Abstract
Objective
Previous studies have reported that Ras-associated domain family 1A (RASSF1A), the most commonly silenced tumor suppressor via promoter methylation, played vital roles in the development of carcinogenesis. The purpose of this meta-analysis was to determine whether RASSF1A promoter methylation increased the risk of thyroid cancer.
Methods
PubMed, Embase, ISI Web of Knowledge, and Chinese National Knowledge Infrastructure databases were searched to obtain eligible studies. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the strength of the associations, using Stata 12.0 software. The methodological quality of included studies was evaluated using Newcastle–Ottawa scale table. Egger’s test and Begg’s test were applied to detect publication biases. TSA 0.9 software was used to calculate the required information size and whether the result was conclusive.
Results
A total of 10 articles with 12 studies that included 422 thyroid cancer patients, identifying the association of RASSF1A promoter methylation with thyroid cancer risk, were collected in this meta-analysis. Overall, RASSF1A promoter methylation significantly increased the risk of thyroid cancer (total, OR=8.27, CI=4.38–15.62, P<0.05; Caucasian, OR=9.25, CI=3.97–21.56, P<0.05; Asian, OR=7.01, CI=2.68–18.38, P<0.05). In the subgroup analysis based on sample type, a significant association between thyroid cancer group and control group was found (normal tissue, OR=9.55, CI=4.21–21.67, P<0.05; adjacent tissue, OR=6.80, CI=2.49–18.56, P<0.05). The frequency of RASSF1A promoter methylation in follicular thyroid carcinoma was higher than in control group (OR=11.88, CI=5.80–24.32, P<0.05). In addition, the results indicated that the RASSF1A promoter methylation was correlated with papillary thyroid carcinoma in Caucasians and Asians (total, OR=8.07, CI=3.54–18.41, P<0.05; Caucasian, OR=11.35, CI=2.39–53.98, P<0.05; Asian, OR=6.67, CI=2.53–17.64, P<0.05). On the basis of the trial sequential analysis, the significant association of RASSF1A promoter methylation with thyroid cancer risk was found, and there was no need to perform further studies.
Conclusion
This meta-analysis confirms that RASSF1A promoter methylation is a risk factor for thyroid tumor.
Introduction
It has been reported that thyroid carcinomas, the most frequently reported endocrine neoplasia, account for only 3%–4% in all human tumors, but the incidence of thyroid cancer is steadily increasing and has the highest increase in incidence within the past two decades.Citation1 Currently, the use of neck ultrasonography is widely used in the diagnosis of thyroid cancer and brings light to the detection of many small early-stage tumors.Citation2 However, the incidence of detection of large tumors and advanced stage tumor patients have also increased in these years.Citation3,Citation4 Thyroid cancer can be categorized into four histotypes: follicular thyroid carcinoma (FTC), papillary thyroid carcinoma (PTC), anaplastic thyroid carcinoma, and medullary thyroid carcinoma (MTC). PTC, the most common type of thyroid cancer, represents 80% of patients, followed by FTC (10%).Citation5,Citation6 PTC derives from the follicular cells of the thyroid, and it has a distinctive papillary architecture. In addition, the nuclear membrane of cells in PTC has several distinctive alternations, such as grooves, pseudoinclusions, and optical clearing. In contrast to the papillary carcinomas, in which the change of nuclear membrane alternations is vital, the diagnosis of FTC depends on whether the thyroid tumor has invaded through the surrounding vessels. MTC has a rare incidence accounting for ~3%, which derives from parafollicular C cells.Citation7 Compared with FTC and PTC – well differentiated cancer – MTC has a more aggressive clinical characteristic.Citation8 MTC often occurs in a form of inherited cancer, which is known as multiple endocrine neoplasia type 2.Citation9 In recent decades, although many genetic studies for thyroid cancer have been performed, no specific biomarkers for the large number of sporadic thyroid cancers have been found.Citation10–Citation12
The Ras-associated domain family 1 (RASSF1) family of proteins, representing one type of Ras effectors, can inhibit the development of cancer. RASSF1A, one of the seven iso-forms of the RASSF1 family and located on 3p21, is the most common epigenetically inactivated tumor suppressor genes via promoter methylation in human cancers.Citation13,Citation14 It can bind Ras protein in a guanosine triphosphate-dependent manner to mediate the cell apoptotic, which has a similar function with Nore1 in mouse.Citation14 In many solid tumors, RASSF1A, as a component of crucial cell signal pathways, plays an important role in the Ras/PI3K/AKT, Ras/RAF/MEK/ERK, and Hippo pathways, and the inactivation of RASSF1A can result in the alternations of clinical characteristic in tumors.Citation15,Citation16 It has been reported that the CpG methylation of RASSF1A promoter can lead to the loss-of-expression of RASSF1A.Citation14 Previous studies have found that the RASSF1A promoter methylation can increase the risk of lung cancer, breast cancer, prostate cancer, ovarian cancer, renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and gastric cancer.Citation17–Citation24 Furthermore, these results indicated that the RASSF1A promoter methylation may be a significant prognostic factor for many human cancers. Despite a number of studies performed on thyroid cancer patients, the relationship of RASSF1A promoter methylation with thyroid cancer risk and pathogenesis remains controversial. To clarify these controversial results, a meta-analysis was performed.
Methods
Publication search
Relevant literature was retrieved from PubMed, Embase, ISI Web of Knowledge, and Chinese National Knowledge Infrastructure databases up until September 2016, without language restrictions. The following keywords were applied: “RASSF1 protein, human (Supplementary Concept),” “Thyroid Neoplasms (Mesh),” “Methylation (Mesh),” “RASSF1A,” “thyroid cancer,” and “hypermethylation” to search eligible studies. Review articles and the references from relevant primary studies were manually searched for identifying additional potential studies.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) studies primarily evaluating the frequency of RASSF1A promoter methylation in thyroid cancer with control group, 2) studies that have a case–control design, 3) studies that had sufficient data of frequency, and 4) patients who had been accurately diagnosed according to the diagnostic criteria. Studies were removed if they met one of the items: 1) reviews, meta-analysis, or animal studies; 2) duplicate data; and 3) study performed in the cell lines.
Data extraction
The following data were extracted from the eligible studies: year of publication, country, ethnicity, frequency of RASSF1A promoter methylation, cancer type, method of methylation detection, and sample type. The information was reviewed by two investigators. If the two investigators had divergence, the disagreement was resolved by consulting with the other authors.
Quality assessment
To ensure the high quality of included literatures, the Newcastle–Ottawa scale (NOS) table was applied to assess the methodological quality of observational studies. The NOS is commonly used in the quality assessment of nonrandomized studies, such as case–control and cohort studies. The scores of quality assessment range from 0 to 9. If the study got a score <5 it was excluded.
Trial sequential analysis
In a cumulative meta-analysis, false-positive and false-negative results may arise, which result from the repeated testing and the adding of other studies. Therefore, it was crucial to lower the risk of type I error and type II error in the repeated statistics. In addition, interim analysis was performed to estimate the required sample size according to the data in the common trials and to avoid wasting of the sample. In this present meta-analysis, the trial sequential analysis was performed to estimate the required information size (RIS) and observe whether the result was conclusive through controlling the risk of type I error and type II error. TSA 0.9 software (www.ctu.dk/tsa) was applied to conduct these analyses by combining the RIS with trial sequential monitoring boundaries. The trial sequential analysis was conducted at the level of 5% risk of type I error and 80% power of statistical test (5% risk of type II error). Moreover, adjusted information size was calculated with a relative risk reduction (−50%) according to the incidence of control and case groups.
Statistical analysis
The frequency of RASSF1A promoter methylation in case and control groups was collected to calculate the pooled odds ratios (ORs) and 95% confidence interval (CI),Citation25 evaluating the strength of association of RASSF1A promoter methylation with thyroid cancer risk or thyroid cancer clinical characteristics. Heterogeneity among studies was measured using Cochran’s Q-test and Higgin’s I2.Citation26,Citation27 If heterogeneity existed in studies (P<0.05, I2>50%), random-effects model was applied, otherwise, fixed-effects model was used (P>0.05, I2<50%).Citation28,Citation29 In addition, subgroup analysis based on ethnicity and sample type were conducted to detect the source of heterogeneity and lower the between-study heterogeneity. Begg’s test and Egger’ test were used to check for the publication bias. When the P-value was <0.05, publication bias was considered as significant. The aggregated sensitivity and specificity were observed by conducting the sensitivity analysis. All meta-analyses were performed using Stata software 12.0 (Stata Corporation, College Station, TX, USA).
Results
Characteristics of studies
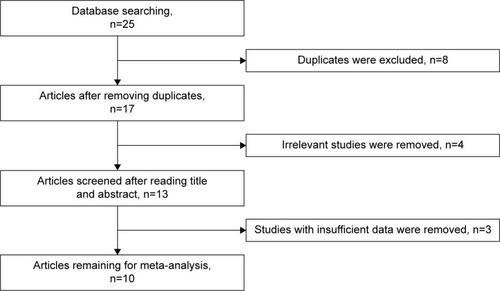
Through searching PubMed, Embase, ISI Web of Knowledge, and Chinese National Knowledge Infrastructure databases, 25 publications were initially retrieved. Eight duplicate articles were removed. After reading titles and abstracts, four articles were excluded due to uncorrelated contents. Furthermore, full-text was read to eliminate irrelevant literatures, and two articles were eliminated because they had no sufficient data of RASSF1A promoter methylation. Finally, 10 publications with 422 patients and 219 controls were identified in this meta-analysis. Of these studies, seven studies were performed in Caucasians,Citation30–Citation36 while three studies were conducted in Asians.Citation37–Citation39 The detection of RASSF1A promoter methylation applied methylation-specific polymerase chain reaction and quantitative methylation-specific polymerase chain reaction. With regard to the type of control sample, normal tissue was used in five studies,Citation31,Citation33,Citation35,Citation36,Citation39 four studies applied benign tissue,Citation31,Citation32,Citation35,Citation38 and adjacent tissue was applied in two studiesCitation34,Citation37 (; and –).
Table 1 Characteristics of eligible studies in the meta-analysis
Quantitative data synthesis
According to this meta-analysis, there was a significant association of RASSF1A promoter methylation with thyroid cancer risk (OR=5.94, 95% CI=2.09–16.89, P<0.05). When stratified by the ethnicity, a significant increased risk of thyroid cancer was detected in Asians, but not in Caucasians. In the stratified analysis by sample type, significantly increased risks were found in both normal tissues and adjacent tissues in detection of RASSF1A promoter methylation in thyroid cancer (normal tissue: OR=9.55, CI=4.21–21.67, P<0.05; adjacent tissue: OR=6.80, CI=2.49–18.56, P<0.05). Furthermore, significant association was also found in both FTC and PTC (for FTC: OR=11.88, CI=5.80–24.32, P<0.05; for PTC: OR=8.07, CI=3.54–18.41, P<0.05). In evaluating the association for pathological stage of thyroid cancer, no significant correlations of RASSF1A promoter methylation with distant metastasis and TNM-stage were observed (–; ).
Figure 2 Forest plot for the association between Ras-associated domain family 1A promoter methylation and risk of thyroid cancer.
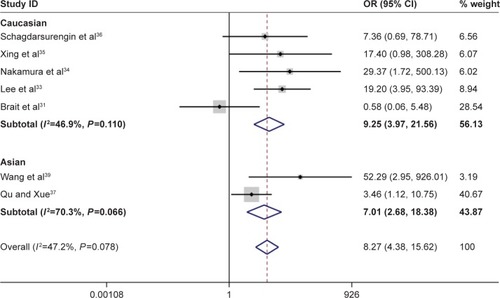
Figure 3 Forest plot for the association between Ras-associated domain family 1A promoter methylation and risk of follicular thyroid carcinoma.
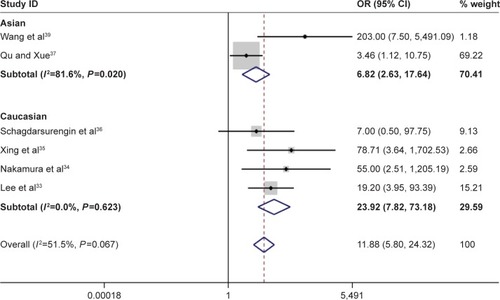
Figure 4 Forest plot for the association between Ras-associated domain family 1A promoter methylation and risk of papillary thyroid carcinoma.
Table 2 Meta-analysis of association between Ras-associated domain family 1A promoter methylation and thyroid cancer risk
Literature qualities
In this meta-analysis, 10 studies were scored by NOS table by two independent authors. The scores of eligible literatures ranged from 6 to 8, suggesting that the quality of included studies were of a high quality and the inclusion criteria were satisfied. The detailed information is shown in .
Publication bias and sensitivity analysis
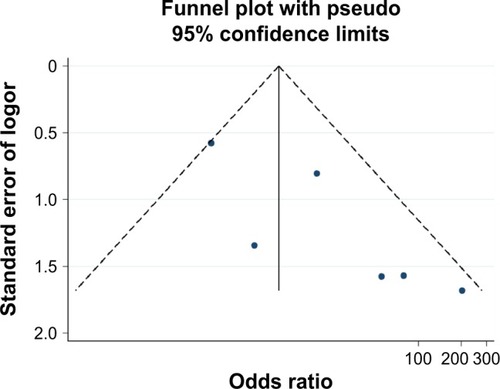
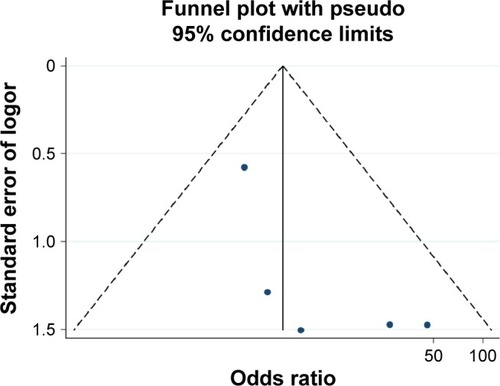
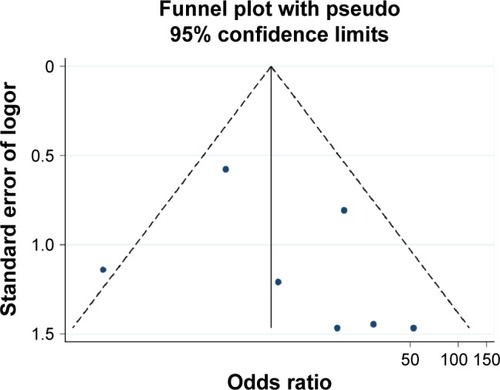
The results of Begg’s test and Egger’s test indicated that no significant publication bias existed in the analysis of association between RASSF1A promoter methylation and thyroid cancer risk or pathological characteristics. (For risk of thyroid: Begg’s test P=0.453, Egger’s test P=0.377; for distant metastasis: Begg’s test P=0.624, Egger’s test P=0.669; for TNM-stage: Begg’s test P=0.117, Egger’s test P=0.441). Sensitivity analysis indicated that the results were stable in this meta-analysis, and the pooled ORs did not have a significant change by omitting each study (–).
Figure 5 Funnel plot of publication biases on the association between Ras-associated domain family 1A promoter methylation and thyroid cancer risk.
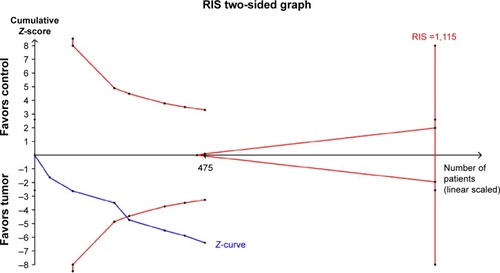
Trial sequential analysis
Trial sequential analysis indicated that Z-curve crossed the traditional boundary and the monitoring boundary for benefit, which indicated that no further studies were performed to study this association of RASSF1A promoter methylation with thyroid cancer risk. The Z-curve did not reach the boundary of RIS, and the RIS was 1,115. In other associations, no trial sequential analysis was conducted for the small number of samples ().
Discussion
Epigenetic alternations play an important role in the regulation of gene expression, especially in mammalian cells. DNA methylation usually occurs in CpG islands – clusters of CpGs of gene promoter region, which can lead to gene activation or inactivation. The hypermethylation of gene promoter represses gene expression by inhibiting gene transcription and further affects the total cell signal pathway. The inactivation of RASSF1A, one of tumor suppressor genes, is critical in the tumorigenesis of thyroid cancer. The changes in gene structure, deletion, or mutation can result in the irreversible loss of RASSF1A function. Alternatively, the cumulative RASSF1A methylation can also influence the expression and affect the progression of cancers, such as tumor differentiation and metastasis. In previous studies of thyroid cancers, many genes methylation have a significant association with the development of thyroid tumor. For example, p16INK4A/CDKN2A, p21CIP1/CDKN1B, and p27KIP1/CDKN1B, considering as tumor suppressors, can regulate the activity of cyclin–CDK complexes in mammalian cells. However, the expression of these genes in which promoter methylation is detected in 30% of thyroid tumor was commonly downregulated in thyroid cancer patients.Citation40,Citation41 In addition, the hypermethylation of thyrotropin receptor gene, receptor, fibroblast growth factor type 2, and receptor, fibroblast growth factor type 1 existed in thyroid cancers.Citation42–Citation44 RASSF1A protein, lacking enzymatic activity, contains a Ras-association domain and can regulate the cell cycle and apoptosis.Citation13,Citation14 Many studies have found that the RASSF1A promoter inactivation was frequent in thyroid cancers, >30% of thyroid tumors,Citation33,Citation34,Citation37,Citation39 but other reports also observed that the RASSF1A promoter methylation did not have significant correlations with thyroid cancer risk.Citation31,Citation35,Citation36 Thus, it is necessary to conduct a meta-analysis to determine the strength of association between RASSF1A promoter methylation and thyroid cancer. In the literature retrieval, a previous meta-analysis was found.Citation45 However, the meta-analysis only explored the association between RASSF1A promoter methylation and PTC risk, and no clinical information was included. The result of the previous study indicated a significant association of RASSF1A promoter methylation with PTC risk. This was the same result in this meta-analysis. To discuss the association of RASSF1A promoter methylation with FTC risk, distant metastasis, and TNM-stage, the meta-analysis was conducted.
In this study, the results indicated that aberrant methylation of RASSF1A promoter was more frequently detected in thyroid cancer than in noncancerous controls. In the included studies, although the results of Wang et alCitation39 and Qu et alCitation37 demonstrated that the frequency of RASSF1A promoter methylation increased the risk of thyroid cancer, the pooled ORs and 95% CI show that there was a significant association between RASSF1A promoter methylation and the risk of thyroid cancer in Asians. Furthermore, a small degree of heterogeneity among studies was noticed in the overall analysis. Therefore, the stratified analysis, on the basis of sample type and ethnicity, was carried out to reduce the heterogeneity. At the same time, the pooled ORs indicated that the significant association was found in the stratified analysis based on ethnicity and sample type. Furthermore, between-study heterogeneity, to a large extent, disappeared in the subgroup analysis. This phenomenon indicated that the sample type and ethnicity were the primary cause of heterogeneity. Interestingly, no significant association between the frequency of RASSF1A promoter methylation in thyroid malignant tumor and the frequency in thyroid benign tumor existed. In other subgroups, the higher frequency of FTC and PTC group was found, compared with the nontumorous group. From all eligible studies in this meta-analysis, no results indicated that the RASSF1A promoter methylation had a significant influence in the metastasis of thyroid tumor.Citation30,Citation36–Citation39 In the meantime, the result of this statistical analysis was similar to the previous studies (for M0-stage and M1-stage: OR=1.18, 95% CI=0.64–2.16, P<0.05). Moreover, no significant association between the frequency of RASSF1A methylation and TNM-stage of thyroid cancer (for I-stage and II–IV-stage: OR=1.79, 95% CI=0.72–4.44, P<0.05) was found. In the included studies, one study found a significant association,Citation38 but two studies obtained negative results.Citation30,Citation38 These results were inconsistent, but now, to a large extent, we could say that the frequency of RASSF1A promoter methylation might not be related with the TNM-stage of thyroid cancer. Certainly, the small sample size may limit the power of statistics. From the trial sequential analysis, although no studies were continuously performed to evaluate the association of RASSF1A promoter methylation with thyroid cancer risk, further studies, investigating the association between RASSF1A promoter methylation and thyroid cancer pathogenesis, were still needed to be carried out.
In this meta-analysis, the publication bias was not detected in the eligible studies. Begg’s test, Egger’s test, and funnel plot were performed, which indicated that the data did not have a considerable discrepancy among studies. Furthermore, consistent results were found in sensitivity analysis. However, several potential limitations must be emphasized in this study. First, with few included studies in this meta-analysis, the influence of small sample size of case and control cannot be ruled out. Second, the value of cut-off point of methylation cannot be achieved, thus the sensitivity and specificity of methylation about thyroid cancer risks cannot be determined. Third, the clinical information was so little that the analysis of association of RASSF1A promoter methylation with the pathogenesis of thyroid cancer patients cannot be performed.
Conclusion
This meta-analysis showed that RASSF1A promoter methylation might play an important role in thyroid cancer initiation and progression. The RASSF1A promoter methylation might be a promising biomarker for the early diagnosis of thyroid cancer. In addition, RASSF1A promoter methylation was associated with the increased risk of distant metastasis and late TNM-stage of thyroid cancer patients. However, in consideration of the limitations acknowledged above, more large-scale, multicenter, and well-designed case–control or cohort researches will provide more insights into the role of RASSF1A promoter methylation in the risk and pathogenesis of thyroid cancer.
Supplementary materials
Table S1 Thyroid cancer risk
Table S2 Meta-analysis table
Table S3 TNM-stage
Table S4 Distant metastasis
Table S5 Papillary thyroid carcinoma
Table S6 Follicular thyroid carcinoma
References
- SantoroAPannoneGCarosiMABRAF mutation and RASSF1A expression in thyroid carcinoma of southern ItalyJ Cell Biochem201311451174118223192464
- BraitMLoyoMRosenbaumECorrelation between BRAF mutation and promoter methylation of TIMP3, RARbeta2 and RASSF1A in thyroid cancerEpigenetics20127771071922694820
- QuFXueWJRASSF1A methylation and its clinical roles in papillary thyroid carcinomaJ Nantong University (Medical Sciences)201232490491
- DaiYLCaiDHChenHZhangZZhangHLiJThe association of the methylation of TSHR and RASSF1A with thyroid cancerShaanxi Med J2011401114461449
- Mohammadi-aslJLarijaniBKhorgamiZQualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARbeta2 genes in papillary thyroid carcinomaMed Oncol20112841123112820535589
- WangXHZhangGCLiuYLSunGFNongWXYaoESThe detection of RASSF1A promoter methylation in thyroid tumor patientsShaanxi Med J200938790792
- LeeJJGeliJLarssonCGene-specific promoter hypermethylation without global hypomethylation in follicular thyroid cancerInt J Oncol200833486186918813801
- NakamuraNCarneyJAJinLRASSF1A and NORE1A methylation and BRAFV600E mutations in thyroid tumorsLab Invest20058591065107515980887
- XingMCohenYMamboEEarly occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesisCancer Res20046451664166814996725
- SchagdarsurenginUGimmOHoang-VuCDralleHPfeiferGPDammannRFrequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinomaCancer Res200262133698370112097277
Disclosure
The authors report no conflicts of interest in this work.
References
- PellegritiGFrascaFRegalbutoCSquatritoSVigneriRWorldwide increasing incidence of thyroid cancer: update on epidemiology and risk factorsJ Cancer Epidemiol2013201396521223737785
- BritoJPDaviesLIs there really an increased incidence of thyroid cancer?Curr Opin Endocrinol Diabetes Obes201421540540825102407
- EnewoldLZhuKRonERising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005Cancer Epidemiol Biomarkers Prev200918378479119240234
- MorrisLGMyssiorekDImproved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysisAm J Surg2010200445446120561605
- EliseiRPincheraAAdvances in the follow-up of differentiated or medullary thyroid cancerNat Rev Endocrinol20128846647522473335
- FaginJAWellsSJBiologic and clinical perspectives on thyroid cancerN Engl J Med2016375111054106727626519
- KebebewEItuartePHSipersteinAEDuhQYClarkOHMedullary thyroid carcinoma clinical characteristics, treatment, prognostic factors, and a comparison of staging systemsCancer20008851139114810699905
- GillilandFDHuntWCMorrisDMKeyCRPrognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the surveillance, epidemiology and end results (SEER) program 1973–1991Cancer19977935645739028369
- RaueFKotzerkeJReinweinDPrognostic factors in medullary thyroid carcinoma: evaluation of 741 patients from the German medullary thyroid carcinoma registerClin Investig1993711712
- RoosLvan DongenJBellCGIntegrative DNA methylome analysis of pan-cancer biomarkers in cancer discordant monozygotic twin-pairsClin Epigenetics20168726798410
- KartalKOnderSKosemehmetogluKKilickapSTezelYGKaynarogluVMethylation status of TSHr in well-differentiated thyroid cancer by using cytologic materialBMC Cancer20151582426519197
- EllisRJWangYStevensonHSGenome-wide methylation patterns in papillary thyroid cancer are distinct based on histological subtype and tumor genotypeJ Clin Endocrinol Metab2014992E329E33724423287
- AllenNPDonningerHVosMDRASSF6 is a novel member of the RASSF family of tumor suppressorsOncogene200726426203621117404571
- HessonLBCooperWNLatifFThe role of RASSF1A methylation in cancerDis Markers2007231–2738717325427
- GuoCTommasiSLiuLYeeJKDammannRPfeiferGPRASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor networkCurr Biol200717870070517379520
- van der WeydenLAdamsDJThe Ras-association domain family (RASSF) members and their role in human tumourigenesisBiochim Biophys Acta200717761588517692468
- YuGSLaiCYXuYBuCFSuZXAberrant methylation of RASSF1A gene contribute to the risk of renal cell carcinoma: a meta-analysisAsian Pac J Cancer Prev201516114665466926107221
- HuangYZWuWWuKXuXNTangWRAssociation of RASSF1A promoter methylation with lung cancer risk: a meta-analysisAsian Pac J Cancer Prev20141523103251032825556469
- JiangDShenYDaiDMeta-analyses of methylation markers for prostate cancerTumour Biol20143510104491045525053593
- SiJGSuYYHanYHChenRHRole of RASSF1A promoter methylation in the pathogenesis of ovarian cancer: a meta-analysisGenet Test Mol Biomarkers201418639440224665911
- AshktorabHRahiHWansleyDToward a comprehensive and systematic methylome signature in colorectal cancersEpigenetics20138880781523975090
- LiYSXieQYangDYZhengYRole of RASSF1A promoter methylation in the pathogenesis of hepatocellular carcinoma: a meta-analysis of 21 cohort studiesMol Biol Rep20144163925393324566681
- ShiDTHanMGaoNTianWChenWAssociation of RASSF1A promoter methylation with gastric cancer risk: a meta-analysisTumour Biol201435294394823982879
- JiangYCuiLChenWDShenSHDingLDThe prognostic role of RASSF1A promoter methylation in breast cancer: a meta-analysis of published dataPLoS One201275e3678022615811
- WoolfBOn estimating the relation between blood group and diseaseAnn Hum Genet195519425125314388528
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- SantoroAPannoneGCarosiMABRAF mutation and RASSF1A expression in thyroid carcinoma of southern ItalyJ Cell Biochem201311451174118223192464
- BraitMLoyoMRosenbaumECorrelation between BRAF mutation and promoter methylation of TIMP3, RARbeta2 and RASSF1A in thyroid cancerEpigenetics20127771071922694820
- Mohammadi-aslJLarijaniBKhorgamiZQualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARbeta2 genes in papillary thyroid carcinomaMed Oncol20112841123112820535589
- LeeJJGeliJLarssonCGene-specific promoter hypermethylation without global hypomethylation in follicular thyroid cancerInt J Oncol200833486186918813801
- NakamuraNCarneyJAJinLRASSF1A and NORE1A methylation and BRAFV600E mutations in thyroid tumorsLab Invest20058591065107515980887
- XingMCohenYMamboEEarly occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesisCancer Res20046451664166814996725
- SchagdarsurenginUGimmOHoang-VuCDralleHPfeiferGPDammannRFrequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinomaCancer Res200262133698370112097277
- QuFXueWJRASSF1A methylation and its clinical roles in papillary thyroid carcinomaJ Nantong University (Medical Sciences)201232490491
- DaiYLCaiDHChenHZhangZZhangHLiJThe association of the methylation of TSHR and RASSF1A with thyroid cancerShaanxi Med J2011401114461449
- WangXHZhangGCLiuYLSunGFNongWXYaoESThe detection of RASSF1A promoter methylation in thyroid tumor patientsShaanxi Med J200938790792
- KhooMLBeasleyNJEzzatSFreemanJLAsaSLOverexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinomaJ Clin Endocrinol Metab20028741814181811932323
- EliseiRShioharaMKoefflerHPFaginJAGenetic and epigenetic alterations of the cyclin-dependent kinase inhibitors p15INK4b and p16INK4a in human thyroid carcinoma cell lines and primary thyroid carcinomasCancer19988310218521939827724
- KhaledHAl LahloubiNRashadNA review on thyroid cancer during pregnancy: multitasking is requiredJ Adv Res20167456557027408758
- StBRZhengLLiuWWinerDAsaSLEzzatSFibroblast growth factor receptors as molecular targets in thyroid carcinomaEndocrinology200514631145115315564323
- KondoTZhengLLiuWKurebayashiJAsaSLEzzatSEpigenetically controlled fibroblast growth factor receptor 2 signaling imposes on the RAS/BRAF/mitogen-activated protein kinase pathway to modulate thyroid cancer progressionCancer Res200767115461547017545628
- JiangJLTianGLChenSJXuLIWangHQPromoter methylation of p16 and RASSF1A genes may contribute to the risk of papillary thyroid cancer: a meta-analysisExp Ther Med20151041549155526622524