information about basic characteristics, pathology, and biochemistry results was obtained from patient medical records. Response to treatment was categorized as 1) complete response (CR) or 2) less than CR. CR was defined as negative serum and urine M-protein by electrophoresis and <5% PCs in the bone marrow. Data on bone marrow angiogenesis, measured as CD34+ micro-vessel density (MVD), were obtained during a previous study and are described in detail in the study by Andersen et al.Citation18 The study was approved by the central Denmark region committee on health research ethics (M-20100171). The requirement for study-specific informed consent was waived due to the retrospective nature of the study and the long follow-up time since inclusion.
Measurement of serum Gal-1
Serum Gal-1 levels were measured using a time-resolved immunofluorometric assay as described previously.Citation10 Microtiter wells were coated with capture antibody (AF1152, R&D Systems) at 2 μg/mL in phosphate-buffered saline (PBS)/pH 8 for 2 h. Plates were washed and blocked for 1 h (PBS, pH 7.4, 1% [v/v] Tween20). The wells were washed and standards or diluted serum (1:4) were added (dilution in assay buffer: PBS, pH 7.4, 25 μM ethylenediaminetetraacetic acid, 0.05% [v/v] human serum albumin, 1% [v/v] Tween20, 0.9% [w/v] NaCl) and incubated for 2 h at room temperature. Following incubation with biotin-conjugated detection antibody (BAF1152, R&D Systems) at 0.1 μg/mL in assay buffer supplemented with europium-labeled streptavidin (PerkinElmer) diluted 1:1,000, DELFIA enhancement solution (PerkinElmer) was added and plates were read in a time-resolved fluorometer (Victor3, PerkinElmer). A standard curve of full-length recombinant Gal-1 (1152-GA, R&D Systems) at concentrations of 100–0.78 ng/mL was fitted using a 4-parameter nonlinear regression curve for each plate. All samples were run in duplicates, with mean %CV of 2.62 in the included samples. Two samples were excluded from further analysis due to %CV>10%. Four donor samples were run in duplicate (CV<3.5%) on every plate, yielding a mean inter-plate CV of 15% (range 7%–21%). Previous measurements showed stability of Gal-1 over a year of repeated freeze/thaw cycles. Furthermore, when correlating the measured Gal-1 concentration with a storage time at −80°C, we found no indication of a time-dependent change in Gal-1 concentration (data not shown). In this study, three and five samples from patients and healthy donors, respectively, showed a concentration above the range of the standard curve (400 ng/mL). These samples were included in the statistical analyses with a concentration of 400 ng/mL.
Statistical analyses
Statistical analyses were done using Stata version 11 for Mac (StataCorp LP, TX, USA). Figures were made using Prism 5 for Mac (GraphPad software Inc., CA, USA). Differences between groups were tested using paired or unpaired t-tests and one-way analysis of variance, when appropriate. Gaussian distribution of data was assessed by Q–Q plots before analysis, and non-normally distributed data were log-transformed before analysis. For investigation of linear associations between parameters, we used Pearson’s correlation analyses. Survival analyses were performed using Cox proportional hazards regression. All Gal-1 concentrations are reported as median values with interquartile range in parentheses. P-values of <0.05 were considered statistically significant.
Results
Serum Gal-1 at the time of diagnosis and after HDT
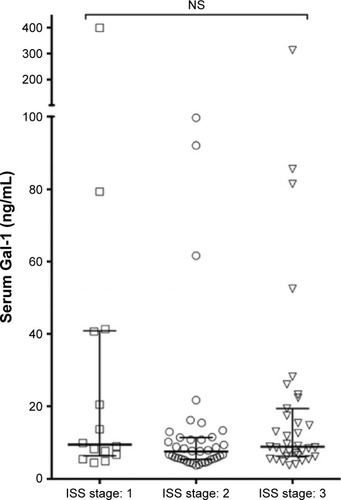
Data on the measurements of Gal-1 concentrations in serum samples from 102 patients with newly diagnosed MM and 30 healthy donors are shown in . The Gal-1 levels displayed relatively large variation for both patients and controls, with median values: MM, 8.48 ng/mL (5.75–14.78) and controls, 11.93 ng/mL (6.23–80.91), which was a borderline statistically significant difference (P=0.05). In , Gal-1 levels are shown in relation to international staging system stage,Citation22 showing no clear association between Gal-1 levels and international staging system stage at the time of diagnosis (P=0.10). Using correlation analyses, we examined associations of Gal-1 with various clinicopathological parameters, but found no statistically significant associations: gender (P=0.82), age at diagnosis (P=0.18), creatinine (P=0.12), albumin (P=0.31), C-reactive protein (P=0.57), beta-2 microglobulin (P=0.93), and bone marrow PC infiltration (P=0.16).
Figure 1 Serum Gal-1 in MM patients pre- and posttreatment.
Abbreviations: Gal-1, galectin-1; HDT, high dose treatment; MM, multiple myeloma; NS, not significant.
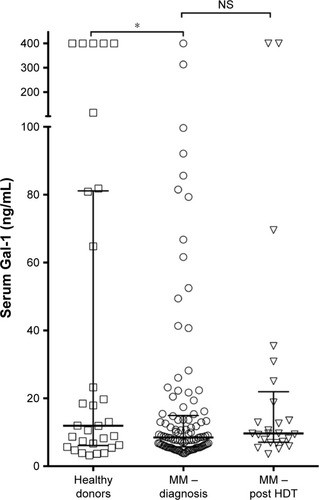
Figure 2 Serum Gal-1 in multiple myeloma patients according to ISS stage.
Abbreviations: Gal-1, galectin-1; ISS, international staging system; NS, not significant.
In the 24 patients for whom we obtained serial samples, we found no statistically significant difference between Gal-1 levels at diagnosis and 3 months after (, P=0.13).
Association of serum Gal-1 levels to treatment response and outcome
For the 41 patients treated with HDT, we had access to data on the response to treatment. In these patients, there was no association with treatment response for either Gal-1 levels at diagnosis and at 3 months after HDT or for the change in Gal-1 during the course of treatment (all P>0.05).
To investigate whether serum Gal-1 was a prognostic marker in the cohort of patients with MM, we performed a Cox proportional hazards regression analyses with Gal-1 as a continuous variable, showing that Gal-1 was not a prognostic factor, for patients treated neither with MP (n=61, hazard ratio [HR] =0.99, P=0.97) nor with HDT (n=41, HR =1.02, P=0.90).
Gal-1 and bone marrow angiogenesis
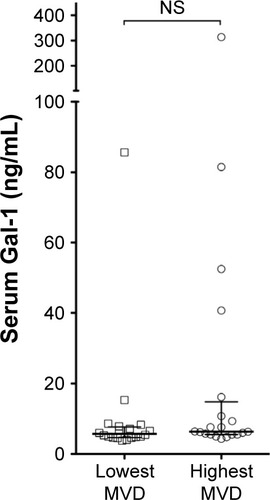
Previously, we have shown that high levels of bone marrow angiogenesis are associated with poor outcome in MM.Citation18 In , serum Gal-1 levels are plotted by angiogenesis level, using our previously published data on bone marrow angiogenesis measured as MVD (split on median). There was a trend toward slightly higher serum Gal-1 levels in patients with highest MVD, but the difference was not statistically significant (P=0.11).
Figure 3 Serum Gal-1 and bone marrow angiogenesis.
Abbreviations: Gal-1, galectin-1; MVD, micro-vessel density; NS, not significant.
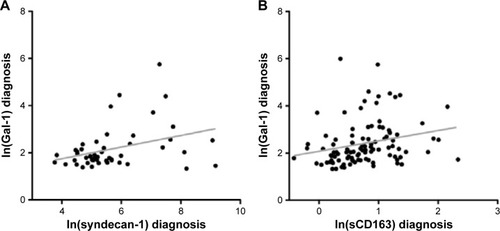
In our previous study, serum levels of syndecan-1 associated positively with bone marrow angiogenesis and with poor patient outcome.Citation18 In this study, we also found a positive linear association of serum Gal-1 with syndecan-1 levels (, n=49, R=0.36, P=0.01).
Figure 4 Associations of serum Gal-1 with macrophage and plasma cell markers.
Abbreviations: Gal-1, galectin-1; sCD138, soluble CD138.
Gal-1 and markers of macrophage activation
Because Gal-1 may be produced by myeloid cells, as reported for patients with CLL,Citation11 and may induce M2 polarization of macrophages, we examined possible associations with monocyte/macrophage-derived sCD163 and sMR. At the time of diagnosis, serum Gal-1 was positively associated with sCD163 (n=102, R=0.24, P=0.02 []). However, there was no association of Gal-1 with sMR (n=102, P=0.92). At 3 months after HDT, there was a borderline significant association with both sCD163 (P=0.07) and sMR (P=0.05; data not shown).
Discussion
Recent years have seen a surge in the interest of galectin involvement in cancer pathophysiology,Citation2 and blood levels of Gal-1 have been associated with more severe disease in hematological malignancies.Citation9,Citation11
However, serum levels of Gal-1 have not previously been reported in patients with MM. In a cohort of 102 patients with newly diagnosed, treatment demanding MM, we found no association between serum Gal-1 at diagnosis, tumor burden (bone marrow PC infiltration), or with overall patient survival. These findings indicate that Gal-1 is not an important serum biomarker in MM, in contrary to what has been shown for cHL and CLL.Citation9,Citation11 This is further supported by the finding that serum Gal-1 levels were not increased in MM patients compared with healthy controls, opposed to the results in cHL and CLL.
In a recent study, high expression of Gal-1 mRNA expression in purified CD138+ bone marrow cells correlated with worse outcome for MM patients.Citation17 Gal-1 is expressed by endothelial cells and has been shown to be a proangiogenic growth factor,Citation19 and in this study, there was a trend toward higher Gal-1 in patients with high MVD, but there was no statistically significant association between the two parameters. Storti et al reported high Gal-1 expression in hypoxic conditions, and inhibition of Gal-1 in MM cells decreased expression of proangiogenic genes and reduced tumor burden and angiogenesis (MVD) in mouse models.Citation17 Thus, this study indicates an important role for Gal-1 in CD138+ cells, but from our results, this may not be translated into measurable Gal-1 protein in serum. Hence, the limitation of this study is that we only had access to blood samples, and not samples of bone marrow. Therefore, future studies on Gal-1 in MM should include bone marrow samples for gene expression analysis, and, especially, protein level analyses on CD138+ cells may provide further insight into the role of Gal-1 in MM pathophysiology.
In CLL and cHL, Gal-1 is expressed by both malignant and tumor-supporting cells.Citation8,Citation11 Gal-1 may influence the activation state of macrophages inducing a less inflammatory (M2-like) phenotype, which has been associated with poor outcome in several human cancers.Citation23 In cHL, tissue expression of Gal-1 was correlated with CD163, which is a marker of M2-activated macrophages.Citation8 In MM, higher infiltration of CD163-expressing macrophages is associated with shorter survival,Citation14 and we have previously shown that sCD163 is associated with overall survival in MM.Citation20 In this study, we found a positive association between the levels of serum Gal-1 and sCD163, which support the previous findings indicating that Gal-1 may be associated with alternative macrophage activation.Citation5
Thus, one explanation for the present results may be that the increased mRNA expression of Gal-1 in MM CD138+ bone marrow cellsCitation17 is not translated into increased protein levels, or that the increase is not reflected in the peripheral blood, resulting in serum levels not being significantly higher in patients with MM than in healthy controls. This should be investigated in future studies.
In conclusion, the serum levels of Gal-1 were not increased in patients with MM (compared with healthy donors) and did not show association with known prognostic factors or patient survival, which is in contrast to published data from patients with other hematological malignancies. However, serum Gal-1 levels were associated with monocyte/macrophage-derived sCD163, supporting the involvement of Gal-1 in alternative (M2-like) activation of macrophages.
Author contributions
MNA contributed in conception and design, collection of data, analysis and interpretation of data, drafting the article, and approval of the version to be published. ML contributed to conception and design, collection of data, revising the manuscript critically, and approval of the version to be published. NA contributed in collection of data, revising the manuscript critically, and approval of the version to be published. IP carried out providing Gal-1 data on healthy donors, revising the manuscript critically, and approval of the version to be published. RH performed Gal-1 assay development and validation, revising the manuscript critically, and approval of the version to be published. MB performed Gal-1 assay development and validation, revising the manuscript critically, and approval of the version to be published. BH contributed in Gal-1 assay development and validation, revising the manuscript critically, and approval of the version to be published. HJM carried out collection of data, revising the manuscript critically, and approval of the version to be published. NFA contributed to conception and design, collection of data, analysis and interpretation of data, revising the manuscript critically, and approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuF-TRabinovichGAGalectins: regulators of acute and chronic inflammationAnn N Y Acad Sci2010118315818220146714
- GiordanoMCrociDORabinovichGAGalectins in hematological malignanciesCurr Opin Hematol201320432733523695449
- RabinovichGAVidalMGalectins and microenvironmental niches during hematopoiesisCurr Opin Hematol201118644345121912250
- BarrionuevoPBeigier-BompadreMIlarreguiJMA novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathwayJ Immunol20061781436445
- YangRYRabinovichGALiuFTGalectins: structure, function and therapeutic potentialExpert Rev Mol Med200810e1718549522
- RubinsteinNAlvarezMZwirnerNWTargeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejectionCancer Cell20045324125115050916
- ToscanoMABiancoGAIlarreguiJMDifferential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell deathNat Immunol20078882583417589510
- KamperPLudvigsenMBendixKProteomic analysis identifies galectin-1 as a predictive biomarker for relapsed/refractory disease in classical Hodgkin lymphomaBlood2011117246638664921505194
- OuyangJPlütschowAPogge von StrandmannEGalectin-1 serum levels reflect tumor burden and adverse clinical features in classical Hodgkin lymphomaBlood2013121173431343323444403
- PetruskeviciusILudvigsenMHjortebjergRClinical relevance of galectin-1 in hematologic malignancies treated with non-myeloablative hemopoietic stem cell transplantationBone Marrow Transplant201651101387139027214079
- CrociDOMorandePEDergan-DylonSNurse-like cells control the activity of chronic lymphocytic leukemia B cells via galectin-1Leukemia20132761413141623257714
- KyleRASteensmaDPHistory of multiple myelomaRecent Results Cancer Res201118332321509678
- ZhengYYangJQianJPSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myelomaLeukemia201227370271022996336
- SuyanıESucakGTAkyürekNTumor-associated macrophages as a prognostic parameter in multiple myelomaAnn Hematol201392566967723334187
- PanchabhaiSKelemenKAhmannGSebastianSManteiJFonsecaRTumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myelomaLeukemia201630495195426202926
- AbrounSOtsuyamaK-IShamsasenjanKGalectin-1 supports the survival of CD45RA(−) primary myeloma cells in vitroBr J Haematol2008142575476518537967
- StortiPMarchicaVAiroldiIGalectin-1 suppression delineates a new strategy to inhibit myeloma-induced angiogenesis and tumoral growth in vivoLeukemia201630122351236327311934
- AndersenNFStandalTNielsenJLSyndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survivalBr J Haematol2005128221021715638855
- GriffioenAWThijssenVLGalectins in tumor angiogenesisAnn Transl Med2014299025405165
- AndersenMNAbildgaardNManieckiMBMøllerHJAndersenNFMonocyte/macrophage-derived soluble CD163: a novel biomarker in multiple myelomaEur J Haematol2014931414724612259
- AndersenMNAndersenNFRødgaard-HansenSHoklandMAbildgaardNMøllerHJThe novel biomarker of alternative macrophage activation, soluble mannose receptor (sMR/sCD206): implications in multiple myelomaLeuk Res201539997197526169445
- GreippPRSan MiguelJDurieBGMInternational staging system for multiple myelomaJ Clin Oncol200523153412342015809451
- SicaAAllavenaPMantovaniACancer related inflammation: the macrophage connectionCancer Lett2008267220421518448242
